Abstract
Background
Children who survive acute traumatic brain injury are at risk of death from subsequent brain swelling and secondary injury. Strict physiologic management in the ICU after traumatic brain injury is believed to be key to survival, and cerebral perfusion pressure is a prominent aspect of post brain injury care. However, optimal cerebral perfusion pressure targets for children are not known. Autoregulation monitoring has been used to delineate individualized optimal perfusion pressures for patients with traumatic brain injury. The methods to do so are diverse, confusing, and not universally validated.
Methods
In this manuscript, we discuss the history of autoregulation monitoring, outline and categorize the methods used to measure autoregulation, and review the available validation data for methods used to monitor autoregulation.
Conclusions
Impaired autoregulation after traumatic brain injury is associated with a poor prognosis. Observational data suggests that optimal neurologic outcome and survival are associated with optimal perfusion pressure defined by autoregulation monitoring. No randomized, controlled, interventional data is available to assess autoregulation monitoring after pediatric traumatic brain injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury (TBI) is a significant cause of global morbidity and mortality [1]. Decreasing the impact of TBI will depend on management strategies to prevent secondary injury that occurs during evolution of the primary injury. These secondary injuries can be worsened by poor control of temperature, arterial blood pressure (ABP), cerebral blood flow (CBF), seizure activity and metabolism, and intracranial pressure (ICP). Maintenance of adequate and appropriate CBF is central to this effort and includes monitoring ICP and ABP to calculate and manipulate cerebral perfusion pressure (CPP) [2]. However, the guidelines for management of CPP after paediatric TBI provide only the weakest level of recommendation for CPP thresholds [3]. Although CPP has been associated with outcome, the use of CPP targets has not been shown to improve outcome. Cerebral autoregulation (CA) is a mechanism that regulates CBF in a state of health. Monitoring CA has been proposed as a method to delineate CPP goals for patients after TBI.
Measuring and monitoring CA are complex, nuanced, and easily confounded. Multiple innate servo mechanisms are operational in the vasculature of the intact brain to constrain and regulate CBF. These include responses to changes in metabolism (neurovascular coupling, or metabolic autoregulation), pressure reactivity (pressure autoregulation), CO2 reactivity, and reactivity to hypoxia and hypoglycaemia. Although any of these homeostatic mechanisms can be considered forms of autoregulation, it is pressure reactivity that is most often termed CA and is the topic of this review. When CA is robust, CBF is independent of changes in CPP, and when CA is impaired, CBF is dependent on CPP, or “pressure-passive”. Pressure passivity is a state of profound vulnerability to injury from hypotension or hypertension. The range of CPP within which CA is effective is bounded at the low end by the lower limit of autoregulation (LLA).
It is intuitive to hypothesise an optimal CPP management above LLA, and within the range of effective CA after TBI, or in any state of critical illness. This review describes ongoing efforts to develop and validate tools that identify optimal CPP and ABP goals using CA monitoring. The historic context of our current understanding of autoregulation is discussed first, followed by an overview of the diverse CA metrics currently used with a goal to organize a somewhat confusing terminology that has evolved from independent research efforts. Finally, the current state of validation and clinical insight learned from these methods is presented.
Historical perspective of autoregulation and autoregulation monitoring
In the 1890s, the importance of ABP in the regulation of cerebral blood flow (CBF) was proposed by Roy and Sherrington who concluded “The higher the arterial pressure, the greater is the amount of blood which passes through the cerebral blood-vessels and vice versa” [4]. However, from the mid-twentieth century, it had become clear that the relationship between ABP and CBF was not linear, and the familiar shape of the CA curve with LLA was published [5, 6].
The testing of autoregulation in humans was facilitated by the availability of intermittent CBF measurements in clinical practice [7]. By decreasing, or increasing ABP and measuring CBF at multiple levels of ABP, the strength of the CA mechanism could be ascertained [8]. For example, if CBF passively followed changes in ABP, CA was deemed diminished, and if CBF remained relatively constant despite the increases or decreases in ABP, CA was deemed intact.
This sort of “testing” of CA, by inducing a decrease (or increase) in ABP while measuring the CBF response, elucidated how CA was affected by various disease states [9, 10]. However, deliberately reducing (or increasing) ABP may expose the vulnerable brain to harm. Therefore, methods were devised to assess CA using spontaneous fluctuations in ABP and CBF facilitated by the development of the transcranial Doppler, a repetitive and continuous monitor of CBF velocity [11,12,13]. It is technically challenging to perform long-term transcranial Doppler interrogations, so the method is more commonly used for periodic assessments.
The pressure reactivity index (PRx) was developed in 1996 as a way to monitor CA from analysis of the relationship between slow ABP and ICP waveforms. The PRx assumes that low-frequency oscillations and transients in the ICP are due to blood volume changes in the cranial vault and that these in turn reflect cerebral vascular resistance (CVR) changes provoked by CA. Both ICP and ABP are routinely monitored continuously, allowing continuous PRx monitoring developed in an adult TBI cohort [14]. Monitoring CA continuously with the PRx facilitates plotting of an optimization curve at the bedside, so the relationship between CA and CPP can be assessed at the point of care [15] (Table 1).
Methods for measuring CA
Prolific and diverse methodologies used to measure CA are published in a body of literature that can be conceptually inaccessible to clinicians. The section that follows delineates, for context, some of the techniques and nomenclature that are commonly encountered in the CA literature.
Static vs. dynamic autoregulation measurements
Static assessments of autoregulation refer to the relationship changes in ABP and CBF at steady state—that is, CBF is assessed after an ABP stimulus, at the time point when CBF is no longer changing. Dynamic autoregulation assesses the time profile of changes in CBF in response to changes in ABP. Dynamic CA assessment requires continuous measurements of both ABP (e.g. from a pressure transducer attached to an arterial line) and a surrogate of CBF or cerebral blood volume (e.g. transcranial Doppler (TCD), NIRS, brain oxygenation, ICP). Dynamic responses are commonly tested from induced ABP changes. Methods to change ABP for the purpose of dynamic CA assessment include deflation of suprasystolic thigh cuffs [25, 26], postural manoeuvres [27], and lower body negative pressure (LBNP) [28].
Lassen’s curve from cohort data
The classic static assessment of autoregulation is the CA curve drawn by Niels Lassen. In his review, Lassen plotted steady-state measurement pairs of ABP and CBF from 11 published studies, demonstrating the autoregulatory plateau and LLA for “man” [5]. From this review, it was widely concluded that the LLA for the human brain is at a mean ABP of 50 mmHg. This hard doctrine persisted in medical practice until recent recognition that the LLA is highly variable across subjects and conditions [29]. Regardless of the methods used to measure autoregulation (steady-state or dynamic), some semblance of a static “Lassen’s curve” has to be generated to identify the LLA and assign ABP and CPP targets using CA monitoring.
Static rate of autoregulation
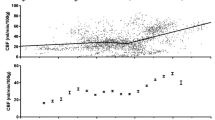
In addition to delineating the LLA from Lassen’s curve, steady-state CA can be assessed with a single, linear metric, the static rate of autoregulation. In this method, a simplified, average change in cerebral vascular resistance (CVR = CPP/CBF) is assessed in response to a simplified, average change in CPP [30]. If the assessments are done at perfusion pressures greater than LLA, then this metric is an assessment of the effectiveness of CA when supported by appropriate CPP. Typically, ABP is increased by the infusion of a vasopressor (such as phenylephrine) to increase the ABP by ~20 mmHg, allowing steady state to occur. Figure 1 shows static assessments of autoregulation in a piglet brain after controlled cortical impact, including Lassen’s curve, an automated determination of LLA by piecewise regression, and determination of the static rate of autoregulation for CPP above LLA [31].
Assessments of static autoregulation in a piglet model of TBI: Ipsilateral (left) and contralateral (right) assessments of autoregulation were made in the same piglet after controlled cortical impact using continuous laser Doppler flowmetry. The lower limit of autoregulation (LLA) was determined from Lassen’s curves (top graphs) with piecewise regression. The static rate of autoregulation (SRoR) for CPP above LLA was determined for each hemisphere as the slope of the line plotting cerebrovascular resistance (CVR) against cerebral perfusion pressure (CPP). Within 6 h of trauma, the two hemispheres of this piglet demonstrated similar LLA and SRoR in the intact range when CPP was greater than LLA. Used with permission [31]
The ARI
A commonly used example of dynamic autoregulation is the autoregulation index (ARI), developed by Tiecks et al. [32]. When a suprasystolic thigh cuff is released, a rapid decrease in total peripheral resistance causes a sudden (>15 mmHg) drop in ABP. With intact CA, this ABP change provokes a change in cerebrovascular resistance (CVR). The time course of this change in CVR is modelled by a second-order linear differential equation to give the change in CVR per second in relation to the change in ABP. In the case where CA is completely impaired, following the decrease in ABP, CVR will remain low, and CBF will fall and remain low. Alternatively, if CA is functioning, CVR will rapidly decrease causing CBF to quickly return to baseline values. These extreme responses and the spectrum in between can be described by a series of nine hypothetical, modelled curves, demarcated as the ARI. ARI 0 is the case with absent autoregulation while ARI 9 is the case with perfect CA. It is not uncommon in the literature to see the term ARI also used to describe CA metrics not derived from the Tiecks model, but using TCD analysed for the static rate of autoregulation described in the precedent section.
Measuring vs. monitoring autoregulation
If the goal of CA assessment is to delineate the optimal perfusion pressure and LLA for a patient at the point of care, then multiple assessments of CA have to be made for comparison across CPP. To facilitate this, intermittent assessments of induced ABP changes have been replaced with repetitive assessments of spontaneous ABP changes. Monitoring CA from spontaneous changes in ABP has a clear advantage of safety and convenience for continuous monitoring. The disadvantage occurs when changes in ABP are inadequate or inconsistent and potentially confounded by non-CA events that change both ABP and CBF (e.g. CO2).
The requirements for CA monitoring are (1) a continuous measure of ABP, the input signal; (2) a continuous measure of the cerebral vasculature, the output signal: CBF, cerebral blood volume, or brain tissue oxygenation; and (3) a mathematical quantification of the relationship between the input and output. The math functions used in no. 3 have included correlation in the time domain, and cross-correlation functions in the frequency domain such as coherence, gain of transfer, and phase shift. More recently, the use of wavelet transforms can give an assessment of CA in both the time and frequency domains simultaneously. The wavelet transform may have an advantage when spontaneous (irregular) ABP waves are used as an input for CA monitoring [33]. Any metric of autoregulation, whether performed in the time or the frequency domain, has a specified range of frequency of interrogation (i.e. the period of ABP oscillations that are used as an input signal). Faster frequencies allow for more rapid monitoring results and are less confounded by drifts in CBF that occur from usually slower, non-CA related events. However, ABP waves sustained less than 30 s may not adequately engage the CA mechanism to allow for accurate CA monitoring [34]. The myriad combinations of 1–3 have generated a dizzying array of metrics in the literature that are neither interchangeable, nor in many cases validated.
Measurements of the cerebral vasculature (output signals)
If a blood pressure change represents an input signal which is either modulated or not due to intact or impaired CA, then the output of this modulation is measured as a change in the cerebral vasculature. The output signal is always an intracranial interrogation of the cerebral vasculature. Using CBF as an output is most intuitive, but autoregulation can also be measured using cerebral blood volume or changes in tissue oxygenation. Continuous CBF surrogates using TCD, laser Doppler, diffuse correlation spectroscopy, and more recently “ultrasound tagged light” are methods that render a measure of CBF velocity or haemoglobin flux. These techniques to monitor CBF predate the era of CA monitoring, but were clinically limited because the metrics of velocity and “flowmetry” are not CBF, and a calibrated CBF is not rendered from most of these techniques. However, math functions such as correlation and phase shift, used to continuously monitor autoregulation, do not require a calibrated signal to give an accurate result. Thus, many of these CBF surrogates are ideal candidates for development of CA monitoring.
Cerebral blood volume can also be used as an output signal to measure CA. Constriction and dilation of the cerebral vasculature mediate CA, and the timing (phase shift) of these vascular diameter changes in relation to the ABP input signal carries information about the health of CA. Cerebral blood volume can be trended using ICP (i.e. the PRx, see Fig. 2), with near-infrared spectroscopy, with ultrasonic time of flight, and with changes in electric impedance across the brain [14, 19, 22, 36].
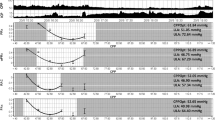
The pressure reactivity index monitored shown in two paediatric subjects with severe traumatic brain injury (TBI). a Arterial blood pressure (ABP) and intracranial pressure (ICP) from a child who survived severe TBI, filtered to remove pulse and respiratory variation, demonstrating low-frequency oscillations (slow waves). The ICP shares a waveform with the ABP at the slow wave frequency. However, these slow waves are phase-inverted. When ABP is increasing, ICP is decreasing and vice versa. b The ICP and ABP from the subject in a show a negative correlation coefficient (the pressure reactivity index or PRx), indicating intact autoregulation. c ABP and ICP slow waves from a non-survivor of TBI are in phase; ABP and ICP increase and decrease at the same time. d The PRx for the non-survivor shows a positive correlation, indicating impaired autoregulation. Used with permission [35]
Two methods to measure tissue oxygenation include the invasive Licox monitor and non-invasive reflectance near-infrared spectroscopy. If tissue oxygenation is used to measure CA, an assumption is made that pressure-passive CBF results in pressure-passive oxygen delivery and consumption by the brain. Reflectance near-infrared spectroscopy has advantages over other interrogations of the cerebral vasculature in that it is both technically easy to apply and non-invasive. The role of this modality for CA monitoring in head trauma is questioned by two theoretical concerns: (1) A regionally specific monitor of CA may give incomplete data for spatially heterogeneous lesions that occur in TBI. (2) The presence of hematoma within the reflectance arc has an undefined effect on the recovery of light through viable vasculature.
Math functions are not interchangeable
All of the math functions used to measure autoregulation ask quantitatively whether or not the output signal from the cerebral vasculature contains the input signal from the ABP or CPP. When the time domain is used (correlation), the resultant metric is intuitive for clinicians without a background in waveform physics. However, when the frequency domain is used (cross-correlation functions), the methods are less understandable to the clinical community. Cross correlations use Fourier transforms, which deconstruct chaotic biologic signals into a combination of sinusoidal waves.
In lay terms, the cross-correlation functions can be understood as follows: Coherent waves share a wave component at a specific frequency. That is, if there is a sine wave component with period x in the input wave, there is a sine wave component with period x in the coherent output wave. The timing is not specified. Thus, coherent waves may be exact replicas of each other or inversions of each other. Phase describes the timing of two coherent waves. When two coherent waves have identical timing, they are “in phase”, or zero degrees phase shifted. When two coherent waves have inverted timing, they are “out of phase” or 180° phase shifted. Gain of transfer describes the magnitude of an output wave that is coherent to an input wave. Coherence at the frequency of interest is required for phase and gain of transfer to have meaning.
These time and frequency domain analytics are related, but not interchangeable. When waves are coherent and in phase, they are positively correlated. When waves are incoherent, they have a correlation of zero. When waves are coherent and out of phase, they have a negative correlation. Thus, a metric that uses coherence may indicate impaired autoregulation for a patient, when a metric that uses phase or correlation may indicate either intact or impaired from the same patient. Given the profusion of methods that have been published and asserted to measure autoregulation, it is reasonable to ask which of these accurately measure autoregulation for which clinical condition. More importantly, can these metrics identify an optimal CPP, and is maintenance of optimal CPP associated with an improvement in outcome?
Animal studies
Validation
Human clinical studies have attempted to validate individual metrics of autoregulation by comparing them with other metrics of autoregulation. Steiner et al. compared the PRx with a static rate of autoregulation from both trans-cranial Doppler and positron emission tomography scanning in head injured patients [37]. Hogue et al. compared the correlation flow index (from ultrasound-tagged near-infrared light) with the mean velocity index derived from trans-cranial Doppler in adults during cardiopulmonary bypass) [38]. The modest agreements published in a variety of these studies are confounded by the lack of a clear gold standard for CA monitoring [39]. The animal model has been used to assert a gold standard from Lassen’s curve such as the one shown in Fig. 1 that unambiguously demonstrates intact and impaired autoregulation in the same animal. This data is not available in humans as the protocol is lethal, and many published methods of quantifying CA have not been examined in this way.
The piglet model has been used to test CA with the time domain using spontaneous fluctuations in CPP and correlation with the output surrogates of CBF, cerebral blood volume, and cortical tissue oxygenation (LDx, PRx, and HVx, COx, respectively). These metrics have similar, moderate, sensitivity and specificity to delineate the LLA in the controlled laboratory setting [19, 21, 40]. In these studies, the time domain method of CA monitoring showed optimization at CPP greater than LLA when data was averaged over 3+ h to create a Lassen’s curve with area under receiver operator curves ranging from 0.8 to 0.9. However, the minute-to-minute measures of autoregulation show a poor signal-to-noise ratio, such that the reliability of a brief measurement of autoregulation using spontaneous CPP waves as input is unacceptably poor.
Speculation to the cause of imprecision in continuous CA monitoring has focused on the erratic, non-periodic and transient nature of CPP waveforms in the low-frequency bandwidth relevant to autoregulation [41, 42]. This has been indirectly confirmed by the experimental creation of a regular, sinusoidal low-frequency oscillation in the ABP of a piglet model. In that model, coherent waves were seen in the ICP with large phase shift above LLA (positive correlation between ABP and ICP) and loss of phase shift below LLA (negative correlation between ABP and ICP). The imprecision seen in PRx measurements with spontaneous ABP oscillations was removed [43]. Until a noise filter is created to remove the minute-to-minute variability in CA monitoring, or until a safe means of inducing regular ABP waveforms is devised, there is a minimum time requirement of CA monitoring to reliably determine the state of CA and delineate LLA. Experimentally, 2–4 h has been used, and clinically, a 4-h window is commonly used [44].
Sex and age factors from animal data
The piglet model has also been useful to elucidate potentially important differences in CA between the sexes and across early developmental ages with respect to pressor choices used to support ABP when hypotension accompanies TBI. Deranged autoregulation after TBI has been linked to an upregulation of the spasmogen endothelin-1 and extracellular signal-related kinase (ERK) isoform of mitogen-activated protein kinase (MAPK). This upregulation and associated disturbance of autoregulation are greater in males compared to females following injury [45]. The infusion of phenylephrine restored CA in newborn piglet females, but did not restore CA in newborn piglet males, with corresponding differences in upregulation of the ERK-MAPK pathway [46]. The infusion of dopamine restored CA in newborn piglets of both sexes and upregulation of the ERK-MAPK pathway [47]. Clinical validation of these findings in human subjects is not yet available, so there is currently no guideline clinical choice of pressor agents that best preserves CA or functional outcome of a child after TBI.
Human clinical studies of CA monitoring
The compelling data for CA monitoring after TBI is from adult studies, and even this data reaches the lowest guideline level of “option”. Adult data is reviewed first, and the limited available paediatric data is summarized separately. The most widely studied metric for monitoring CA in this body of literature is the PRx.
CA and survival
Impaired pressure-reactivity (positive PRx) is an independent predictor of fatal outcome following head injury [42]. Multiple adult series have used the PRx to demonstrate a link between CA and outcome, and the largest database that has demonstrated relationships between PRx and outcome is from Cambridge University, a collection of ICP and ABP recordings from neurocritical care subjects that has been ongoing for two decades. In one series of 307 adult subjects with TBI from the Cambridge database, PRx was a significant predictor of mortality [48]. Higher PRx in the first 6 h after injury was associated with mortality in a subset of 171 subjects with severe TBI in the Cambridge database [49]. In a larger subset, Budohoski et al. reported the PRx to be lower in the first 24 h after TBI for survivors compared to those who died [50]. In the same database, an association has been demonstrated between autoregulation, outcome, and age. Specifically, an age-related decline in cerebrovascular autoregulation after TBI is associated with a deterioration in outcome in elderly patients following head trauma [51].
CPPOPT from PRx
Showing that survival is associated with a low PRx is not actionable beyond prognosis, nor does it show that any specific effort to optimize PRx improves outcome. Demonstration of an optimization curve of PRx when plotted against CPP was published in 2002 by Steiner et al. [15]. The term “optimal CPP” (CPPOPT) was put forward to mean the CPP at which PRx was most negative and CA was optimized. In that study, it was observed that patients with greater difference between average CPP and CPPOPT had significantly worse scores on the Glasgow outcome scale. A natural conclusion was proposed that PRx could be monitored not for its behaviour in time, but as a function of CPP prompting haemodynamic intervention to achieve the now visible CPPOPT for individual patients (see Fig. 3). Subsequently, an algorithm was developed to analyse these PRx/CPP plots to render a single CPP value representing CPPOPT. Aries et al. used this algorithm retrospectively on the Cambridge database which at that time included a cohort of 327 subjects with severe TBI and continuous monitoring of ICP and ABP. Exposure to CPP less than CPPOPT was a significant risk for death, and survival was not associated with CPP greater than CPPOPT. Further, exposure to CPP greater than CPPOPT was a significant risk for survival with poor neurologic outcome. Optimal outcome (survival with neurologic recovery) was associated with a CPP equal to CPPOPT [44]. A randomized trial of PRx monitoring to guide CPP targets prospectively in a randomized trial is currently underway (clinicaltrials.gov record no. NCT02982122).
Pressure reactivity index (PRx) optimization curves. a Intracranial pressure (ICP) and arterial blood pressure (ABP) are shown in time for a child who survived severe TBI. Below these panels, the PRx is shown from the same time period as a function of cerebral perfusion pressure (CPP). At CPP of 75 mmHg, the lowest value of PRx is observed, indicating optimal pressure reactivity at that CPP (CPPOPT). b ICP and ABP are shown for a child who did not survive TBI, as shown in the progressive and terminal ICP elevation. This child also shows an optimization curve with CPPOPT occurring at CPP near 50 mmHg, but the CPP was not managed within this range. Used with permission [35]
CA monitoring after paediatric TBI
The argument for monitoring CA in paediatric patients with TBI is derived from adult studies. Limited studies of CA in smaller cohorts of paediatric patients are available. Most of these have a wide age range for enrolment, but lack the numbers to confidently analyse the effect of age on CA and especially CPPOPT after TBI.
Vavilala et al. showed an association between CA after TBI (using ARI) and 6-month Glasgow outcome score in a cohort of 28 children [23]. At the same centre, Freeman et al. found associations between younger age, impaired CA (using a form of ARI that is similar to the static rate of autoregulation), and Glasgow outcome score in a prospective cohort of paediatric subjects with TBI [52].
Figaji et al. studied the relationship between CA (using ARI) and the response of ICP and brain tissue oxygenation to a change in ABP [53]. It was shown in 24 patients that impaired CA (lower ARI) was associated with pressure-passive ICP and pressure-passive tissue oxygenation. One interpretation of this study is a demonstration of the internal consistency of three ways of viewing CA. When the ARI from TCD shows a pressure-passive state, it is associated with pressure passivity in other neuromonitoring modalities. In that study, however, a low ARI was not associated with a higher mortality. The tissue oxygenation response in that study was noted to be less straightforward than the ICP response to increased ABP. In nearly all cases, tissue oxygen tension increased with a rise in ABP, but the increase was greater in subjects with a low ARI.
Three small studies have examined the PRx and optimization plots in paediatric patients. Brady et al. showed an association between impaired CA (high PRx) and death in a cohort of 21 paediatric subjects with severe TBI [35]. In that cohort, PRx was lower (intact autoregulation) at higher CPP, and at CPP less than 40 mmHg, CA was universally impaired (positive PRx). Non-survivors had greater exposure to lower CPP. Lewis et al. studied 36 paediatric subjects with PRx monitoring, showing an association between impaired CA and poor Glasgow outcome score [54]. Increased exposure by both duration and magnitude of CPP less than CPPOPT was associated with worse outcome in that cohort. Young et al. published data from a cohort of 12 paediatric subjects with TBI showing that exposure to CPP 10 mmHg less than CPPOPT derived from a PRx optimization curve was associated with death [55].
Conclusions and treatment implications
CA is a vital protective homeostatic mechanism for the brain, protecting the brain from injurious blood flow that is either excessive or inadequate. In recent decades, the technology to quantify the state of CA has expanded from intermittent, technically challenging metrics to continuous monitoring modalities. CA assessment tools have shifted from prognostic to therapeutic instruments by identifying a specific CPP target for a monitored subject. The rapid pace of technology development and publication in the field of CA has created a complex nomenclature and a complex array of metrics, many of which have been published without a clear validation.
Validation studies that have been conducted include comparison against a lethal gold standard in animal models and observational links to outcome. The most widely studied monitor of autoregulation to date is the PRx. It has been shown to be moderately sensitive and specific in animal models for delineating an CPPOPT above LLA. These animal studies have highlighted the problem with imprecision in the PRx. Currently, imprecision in the PRx is mitigated by averaging over long (hours) windows of time to delineate CPPOPT. Future improvements can be expected in the PRx with filtering algorithms or possibly an ABP manipulation scheme.
In large adult studies and small paediatric studies, the PRx has been consistently associated with outcome: a positive PRx indicating impaired CA is associated with both death and neurologic disability. All of these studies have been observational. The more compelling aspect of the PRx clinical data is the delineation of CPPOPT from PRx optimization curves. In adult studies that are large by TBI research standards, exposure to CPP less than CPPOPT is associated with death. Exposure to CPP greater than CPPOPT is associated with permanent neurologic disability. In guideline parlance, this collective evidence rises only to the level of option: CA monitoring is still based on physiologic rationale and observational data. Interventional trials with the PRx are forthcoming, but they do not include a paediatric enrolment. Therefore, paediatric intensivists and neurosurgeons will be left with extrapolation from adult data when it becomes available.
References
Roozenbeek B, Maas AIR, Menon DK (2013) Changing patterns in the epidemiology of traumatic brain injury. Nat Rev Neurol 9:231–236. doi:10.1038/nrneurol.2013.22
Donnelly J, Budohoski KP, Smielewski P, Czosnyka M (2016) Regulation of the cerebral circulation: bedside assessment and clinical implications. Crit Care 20:129. doi:10.1186/s13054-016-1293-6
Chapter 5. Cerebral perfusion pressure thresholds: Pediatric Critical Care Medicine. In: LWW. http://journals.lww.com/pccmjournal/Fulltext/2012/01001/Chapter_5__Cerebral_perfusion_pressure_thresholds.6.aspx. Accessed 26 Apr 2017
Roy CS, Sherrington CS (1890) On the regulation of the blood-supply of the brain. J Physiol 11:85–158.17. doi:10.1152/japplphysiol.00257.2010
Lassen N (1959) Cerebral blood flow and oxygen consumption in man. Physiol Rev 39:183–238
Lassen NA (1964) Autoregulation of cerebral blood flow. Circ Res 15(SUPPL):201–204
Kety SS, Schmidt CF (1944) The quantitative determination of cerebral blood flow in man by the use of nitrous oxide in low concentrations. Am J Med Sci 208:809. doi:10.1097/00000441-194412000-00013
Paulson OB, Strandgaard S, Edvinsson L (1990) Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2:161–192. doi:10.1161/01.STR.15.3.413
Bentsen N, Larsen B, Lassen NA (1975) Chronically impaired autoregulation of cerebral blood flow in long-term diabetics. Stroke J Cereb Circ 6:497–502. doi:10.1161/01.STR.6.5.497
Strandgaard S, Olesen J, Skinhøj E, Lassen NA (1973) Autoregulation of brain circulation in severe arterial hypertension. Br Med J 1:507–510
Diehl RR, Linden D, Lücke D, Berlit P (1998) Spontaneous blood pressure oscillations and cerebral autoregulation. Clin Auton Res Off J Clin Auton Res Soc 8:7–12
Zhang R, Zuckerman JH, Giller CA, Levine BD (1998) Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Phys 274:H233–H241. doi:10.1016/j.preghy.2015.09.001
Giller CA (1990) The frequency-dependent behavior of cerebral autoregulation. Neurosurgery 27:362–368. doi:10.1227/00006123-199009000-00004
Czosnyka M, Smielewski P, Kirkpatrick P et al (1997) Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery 41:11–17
Steiner LA, Czosnyka M, Piechnik SK et al (2002) Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 30:733–738. doi:10.1097/00003246-200204000-00002
Czosnyka M, Smielewski P, Kirkpatrick P et al (1996) Monitoring of cerebral autoregulation in head-injured patients. Stroke J Cereb Circ 27:1829–1834. doi:10.1161/01.STR.27.10.1829
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J (2006) Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity. Crit Care Med 34:1783–1788. doi:10.1097/01.CCM.0000218413.51546.9E
Brady KM, Lee JK, Kibler KK et al (2007) Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke 38:2818–2825. doi:10.1161/STROKEAHA.107.485706
Lee JK, Kibler KK, Benni PB et al (2009) Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke 40:1820–1826. doi:10.1161/STROKEAHA.108.536094
Lam JM, Hsiang JN, Poon WS (1997) Monitoring of autoregulation using laser Doppler flowmetry in patients with head injury. J Neurosurg 86:438–445. doi:10.3171/jns.1997.86.3.0438
Brady KM, Lee JK, Kibler KK et al (2008) Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke 39:2531–2537. doi:10.1161/STROKEAHA.108.514877
Ragauskas A, Daubaris G, Petkus V et al (2005) Clinical study of continuous non-invasive cerebrovascular autoregulation monitoring in neurosurgical ICU. Acta Neurochir Suppl 95:367–370
Massaro AN, Govindan RB, Vezina G et al (2015) Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J Neurophysiol 114:818–824. doi:10.1152/jn.00353.2015
Hori D, Hogue C, Adachi H et al (2016) Perioperative optimal blood pressure as determined by ultrasound tagged near infrared spectroscopy and its association with postoperative acute kidney injury in cardiac surgery patients. Interact Cardiovasc Thorac Surg 22:445–451. doi:10.1093/icvts/ivv371
Aaslid R, Newell DW, Stooss R et al (1991) Assessment of cerebral autoregulation dynamics from simultaneous arterial and venous transcranial Doppler recordings in humans. Stroke 22:1148–1154. doi:10.1161/01.STR.22.9.1148
Tiecks FP, Douville C, Byrd S et al (1996) Evaluation of impaired cerebral autoregulation by the Valsalva maneuver. Stroke J Cereb Circ 27:1177–1182. doi:10.1161/01.STR.27.7.1177
Smirl JD, Tzeng Y-C, Monteleone BJ, Ainslie PN (2014) Influence of cerebrovascular resistance on the dynamic relationship between blood pressure and cerebral blood flow in humans. J Appl Physiol Bethesda Md (1985). doi:10.1152/japplphysiol.01266.2013
Tan CO (2012) Defining the characteristic relationship between arterial pressure and cerebral flow. J Appl Physiol 113:1194–1200
Drummond JC (1997) The lower limit of autoregulation: time to revise our thinking? Anesthesiology 86:1431–1433
Strebel S, Lam AM, Matta B et al (1995) Dynamic and static cerebral autoregulation during isoflurane, desflurane, and propofol anesthesia. Anesthesiology. doi:10.1097/00132586-199610000-00054
Mytar J, Kibler KK, Easley RB et al (2012) Static autoregulation is intact early after severe unilateral brain injury in a neonatal swine model. Neurosurgery 71:138–145. doi:10.1227/NEU.0b013e318251795a
Tiecks FP, Lam AM, Aaslid R, Newell DW (1995) Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26:1014–1019. doi:10.1161/01.STR.26.6.1014
Addison PS (2015) A review of wavelet transform time–frequency methods for NIRS-based analysis of cerebral autoregulation. IEEE Rev Biomed Eng 8:78–85. doi:10.1109/RBME.2015.2436978
Fraser CD, Brady KM, Rhee CJ et al (2013) The frequency response of cerebral autoregulation. J Appl Physiol Bethesda Md (1985) 115:52–56. doi:10.1152/japplphysiol.00068.2013
Brady KM, Shaffner DH, Lee JK et al (2009) Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics 124:e1205–e1212. doi:10.1542/peds.2009-0550
Brady KM, Mytar JO, Kibler KK et al (2010) Monitoring cerebrovascular pressure reactivity with rheoencephalography. J Phys Conf Ser 224:12089. doi:10.1088/1742-6596/224/1/012089
Steiner LA, Coles JP, Johnston AJ et al (2003) Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke 34:2404–2409. doi:10.1161/01.STR.0000089014.59668.04
Hori D, Hogue CW, Shah A et al (2015) Cerebral autoregulation monitoring with ultrasound-tagged near-infrared spectroscopy in cardiac surgery patients. Anesth Analg 121:1187–1193. doi:10.1213/ANE.0000000000000930
Brady K, Andropoulos DB, Kibler K, Easley RB (2015) A new monitor of pressure autoregulation: what does it add? Anesth Analg 121:1121–1123. doi:10.1213/ANE.0000000000000952
Brady KM, Mytar JO, Kibler KK et al (2010) Noninvasive autoregulation monitoring with and without intracranial pressure in the naive piglet brain. Anesth Analg 111:191–195. doi:10.1213/ANE.0b013e3181e054ba
Diedler J, Zweifel C, Budohoski KP et al (2011) The limitations of near-infrared spectroscopy to assess cerebrovascular reactivity: the role of slow frequency oscillations. Anesth Analg 113:849–857. doi:10.1213/ANE.0b013e3182285dc0
Czosnyka M, Brady K, Reinhard M et al (2009) Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care 10:373–386. doi:10.1007/s12028-008-9175-7
Brady KM, Easley RB, Kibler K et al (2012) Positive end-expiratory pressure oscillation facilitates brain vascular reactivity monitoring. J Appl Physiol Bethesda Md (1985) 113:1362–1368. doi:10.1152/japplphysiol.00853.2012
Aries MJH, Czosnyka M, Budohoski KP et al (2012) Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med 40:2456–2463. doi:10.1097/CCM.0b013e3182514eb6
Armstead WM, Kiessling JW, Riley J et al (2011) Phenylephrine infusion prevents impairment of ATP- and calcium-sensitive potassium channel-mediated cerebrovasodilation after brain injury in female, but aggravates impairment in male, piglets through modulation of ERK MAPK upregulation. J Neurotrauma 28:105–111. doi:10.1089/neu.2010.1581
Armstead WM, Riley J, Vavilala MS (2012) TBI sex dependently upregulates ET-1 to impair autoregulation, which is aggravated by phenylephrine in males but is abrogated in females. J Neurotrauma 29:1483–1490. doi:10.1089/neu.2011.2248
Armstead WM, Riley J, Vavilala MS (2013) Dopamine prevents impairment of autoregulation after traumatic brain injury in the newborn pig through inhibition of up-regulation of endothelin-1 and extracellular signal-regulated kinase mitogen-activated protein kinase. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 14:e103–e111. doi:10.1097/PCC.0b013e3182712b44
Lang EW, Kasprowicz M, Smielewski P et al (2016) Outcome, pressure reactivity and optimal cerebral perfusion pressure calculation in traumatic brain injury: a comparison of two variants. Acta Neurochir Suppl 122:221–223. doi:10.1007/978-3-319-22533-3_44
Gao L, Smieleweski P, Czosnyka M, Ercole A (2017) Early asymmetric cardio-cerebral causality and outcome after severe traumatic brain injury. J Neurotrauma. doi:10.1089/neu.2016.4787
Budohoski KP, Czosnyka M, de Riva N et al (2012) The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery 71:652–660. doi:10.1227/NEU.0b013e318260feb1
Czosnyka M, Balestreri M, Steiner L et al (2005) Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg 102:450–454. doi:10.3171/jns.2005.102.3.0450
Freeman SS, Udomphorn Y, Armstead WM et al (2008) Young age as a risk factor for impaired cerebral autoregulation after moderate to severe pediatric traumatic brain injury. Anesthesiol J Am Soc Anesthesiol 108:588–595. doi:10.1097/ALN.0b013e31816725d7
Figaji AA, Zwane E, Fieggen AG et al (2009) Pressure autoregulation, intracranial pressure, and brain tissue oxygenation in children with severe traumatic brain injury: clinical article. J Neurosurg Pediatr 4:420–428. doi:10.3171/2009.6.PEDS096
Lewis PM, Czosnyka M, Carter BG et al (2015) Cerebrovascular pressure reactivity in children with traumatic brain injury. Pediatr Crit Care Med 16:739–749. doi:10.1097/PCC.0000000000000471
Young AMH, Donnelly J, Czosnyka M et al (2016) Continuous multimodality monitoring in children after traumatic brain injury—preliminary experience. PLoS One 11:e0148817. doi:10.1371/journal.pone.0148817
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare related to this manuscript.
Rights and permissions
About this article
Cite this article
Donnelly, J.E., Young, A.M.H. & Brady, K. Autoregulation in paediatric TBI—current evidence and implications for treatment. Childs Nerv Syst 33, 1735–1744 (2017). https://doi.org/10.1007/s00381-017-3523-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3523-x