Abstract
Neonatal units have started to switch from using conventional soy-based to alternate lipid emulsions, like SMOFlipid. SMOFlipid has been associated with an improvement in biochemical parameters and delays progression of parenteral nutrition-associated liver disease (PNALD). This retrospective epoch study aimed to compare clinically relevant neonatal outcomes in preterm infants (< 32 weeks), receiving SMOFlipid versus Intralipid. We compared clinical outcomes in two epochs—epoch 1 (Intralipid, October 2013–June 2015) versus epoch 2 (SMOFlipid, July 2015–March 2017). Primary outcome studied was mortality and rates of severe neonatal morbidities. Univariate and multivariate regression was conducted to determine risk for mortality and PNALD. A total of 222 infants (epoch 1, 123 versus epoch 2, 99) were included in the study. A higher incidence of late onset sepsis (56 versus 30%, p < 0.005) was observed in epoch 1. There was no significant difference in mortality or rates of any other severe neonatal morbidity. The type of lipid emulsion did not have a significant effect on mortality or PNALD on regression analysis.
Conclusion: Use of SMOFlipid as the primary lipid emulsion seems to have minimal effect on rates of clinically important neonatal outcomes; however, long-term effects need to be further evaluated.
What is Known: • Many neonatal units have started replacing traditional soy-based lipid formulations with SMOFlipid (ω-3 enriched lipid emulsion), as the primary lipid component in parenteral nutrition for preterm infants. • While there is evidence associating improved liver function and balanced essential fatty acid levels in infants receiving SMOFlipid, there is a lack of evidence evaluating relevant clinical outcomes in infants receiving SMOFlipid versus traditional lipid formulations. |
What is New: • The influence of SMOFlipid on a series of clinical outcomes in an at-risk preterm population is presented. • SMOFlipid appears to be well tolerated in preterm infants with minimal side effects, and some growth benefits seen. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Preterm infants often require parenteral feeding to meet energy requirements and provide essential nutrients, optimising postnatal growth and development [15, 24]. Total parenteral nutrition (TPN) supplies nutrients, caloric requirements, as well as essential and non-essential fatty acids vital for development [9, 16, 27].
Intravenous fat emulsions provide necessary lipids required to form key structural components in nearly all tissues and serve as precursors for prostaglandins, prostacyclins and other eicosanoids. These eicosanoids are regulators of various cellular processes, including inflammation, immune response and platelet aggregation [17, 27]. Parenteral nutrition can present with adverse neonatal outcomes that may be related to the type of fat emulsion, rate of infusion and total duration of administration [17]. Hence, there has been a widespread interest in developing alternate lipid formulations with goals of optimising neonatal outcomes.
Intralipid (Baxter Australia, NSW), a pure soy-based emulsion, has been the traditional lipid emulsion used in neonatal intensive care units (NICUs) around the world. Pure soy-based lipid preparations contain minimal long-chain polyunsaturated fatty acid (LCPUFA) derivatives of ω-6 and ω-3 fatty acids (arachidonic acid (ARA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA)). EPA and ARA play an important immunomodulatory role in activation of pro-inflammatory (mainly ARA derived) and anti-inflammatory pathways (mainly EPA derived) [30]. Soybean emulsions contain a high ratio of ω-6: ω-3 fatty acids, which in turn leads to lesser proportions of ω-3 fatty acid derivatives, DHA and EPA. DHA and EPA are important substances for neural and retinal development with key anti-inflammatory properties [23]. As the preterm infant is unable to convert and generate a sufficient supply of vital LCPUFA themselves, the impact of fatty acid deficits upon neonatal growth and clinical outcomes can be significant [15, 27].
Alternate lipid emulsions are being used in a number of neonatal units—including combinations of clinoleic acid, fish oil, medium and long chain triglycerides. In the last decade, SMOFlipid (Fresenius Kabi Australia Pty Ltd), a formula consisting of soybean, medium chain triglycerols (MCT), olive oil and fish oil has become a popular lipid emulsion as it provides immunomodulatory benefits and a balanced ω-6:ω-3 fatty acid ratio [30]. MCTs are commonly derived from coconut oil and are shown to lower ω-6 fatty acid levels. MCT and soy oil mixtures are also less susceptible to lipid peroxidation, thus associated with reduced systematic inflammatory response and positive effect on leukocyte and reticuloendothelial function [30].
Another alternative source, olive oil, has been found to reduce high content of ω-6 fatty acid and provide additional monosaturated fatty acids and vitamin E, important to prevent cellular damage [30, 31]. Fish oil is believed to modulate various anti-inflammatory pathways as it contains a high proportion of ω-3 fatty acid which is rarely found in other plant oil-based emulsions [23, 30]. Hence, due to its lesser phytosterol content [8, 10] and perceived anti-inflammatory benefits, SMOFlipid has been conventionally used in surgical patients and infants with intestinal failure-associated liver disease [19]. SMOFlipid has been found to have a positive effect in lowering total bilirubin, with the fish oil component associated to the reversal of parenteral nutrition-associated liver disease (PNALD) in some infants [7, 14, 18, 20].
SMOFlipid has shown to be safe and is associated with increased levels of DHA and EPA, with conversely lower arachidonic acid levels in both erythrocyte membranes and plasma of premature infants as compared to pure soy emulsions. Studies have compared biochemical markers in premature infants receiving a pure soy-based against alternate lipid emulsions with statistically significant differences seen in serum composition [6, 21, 28, 29, 32]. However, there is limited evidence to suggest any clinically significant differences amongst infants receiving SMOFlipid versus a soy-based lipid emulsion when used routinely in preterm infants [13].
Despite many previous intervention studies comparing SMOFlipid to alternate lipid emulsions, there is insufficient data upon the clinical impact. Our aim was to conduct an epoch study to evaluate the incidence of common neonatal outcomes between two time periods in order to directly compare SMOFlipid with Intralipid.
Methods
Study design
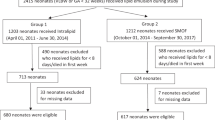
This was a retrospective cohort study analysing data from the neonatal unit (Monash Newborn) at Monash Children’s Hospital, Melbourne. Monash Newborn is a tertiary combined medical and surgical neonatal unit with approximately 4000 births per annum. Appropriate ethics approval was obtained from the Hospital Human Research Ethics Committee to review data. In July 2015, Monash Newborn moved from using Intralipid to SMOFlipid as the primary lipid emulsion for parenteral nutrition in preterm neonatal infants. We thus outlined two epoch groups to help compare the time periods in which SMOFlipid and Intralipid were used.
The two epoch periods were defined as:
-
Epoch 1: 20 months period prior to the introduction of SMOFlipid, where Intralipid was primarily used: October 2013–June 2015
-
Epoch 2: 20 months period after the introduction of SMOFlipid: July 2015–March 2017
We collaborated with the hospital pharmacy to acquire TPN data of the preterm infants (< 32 weeks) that required either Intralipid or SMOFlipid for at least 14 days from both epoch periods. Hospital electronic databases (medical records, pathology and imaging) were then accessed to retrieve clinical data from the two cohorts. Chart reviews of individual patients were also conducted to ensure accuracy and completeness of information. Baseline characteristics of each epoch group were compared in respect to gestational age at birth, maternal age, sex, birth weight, length, head circumference, antenatal and immediate postnatal characteristics.
Inclusion criteria
The inclusion criteria were preterm infants born prior to 32 weeks of gestation and requiring a single parenteral lipid emulsion formula for a minimum of 14 consecutive days.
Exclusion criteria
The exclusion criteria were infants with severe congenital malformation, metabolic disorders or medical records with insufficient/missing data. Infants with insufficient medical records (15 in total) were typically preterms that had been transferred to an alternative health network at parents’ request.
Intervention
Infants requiring TPN were administered either SMOFlipid or Intralipid emulsion for duration of at least 14 days starting within 24 h of birth. Central venous access was used to administer TPN in most cases and either lipid emulsion was titrated up to a rate of 3–4 g/kg/day. Both lipid emulsions were administered in a separate syringe alongside standardised solution containing protein, glucose, minerals and trace elements. Parenteral nutrition continued in both epochs until full enteral feeds were tolerated with no difference in standard cardio-respiratory care between the epochs.
Outcomes
Primary outcomes compared between the epoch groups included mortality, rates of PNALD (defined via abnormal biochemical markers), patent ductus arteriosus requiring treatment, bronchopulmonary dysplasia (defined as need for oxygen or respiratory support at 36 weeks postconceptional age), severe intraventricular haemorrhage (grade 3–4), cystic periventricular leukomalacia, retinopathy of prematurity requiring laser treatment, necrotising enterocolitis (all stages) and late-onset culture-proven sepsis. Composite outcome of mortality or any of the above serious morbidities was also compared. Secondary outcomes compared included duration of invasive ventilation, duration of total pressure support, duration of oxygen requirement, need for home oxygen, any stage of retinopathy of prematurity, any grade of intraventricular haemorrhage, periventricular leukomalacia, days till enteral feeding tolerated, length of stay and growth parameters at 36 weeks.
Based on an estimated 80% incidence of composite outcome (death or major morbidity) in this infant population, to expect a 20% improvement in composite outcomes (with SMOFlipid) with an alpha error of 0.05 at 0.8 power, a minimum of 81 infants would be needed in each epoch.
All statistical analysis was performed using STATA 14 (StataCorp LP, TX, USA). Continuous variables were expressed as mean (standard deviation (SD)) or median (interquartile range (IQR)). Normally distributed data were analysed by Students t test and skewed data by Wilcoxon rank-sum test. Categorical variables were analysed by chi-squared test. Multivariate and univariate logistic regression analysis adjusted for baseline variables was undertaken to determine the significant risk factors for mortality and PNALD, expressed as adjusted odds ratio (OR) and 95% confidence (CI). Statistical significance was set at p < 0.05.
Results
During the study period, 1113 preterm infants (less than 32 weeks’ gestation) were admitted to the NICU. Overall, 222 infants were enrolled in the study who had received TPN lipid for 14 or more days, with 123 in epoch 1 receiving Intralipid and 99 infants in epoch 2 receiving SMOFlipid (Fig. 1). The mean gestational age (SD) (26.7 (2.1) versus 26.7 (2.0) weeks; p = 0.9) and birth weight (935.6 (342) versus 935.5 (25) grams; p = 1.0) were very similar in the two epoch groups. Both groups had similar baseline characteristics (Table 1), with no statistically significant disparity between sex, duration of lipid administration and rates of IUGR. There was a slightly significant difference seen in rates (%) of premature rupture of membranes (PROM) (42 versus 21%; p < 0.05) and antepartum haemorrhage (APH) (10 versus 20%; p < 0.05) between the epochs.
Table 2 shows the results of the primary outcomes in both epochs. There was no significant difference in the rates of bronchopulmonary dysplasia (57 versus 67%; p = 0.3, risk difference = 0.1 (− 0.1, 0.2)), PNALD (7 versus 11%; p = 0.3, risk difference = 0.03 (− 0.03, 0.1)), necrotising enterocolitis (22 versus 19%; p = 0.6, risk difference = − 0.02 (− 0.1, 0.1)) or mortality (6 versus 4%; p = 0.4, risk difference = − 0.01 (− 0.1, 0.03)) between the two epoch groups. A noticeably larger incidence of late onset sepsis was observed in epoch 1 as compared to epoch 2 (56 versus 30%; p < 0.005, risk difference = − 0.3 (− 0.4, − 0.1)), yet composite outcomes (death and/or any major morbidity) (85 versus 80%; p = 0.3, risk difference = − 0.1 (− 0.2, 0.04)) remained similar between groups.
Secondary outcomes are presented in Table 3. Retinopathy of prematurity (ROP) (any stage) was observed less frequent in epoch 1 versus epoch 2 which was statistically significant (39 versus 54%; p = 0.03, risk difference = 0.1 (0.01, 0.3)), yet there was no difference amongst rates of infants requiring laser therapy for management of ROP. On the other hand, rates of intraventricular haemorrhage (any grade) were shown to be statistically greater in epoch 1 versus epoch 2 (44 versus 30%; p = 0.03, risk difference = − 0.1 (− 0.3, − 0.01), yet no significant difference was seen in rates of intraventricular haemorrhage grades 3–4. There was a trend towards higher use of postnatal steroids (24 versus 18%, p = 0.06, risk difference = 0.1 (− 0.007, 0.2)) in epoch 2, concurrent with the slightly higher rate of BPD. When comparing growth outcomes, infants receiving SMOFlipid were found to have a greater weight at 36 weeks postconception (2016 versus 2141 g; p < 0.05, risk difference = 125.0 (3.0, 247.1), alongside a significant increase in weight at 36 weeks.
Univariate and multivariate logistic regression analyses were performed to investigate the association between clinical risk factors and two outcomes of interest, mortality and PNALD (Table 4). Both regression models found that the type of lipid did not influence rates of death (univariate odds ratio = 1.4 (CI 0.4, 5.0); multivariate odds ratio = 0.6 (CI 0.4, 51.3)) or PNALD (univariate odds ratio = 0.6 (CI 0.3, 1.6); multivariate odds ratio = 0.6 (CI 0.2, 1.9)). The univariate model established intrauterine growth restriction as the only significant risk factor for mortality associated with a sixfold increased risk of death (odds ratio = 6.4 (CI 1.9, 22.3)). Univariate analysis identified total lipid duration and necrotising enterocolitis as significant risk factors for development of PNALD. There was found to be a 10% increased risk of PNALD with every additional day of lipid administration (odds ratio = 1.1 (CI 1.05–1.1)) and a near sixfold increase in rates of PNALD associated with necrotising enterocolitis (odds ratio = 5.8 (2.2, 15.1). Total lipid duration was the only significant risk factor identified for PNALD (odds ratio = 1.1 (CI 1.04–1.1)) in the multivariate analysis. In addition, multivariate regression identified gestational age to be the only significant risk for mortality with a threefold increased risk of death with every week decrease in gestation (odds ratio = 3.0 (CI 1.2, 7.9)).
Discussion
We compared clinically relevant outcomes in preterm infants receiving SMOFlipid as the primary lipid emulsion to those receiving Intralipid emulsion in this epoch-based study. SMOFlipid is increasingly being used as the primary lipid component in parenteral nutrition, albeit with limited data evaluating its clinical impact. Thus, this being one of the first studies to primarily compare clinical outcomes between these two commonly used lipid emulsions in preterm infants.
A recent Cochrane review [13] concentrating on clinical outcomes in infants receiving SMOFlipid showed no notable difference in significant outcomes including mortality, bronchopulmonary dysplasia (BPD), sepsis, growth rates and parenteral nutrition-associated liver disease (PNALD). However, most studies included in the systematic review [13] focused on biochemical markers as primary outcomes, with the largest study consisting of only 96 participants [29]. All studies attested SMOFlipid to be safe and well tolerated in the neonatal population as compared to other lipid formulations. However, it was concluded that larger studies were required to investigate clinically relevant outcomes in targeted neonatal population groups.
This study investigated the incidence of important clinical outcomes in very preterm infants born less than 32 weeks receiving Intralipid versus SMOFlipid. Infants received minimum of 14 days’ continuous lipid emulsion as a component of parenteral nutrition to ensure adequate exposure to the lipid formulations. Previous trials show an influence on biochemical parameters in infants receiving lipid emulsion for at least 7–14 days [5, 6, 21, 29, 32], thus we postulated this to be an adequate period to produce meaningful impacts on clinical outcomes. Participants from both epoch periods were well matched with no significant differences in nearly all demographic features. The difference seen in rates of PROM and APH between cohorts is most likely coincidental as there is no recognisable difference in diagnosis of either complication. Prenatal and postnatal care remained largely consistent between both epochs, although there was a change in central line care practice in late 2014 based upon a quasi-experimental study at the study site [26]. The new checklist initiative was associated with reduced central line-associated bloodstream infections which most likely contributed towards lesser rates of late-onset sepsis seen in the SMOFlipid cohort.
No previous trials have recognised any mortality benefit associated with any alternate lipid emulsion formula [13]. Intrauterine growth restriction and gestational age were the only predictors of mortality identified in this study, which remains consistent with published data.
An interesting trend was seen in the respiratory outcomes between the groups, with infants receiving SMOFlipid observed to have slightly higher rates of bronchopulmonary dysplasia, require longer duration of invasive ventilation, total days of pressure support and oxygen use (none of them however statistically significant). With significantly lesser rates of culture-proven sepsis in the SMOFlipid cohort, it may have been expected to see better rates of bronchopulmonary dysplasia and related respiratory outcomes. These figures stand similar to findings of the recently concluded N3RO trial [4] and may warrant larger randomised control trials directly comparing respiratory outcomes in SMOFlipid versus Intralipid. N3RO trial specifically focused on rates of bronchopulmonary dysplasia in neonates receiving enteral supplemental docosahexaenoic acid (DHA) versus control soy lipid emulsion. The results of the study challenged traditional perspectives and found DHA supplementation to possibly increase risk of bronchopulmonary dysplasia in preterm infants. The N3RO trial and this study have both focused upon vulnerable preterm infants requiring longer durations of parenteral nutrition. Hence, the population groups investigated have been at higher risk for developing bronchopulmonary dysplasia as well as other adverse clinical outcomes. This data may raise concern regarding the potential impact of SMOFlipid on respiratory outcomes in a susceptible preterm population.
Lesser rates of retinopathy of prematurity (ROP) (any stage) were seen in the group receiving Intralipid, yet no difference was noted in rates of ROP requiring laser therapy. One study has previously reported lower rates of ROP (stage 1–2) in association with use of SMOFlipid [2], yet no evidence exists to favour either lipid emulsion in the progression of ROP to advanced stages [13].
Differences were also recognised in rates of intraventricular haemorrhage (any grade), with lesser incidence associated to SMOFlipid. Despite having potential neurodevelopment benefits [12, 23], SMOFlipid did not impact on the progression of haemorrhage as rates of severe IVH (grade 3–4) and periventricular leukomalacia remained similar. These results stand consistent with previous trials [13].
PNALD is one of the most well-reported adverse effects associated to parenteral nutrition, with evidence suggesting SMOFlipid may aid in delaying progression or even reversal of the complication [7, 18]. Additionally, SMOFlipid has been associated with increased levels of eicosapentaenoic acid (EPA) and DHA, whilst decreasing plasma bilirubin [6, 32]. Both our univariate and multivariate regression models identified lipid duration to be a significant risk factor in the development of PNALD with the choice of lipid being inconsequential. In our univariate model, however, necrotising enterocolitis was associated with a notable sixfold increase in the rate of PNALD. Further investigations focusing on at risk populations would be needed to assess the possible influence of alternate lipid emulsions and necrotising enterocolitis on PNALD rates.
Average birthweights in both epochs were near identical, yet infants receiving SMOFlipid had a significantly greater body weight at 36 weeks’ postconception. Another randomised control trial has associated SMOFlipid with a greater rate of weight gain [29], whilst majority shows inconsequential difference [13, 21, 22]. It is difficult to attribute better growth outcomes to either lipid emulsion as the greater incidence of late-onset sepsis may be confounding results. As mentioned, a number of studies have associated infants receiving SMOFlipid with increased levels of EPA and DHA, yet concurrently having lower levels of plasma arachidonic acid (ARA) [32]. Interestingly, previous data has correlated ARA levels with infantile growth [3], whilst other data suggests that ω-3 supplementation in pregnancy may be associated with greater head circumference [25]. European guidelines currently recommend formulations with balanced components of ω-3 and ω-6 fatty acids [1, 11, 15], as further research is necessary to comprehend the role of fatty acid components in growth [12].
It is important to emphasise that our primary focus on clinical outcomes increases the relevance of our study to clinical practice. Furthermore, the study targeted an at-risk population which further raises the clinical relevance as a comprehensive range of common neonatal complications was measured. Each of the infantile outcomes had been predefined, with definitions remaining constant through the two cohorts to minimise variation in detection.
As a retrospective study, limitations include potential selection bias and inability to accurately control exposure to lipid formulations. The study did not focus on biochemical parameters which may have added some significance in correlation to clinical outcomes. Long-term follow-up may add further value to assess development of infantile complications as data from the study is limited to discharge date.
Conclusions
SMOFlipid is fast becoming the primary lipid emulsion of choice replacing soybean-based formulations in neonatal units around the word. With evidence suggesting it may provide a more balanced nutritional supply, there is still a need to further evaluate its clinical impact and particular effect on respiratory outcomes, parenteral nutrition-associated liver disease and growth.
Abbreviations
- APH:
-
Antepartum haemorrhage
- ARA:
-
Arachidonic acid
- BPD:
-
Bronchopulmonary dysplasia
- CI:
-
Confidence interval
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- IQR:
-
Interquartile range
- IUGR:
-
Intrauterine growth restriction
- IVH:
-
Intraventricular haemorrhage
- LCPUFA:
-
Long-chain polyunsaturated fatty acids
- MCT:
-
Medium chain triglycerides
- NEC:
-
Necrotising enterocolitis
- NICU:
-
Neonatal intensive care unit
- OFC:
-
Occipitofrontal circumference
- OR:
-
Odds ratio
- PROM:
-
Premature rupture of membranes
- ROP:
-
Retinopathy of prematurity
- SD:
-
Standard deviation
- TPN:
-
Total parenteral nutrition
References
Agostoni C, Buonocore G, Carnielli VP, De Curtis M, Darmaun D, Decsi T, Domellof M, Embleton ND, Fusch C, Genzel-Boroviczeny O, Goulet O, Kalhan SC, Kolacek S, Koletzko B, Lapillonne A, Mihatsch W, Moreno L, Neu J, Poindexter B, Puntis J, Putet G, Rigo J, Riskin A, Salle B, Sauer P, Shamir R, Szajewska H, Thureen P, Turck D, van Goudoever JB, Ziegler EE (2010) Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J Pediatr Gastroenterol Nutr 50(1):85–91. https://doi.org/10.1097/MPG.0b013e3181adaee0
Beken S, Dilli D, Fettah ND, Kabatas EU, Zenciroglu A, Okumus N (2014) The influence of fish-oil lipid emulsions on retinopathy of prematurity in very low birth weight infants: a randomized controlled trial. Early Hum Dev 90(1):27–31. https://doi.org/10.1016/j.earlhumdev.2013.11.002
Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA (1993) Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci U S A 90(3):1073–1077
Collins CT, Gibson RA, Makrides M, McPhee AJ, Sullivan TR, Davis PG, Thio M, Simmer K, Rajadurai VS (2016) The N3RO trial: a randomised controlled trial of docosahexaenoic acid to reduce bronchopulmonary dysplasia in preterm infants < 29 weeks' gestation. BMC Pediatr 16:72. https://doi.org/10.1186/s12887-016-0611-0
D'Ascenzo R, D'Egidio S, Angelini L, Bellagamba MP, Manna M, Pompilio A, Cogo PE, Carnielli VP (2011) Parenteral nutrition of preterm infants with a lipid emulsion containing 10% fish oil: effect on plasma lipids and long-chain polyunsaturated fatty acids. J Pediatr 159(1):33–38.e31. https://doi.org/10.1016/j.jpeds.2010.12.052
Deshpande G, Simmer K, Deshmukh M, Mori TA, Croft KD, Kristensen J (2014) Fish Oil (SMOFlipid) and olive oil lipid (Clinoleic) in very preterm neonates. J Pediatr Gastroenterol Nutr 58(2):177–182. https://doi.org/10.1097/mpg.0000000000000174
Diamond IR, Grant RC, Pencharz PB, de Silva N, Feldman BM, Fitzgerald P, Sigalet D, Dicken B, Turner J, Marchand V, Ling SC, Moore AM, Avitzur Y, Wales PW (2016) Preventing the progression of intestinal failure–associated liver disease in infants using a composite lipid emulsion: a pilot randomized controlled trial of SMOFlipid. J Parenter Enter Nutr 41(5):866–877. https://doi.org/10.1177/0148607115626921
Fell GL, Nandivada P, Gura KM, Puder M (2015) Intravenous lipid emulsions in parenteral nutrition. Adv Nutr (Bethesda, Md) 6(5):600–610. https://doi.org/10.3945/an.115.009084
Fusch C, Bauer K, Böhles HJ, Jochum F, Koletzko B, Krawinkel M, Krohn K, Mühlebach S, Working group for developing the guidelines for parenteral nutrition of The German Society for Nutritional M (2009) Neonatology/Paediatrics—Guidelines on Parenteral Nutrition, Chapter 13. GMS German Medical Science 7:Doc15. doi:https://doi.org/10.3205/000074
Guthrie G, Kulkarni M, Vlaardingerbroek H, Stoll B, Ng K, Martin C, Belmont J, Hadsell D, Heird W, Newgard CB, Olutoye O, van Goudoever J, Lauridsen C, He X, Schuchman EH, Burrin D (2016) Multi-omic profiles of hepatic metabolism in TPN-fed preterm pigs administered new generation lipid emulsions. J Lipid Res 57(9):1696–1711. https://doi.org/10.1194/jlr.M069526
Hojsak I, Colomb V, Braegger C, Bronsky J, Campoy C, Domellof M, Embleton N, Fidler Mis N, Hulst JM, Indrio F, Lapillonne A, Mihatsch W, Molgaard C, van Goudoever J, Fewtrell M (2016) ESPGHAN Committee on Nutrition position paper. Intravenous lipid emulsions and risk of hepatotoxicity in infants and children: a systematic review and meta-analysis. J Pediatr Gastroenterol Nutr 62(5):776–792. https://doi.org/10.1097/mpg.0000000000001121
Huffman SL, Harika RK, Eilander A, Osendarp SJM (2011) Essential fats: how do they affect growth and development of infants and young children in developing countries? A literature review. Matern Child Nutr 7(s3):44–65. https://doi.org/10.1111/j.1740-8709.2011.00356.x
Kapoor V, Glover R, Malviya MN (2015) Alternative lipid emulsions versus pure soy oil based lipid emulsions for parenterally fed preterm infants. Cochrane Database Syst Rev 12. https://doi.org/10.1002/14651858.CD009172.pub2
Kelly DA (2010) Preventing parenteral nutrition liver disease. Early Hum Dev 86(11):683–687. https://doi.org/10.1016/j.earlhumdev.2010.08.012
Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R (2005) 1. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr 41(Suppl 2):S1–87
Lee EJ, Simmer K, Gibson RA (1993) Essential fatty acid deficiency in parenterally fed preterm infants. J Paediatr Child Health 29(1):51–55
Mirtallo JM, Dasta JF, Kleinschmidt KC, Varon J (2010) State of the art review: intravenous fat emulsions: current applications, safety profile, and clinical implications. Ann Pharmacother 44(4):688–700. https://doi.org/10.1345/aph.1M626
Muhammed R, Bremner R, Protheroe S, Johnson T, Holden C, Murphy MS (2012) Resolution of parenteral nutrition-associated jaundice on changing from a soybean oil emulsion to a complex mixed-lipid emulsion. J Pediatr Gastroenterol Nutr 54(6):797–802. https://doi.org/10.1097/MPG.0b013e3182447daf
Nandivada P, Carlson SJ, Chang MI, Cowan E, Gura KM, Puder M (2013) Treatment of parenteral nutrition-associated liver disease: the role of lipid emulsions. Adv Nutr (Bethesda, Md) 4(6):711–717. https://doi.org/10.3945/an.113.004770
Pichler J, Simchowitz V, Macdonald S, Hill S (2014) Comparison of liver function with two new/mixed intravenous lipid emulsions in children with intestinal failure. Eur J Clin Nutr 68(10):1161–1167. https://doi.org/10.1038/ejcn.2014.118
Rayyan M, Devlieger H, Jochum F, Allegaert K (2012) Short-term use of parenteral nutrition with a lipid emulsion containing a mixture of soybean oil, olive oil, medium-chain triglycerides, and fish oil: a randomized double-blind study in preterm infants. JPEN J Parenter Enteral Nutr 36(1 Suppl):81s–94s. https://doi.org/10.1177/0148607111424411
Savini S, D'Ascenzo R, Biagetti C, Serpentini G, Pompilio A, Bartoli A, Cogo PE, Carnielli VP (2013) The effect of 5 intravenous lipid emulsions on plasma phytosterols in preterm infants receiving parenteral nutrition: a randomized clinical trial. Am J Clin Nutr 98(2):312–318. https://doi.org/10.3945/ajcn.112.056556
Simopoulos AP (2002) Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr 21(6):495–505
Su B-H (2014) Optimizing nutrition in preterm infants. Pediatr Neonatol 55(1):5–13. https://doi.org/10.1016/j.pedneo.2013.07.003
Szajewska H, Horvath A, Koletzko B (2006) Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: a meta-analysis of randomized controlled trials. Am J Clin Nutr 83(6):1337–1344
Taylor JE, McDonald SJ, Earnest A, Buttery J, Fusinato B, Hovenden S, Wallace A, Tan K (2017) A quality improvement initiative to reduce central line infection in neonates using checklists. Eur J Pediatr 176(5):639–646. https://doi.org/10.1007/s00431-017-2888-x
Uauy R, Hoffman DR (2000) Essential fat requirements of preterm infants. Am J Clin Nutr 71(1 Suppl):245s–250s
Uthaya S, Liu X, Babalis D, Dore CJ, Warwick J, Bell J, Thomas L, Ashby D, Durighel G, Ederies A, Yanez-Lopez M, Modi N (2016) Nutritional Evaluation and Optimisation in Neonates: a randomized, double-blind controlled trial of amino acid regimen and intravenous lipid composition in preterm parenteral nutrition. Am J Clin Nutr 103(6):1443–1452. https://doi.org/10.3945/ajcn.115.125138
Vlaardingerbroek H, Vermeulen MJ, Carnielli VP, Vaz FM, van den Akker CH, van Goudoever JB (2014) Growth and fatty acid profiles of VLBW infants receiving a multicomponent lipid emulsion from birth. J Pediatr Gastroenterol Nutr 58(4):417–427. https://doi.org/10.1097/mpg.0000000000000280
Waitzberg DL, Torrinhas RS, Jacintho TM (2006) New parenteral lipid emulsions for clinical use. JPEN J Parenter Enteral Nutr 30(4):351–367
Wanten GJ, Calder PC (2007) Immune modulation by parenteral lipid emulsions. Am J Clin Nutr 85(5):1171–1184
Zhao Y, Wu Y, Pei J, Chen Z, Wang Q, Xiang B (2015) Safety and efficacy of parenteral fish oil-containing lipid emulsions in premature neonates. J Pediatr Gastroenterol Nutr 60(6):708–716. https://doi.org/10.1097/mpg.0000000000000665
Acknowledgements
The authors wish to thank Ms. Megan Williams, Pharmacist, Monash Health.
Author information
Authors and Affiliations
Contributions
NC performed the data collection and analysis, wrote the first draft and approved the final version of the manuscript. KT assisted in data analysis, critically reviewed and approved the final version of the manuscript. AM formulated the research question, critically reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Communicated by Patrick Van Reempts
Rights and permissions
About this article
Cite this article
Choudhary, N., Tan, K. & Malhotra, A. Inpatient outcomes of preterm infants receiving ω-3 enriched lipid emulsion (SMOFlipid): an observational study. Eur J Pediatr 177, 723–731 (2018). https://doi.org/10.1007/s00431-018-3112-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-018-3112-3