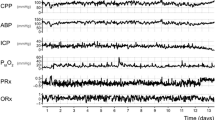
Abstract
Background
The Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II randomized controlled trial used a tier-based management protocol based on brain tissue oxygen (PbtO2) and intracranial pressure (ICP) monitoring to reduce brain tissue hypoxia after severe traumatic brain injury. We performed a secondary analysis to explore the relationship between brain tissue hypoxia, blood pressure (BP), and interventions to improve cerebral perfusion pressure (CPP). We hypothesized that BP management below the lower limit of autoregulation would lead to cerebral hypoperfusion and brain tissue hypoxia that could be improved with hemodynamic augmentation.
Methods
Of the 119 patients enrolled in the Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II trial, 55 patients had simultaneous recordings of arterial BP, ICP, and PbtO2. Autoregulatory function was measured by interrogating changes in ICP and PbtO2 in response to fluctuations in CPP using time-correlation analysis. The resulting autoregulatory indices (pressure reactivity index and oxygen reactivity index) were used to identify the “optimal” CPP and limits of autoregulation for each patient. Autoregulatory function and percent time with CPP outside personalized limits of autoregulation were calculated before, during, and after all interventions directed to optimize CPP.
Results
Individualized limits of autoregulation were computed in 55 patients (mean age 38 years, mean monitoring time 92 h). We identified 35 episodes of brain tissue hypoxia (PbtO2 < 20 mm Hg) treated with CPP augmentation. Following each intervention, mean CPP increased from 73 ± 14 mm Hg to 79 ± 17 mm Hg (p = 0.15), and mean PbtO2 improved from 18.4 ± 5.6 mm Hg to 21.9 ± 5.6 mm Hg (p = 0.01), whereas autoregulatory function trended toward improvement (oxygen reactivity index 0.42 vs. 0.37, p = 0.14; pressure reactivity index 0.25 vs. 0.21, p = 0.2). Although optimal CPP and limits remained relatively unchanged, there was a significant decrease in the percent time with CPP below the lower limit of autoregulation in the 60 min after compared with before an intervention (11% vs. 23%, p = 0.05).
Conclusions
Our analysis suggests that brain tissue hypoxia is associated with cerebral hypoperfusion characterized by increased time with CPP below the lower limit of autoregulation. Interventions to increase CPP appear to improve autoregulation. Further studies are needed to validate the importance of autoregulation as a modifiable variable with the potential to improve outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cerebral autoregulation refers to the capacity of cerebral arterioles to dynamically adjust their diameter over a range of arterial blood pressures (BPs) to maintain stable cerebral perfusion. Outside the limits of autoregulation (LA), cerebral blood flow (CBF) passively follows changes in pressure. This mechanism ensures that blood flow matches the brain’s metabolic demands and protects it from hypoperfusion or hyperperfusion [1]. Impaired autoregulation occurs in various acute and chronic cerebrovascular conditions, and cerebral autoregulation status has been recognized as an important parameter in managing patients with acute brain injury [2, 3].
Observational studies have shown that BP management outside personalized LA is associated with an increased risk of neurologic complications and poor functional outcomes after traumatic brain injury (TBI) [4, 5]. However, individualized thresholds at which cerebral hypoperfusion leads to secondary brain injury are unclear, as are the precise interactions between cerebral autoregulatory function, BP, and brain tissue hypoxia. Finally, it remains unknown if BP interventions during periods of autoregulatory dysfunction can restore autoregulatory function by shifting patients to a more favorable position on their autoregulatory curve.
This study aimed to explore the pathophysiologic underpinnings of cerebrovascular control after TBI and determine whether BP management below the lower LA (LLA) would lead to cerebral hypoperfusion and brain tissue hypoxia, which can be reversed with hemodynamic augmentation.
Methods
Study Design
This study is a secondary analysis of the Brain Oxygen Optimization in Severe TBI Phase II (BOOST-II) study. BOOST-II was a prospective, randomized, controlled, multicenter trial that screened and enrolled patients with severe TBI at ten level I trauma centers across the United States. Study details, including patient inclusion and exclusion criteria, have been previously published [6]. In brief, enrolled patients underwent placement of intraparenchymal intracranial pressure (ICP) and brain tissue oxygenation (PbtO2) monitors and digital data recorders (Moberg ICU Solutions, Ambler, PA) continuously recorded the physiologic data. PbtO2 monitors were kept in place for a minimum of 48 h if no abnormalities were found and a maximum of 5 days. The removal was at the discretion of the treating physician. The trial was approved by the local institutional review board at each participating center, and proxy informed consent was obtained before any research procedures.
Using a tier-based management protocol, physiologic interventions addressing isolated intracranial hypertension events (type-B scenario), isolated brain hypoxia events (type-C scenario), or simultaneous occurrences of both events (type-D scenario) were implemented (Table S1). Following randomization, the control group (ICP-only management) was medically managed for type-B events with a standard-of-care stepwise intervention strategy triggered by an ICP ≥ 20 mm Hg for > 5 min (Table S2). The intervention group (ICP and PbtO2 management) received tiered treatments to correct either elevated ICP or a reduction in PbtO2 (< 20 mm Hg, > 5 min). Treatment was directed to each episode. Tier 1 treatments were initiated within 15 min of the start of an episode and escalated to tier 2 interventions if the first tier 1 treatment was ineffective after 60 min (or earlier if deemed necessary by the treating physician). Tier 3 treatments were optional and at the discretion of the treating physician.
Brain Tissue Hypoxia
To study the role of hemodynamic interventions on brain physiology and cerebral autoregulatory function, we searched the case report forms for episodes of brain tissue hypoxia (PbtO 2 ≤ 20 mm Hg, type-C or type-D scenario) that were treated with hemodynamic augmentation as per the BOOST protocol, including (1) cerebral perfusion pressure (CPP) increases to a maximum of 70 mm Hg with fluid bolus (tier 1), (2) hemodynamic optimization (tier 1), and (3) CPP increases to a maximum of 70 mm Hg with a vasopressor (tier 2).
Physiologic Data Recordings
Digital data recordings captured using the Moberg CNS Monitor were obtained from the BOOST-II investigators. These recordings were analyzed using ICM + software (V9.1; Cambridge Enterprises, Cambridge, UK) and MATLAB (R2016b; MathWorks, Inc). Patient files with at least 6 h of simultaneous recordings of ICP, PbtO2, and arterial BP or CPP were included in the analysis. The sampling frequency was 500 Hz for waveform data and 0.5 Hz for numerical values. Artifacts were removed by using visual inspection and a custom MATLAB data verification script.
Calculation of Autoregulatory Indices
Each physiological data signal was first averaged by using a 10-s filter to diminish noise. Cerebral autoregulatory function was calculated by interrogating changes in ICP or PbtO2 in response to fluctuations in CPP using time-correlation analysis. A rolling Pearson correlation coefficient between 30 consecutive time-averaged values of ICP or PbtO2 and BP was used to calculate the pressure reactivity index (PRx) and the oxygen reactivity index (ORx), respectively. CPP has a near-zero or negative correlation with ICP or PbtO2 when autoregulation is intact. In contrast, CPP positively correlates with ICP or PbtO2 when autoregulation is impaired, and ICP or PbtO2 passively follows changes in CPP.
Calculation of Optimal BP and LA
The CPP range at which autoregulation was most preserved (i.e., optimal CPP [CPPopt]) and LA were calculated for each patient according to a previously published algorithm using the ICM + software [4, 5, 7]. In brief, the CPP signal was recorded alongside the PRx and ORx over a 4-h monitoring period and divided into sections of 5 mm Hg (e.g., 60–65 mm Hg). Corresponding PRx and ORx values were then averaged within these groups, and a parabolic curve was fitted to the binned CPP data. The CPPopt was identified as the CPP with the lowest associated PRx/ORx value at the curve’s vertex. This value corresponds to the midpoint of the autoregulatory (i.e., Lassen) curve. The LA were calculated by applying a threshold for impaired autoregulation (PRx ± 0.25, ORx ± 0.3). The CPP values at which the autoregulation switches from intact to impaired provide estimates of the lower LA (LLA) and upper LA limits (ULA). All parameters were continuously calculated throughout the monitoring period by using a multiwindow weighted algorithm (Fig. 1) [8, 9].
Time series of physiologic variables (BP, ICP, CPP, and PbtO2) and autoregulatory indices (ORx and PRx) from an example patient after analyzing the data with ICM + software. a Continuous time trend of CPPopt (red line), ULA and LLA (pink area surrounding CPPopt) computed for an individual patient over a 2-day monitoring period. b The superimposed green line indicates the patient’s actual CPP. BP, blood pressure, CPP, cerebral perfusion pressure, CPPopt, optimal cerebral perfusion pressure, ICP, intracranial pressure, LLA, lower limit of autoregulation, ORx, oxygen reactivity index, PbtO2, brain tissue oxygenation, PRx, pressure reactivity index, ULA, upper limit of autoregulation (Color figure online)
Statistical Analysis
Baseline characteristics were summarized by means and standard deviations for normally distributed continuous variables and numbers (%) for categorical variables. Glasgow Outcome Scale—Extended (GOS-E) scores were dichotomized into favorable (GOS-E 5–8) and unfavorable (GOS-E 1–4) outcomes [10]. The percentage of time that CPP was below the LLA, within LA, and above the ULA was calculated for each patient. In addition, the percentage of time a patient’s CPP exceeded or fell below a fixed distance (± 5 mm Hg and ± 10 mm Hg) from CPPopt was calculated. Physiologic variables and autoregulatory parameters were averaged over 60 min before and after the completion of all interventions aimed at optimizing the CPP in response to brain tissue hypoxia. Averaged physiologic and autoregulatory values before and after completed interventions were compared using paired Student’s t-tests. Clinical covariates in each patient outcome group were compared using Student’s t-test or Fisher’s exact test, as appropriate. MATLAB was used for calculating averaged values, and statistical analyses were conducted using R (V3.6.2, The R Foundation), with statistical significance set at p < 0.05.
Results
Study Participant Characteristics
Of the 119 patients enrolled in BOOST-II, time series data files were obtained for 85 patients. Arterial BP waveform data were recorded in 32 patients, numeric data were recorded in 50 patients, and no BP data were found for three patients. Of the 82 patients with recorded BP data, 27 were excluded from the analysis due to insufficient BP data (less than 6 h), and 55 patients with simultaneous arterial BP, ICP, and PbtO2 recordings were included in our analysis (age 38.2 ± 18.2 years, 76% men, monitoring time 92.8 ± 42.4 h). Baseline characteristics for the final cohort are shown in Table 1. No significant differences were seen between the analyzed, excluded, and unavailable data patient groups (Table S3). Six-month GOS-E scores were available in 47 patients. Seventeen (36%) patients achieved a favorable outcome at 6 months. No significant differences were seen between the groups across all hemodynamic measures (Table S4).
Autoregulatory Indices and LA
Individualized LA were successfully computed in all 55 patients. On average, optimal CPP and LA could be calculated for 66% of the total monitoring time. The mean CPPopt across all patients was 78 ± 14 mm Hg; ULA and LLA were 90 ± 14 mm Hg and 66 ± 12 mm Hg, respectively. Patients spent 63 ± 21% of their monitored time within their personalized LA. Similar results were obtained when PRx was used as the autoregulatory index to compute CPPopt and LA (Table S5).
PbtO2 During Episodes of Cerebral Hypoperfusion
Data recordings of each patient were analyzed for episodes of cerebral hypoperfusion when CPP decreased below the LLA for at least 5 min. We identified a total of 760 episodes in 47 patients, with a mean duration of 16.6 ± 9.3 min. The mean PbtO2 during these episodes was lower when compared with times with CPP within LA (22.62 ± 10.55 vs. 26.10 ± 10.83, p = 0.11) or above the ULA (22.62 ± 10.55 vs. 30.07 ± 13.94, p = 0.004; Fig. 2). Similar results were found when PRx was used as the autoregulatory index to compute LA (Fig. S1).
Mean PbtO2 levels during episodes of cerebral hypoperfusion (CPP < LLA) compared with mean PbtO2 levels during when CPP was within LA and when CPP was above the ULA using ORx as the autoregulatory index to compute LA. Bars represent median and IQR. CPP, cerebral perfusion pressure, IQR, interquartile range, LA, limits of autoregulation, ORx, oxygen reactivity index, PbtO2, brain tissue oxygenation, ULA, upper limit of autoregulation
Intervention Analysis
Among patients enrolled in the intervention arm (treatment based on ICP plus PbtO2 monitoring), we identified 35 episodes of brain tissue hypoxia (PbtO2 < 20 mm Hg) in six patients who were treated with CPP augmentation. Thirty-one (88.6%) episodes were type-C scenarios (low PbtO2, normal ICP), and four (11.4) were type-D scenarios (low PbtO2, elevated ICP). Interventions included fluid boluses (n = 16), hemodynamic optimization (n = 15), and treatment with vasopressors (n = 4).
Following the intervention, mean CPP increased from 73 ± 14 to 79 ± 19 mm Hg (p = 0.15), and mean PbtO2 improved from 18.4 ± 5.6 to 21.9 ± 5.6, p = 0.01, while autoregulatory function trended toward improvement (ORx 0.42 vs. 0.37, p = 0.14; PRx 0.25 vs. 0.21, p = 0.2). LA and CPPopt remained relatively unchanged. However, there was a significant decrease in the percent time with CPP below the LLA in the 60 min after compared with before an intervention (11 ± 18% vs. 23 ± 24%, p = 0.05). Figure 3 shows a 2-h time series of PbtO2, CPP, and LA in an example patient who was treated with CPP augmentation for an episode of brain tissue hypoxia. A complete list of physiologic variables and autoregulatory parameters before and after hemodynamic interventions using ORx is provided in Table 2.
Representative example of the hemodynamic augmentation analysis. A 2-h continuous data recording is shown as a representative example from a patient receiving hemodynamic augmentation for brain tissue hypoxia. Autoregulatory function and the percent time with CPP outside personalized LA were calculated before, during, and after all interventions that overlapped in time with autoregulation monitoring, which were directed to optimize CPP in the BOOST-II study. Prior to the intervention the CPP (magenta line) decreases below the LLA (blue area) resulting in brain tissue hypoxia (PbtO2 < 20 mm Hg, black line). After initiation of the intervention, CPP increases returning to within limits of autoregulation. This is correlated with an improvement in PbtO2. BOOST-II, Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II, CPP, cerebral perfusion pressure, LA, limits of autoregulation, LLA, lower limit of autoregulation, PbtO2, brain tissue oxygenation, ULA, upper limit of autoregulation (Color figure online)
Discussion
Our secondary analysis of the BOOST-II clinical trial showed lower PbtO2 levels when CPP decreased below personalized LA. Furthermore, sustained episodes of brain tissue hypoxia that triggered a study intervention correlated with cerebral hypoperfusion characterized by an increased time with CPP below the LLA. Treatment with CPP augmentation was associated with improved autoregulation and better PbtO2 levels.
These results provide support for autoregulation-guided BP management. Changes in PbtO2 are closely tied to CBF and metabolism [11, 12]. Thus, a reduction in CPP below the LLA results in a decrease in CBF and, consequently, lower PbtO2 levels. Our study corroborates the results of a recently published observational study of 131 patients with aneurysmal subarachnoid hemorrhage [13]. Using spline regression, Megjhani et al. [13] demonstrated a nonlinear relationship between PbtO2 and delta CPP, defined as the deviation of CPP from CPPopt. PbtO2 was most stable when CPP was close to or above CPPopt, whereas CPP below CPPopt corresponded to decreasing PbtO2 values.
There is general agreement that low CPP should be avoided after TBI because it may lead to cerebral hypoperfusion, brain tissue hypoxia, and cerebral metabolic crisis [14]. However, individual thresholds below which hypoperfusion may cause secondary brain injury are not well defined. Current guidelines favor a CPP target of 60–70 mm Hg [15]. In our study, the mean CPP in the hour before the hemodynamic intervention was 73 ± 14 mm Hg, which exceeded the guideline-recommended target range. More importantly, we observed a considerable variation in the LLA across our patient population (Fig. S2). This means some patients experienced brain tissue hypoxia at CPP levels much higher than those typically targeted in clinical care. Thus, having a fixed CPP target range is unlikely to be helpful for all patients. Some patients may require higher CPP levels to prevent brain tissue hypoxia and secondary brain injury. Invasive multimodality monitoring has been used to characterize patient-level pathophysiology and guide hemodynamic management; however, it is only indicated in patients with severe TBI or other forms of critical neurologic injury.
The Seattle International Severe Traumatic Brain Injury Consensus Conference recently published two management algorithms to assist clinicians in treating patients with severe TBI [16, 17]. Both included the interrogation of autoregulation via CPP manipulation to optimize ICP and PbtO2, emphasizing the growing recognition of autoregulatory function as a critical modifiable variable in TBI management. However, if autoregulation is impaired, a mean arterial pressure challenge could be harmful by increasing ICP. Furthermore, the infrequent assessments do not allow for continuous optimization of brain physiology.
Over the last decade, the continuous estimation of CPPopt and LA has gained interest as a treatment strategy to define personalized CPP targets after TBI. Advances in data acquisition technologies and real-time signal processing have helped translate the mathematical concept into a real-time bedside clinical decision-making tool [8, 18]. Our analysis showed that BP management below personalized LA is associated with lower PbtO2 levels, thereby providing additional support for using the CPPopt method to target a personalized CPP to reduce the risk of cerebral metabolic failure. In patients not undergoing PbtO2 monitoring, this approach could lead to less brain tissue hypoxia and improved outcomes. CPP optimization can be performed by using the ICP-based PRx or autoregulatory indices based on noninvasive technologies, such as transcranial Doppler and near-infrared spectroscopy. These noninvasive alternatives show promise in identifying personalized BP targets [19, 20].
The BOOST-II trial design allowed us to investigate the impact of hemodynamic interventions on autoregulatory function. Our findings indicate that peripheral BP manipulation can effectively maintain a patient’s CPP near their computed optimum value. We found that during periods when BP falls below the LLA, hemodynamic interventions do not alter the LA. Instead, they can restore autoregulatory function by shifting patients to a more favorable position on their autoregulatory curve (Fig. S3). However, it’s important to note that in this study, the assessment of the effects of CPP augmentation on autoregulatory function was limited by the study protocol as hemodynamic interventions were triggered by low levels of PbtO2 and not by reductions in BP below the LLA.
A recently published phase II clinical trial demonstrated the feasibility and safety of individualized CPP optimization [21]. The Targeting Autoregulation-Guided Cerebral Perfusion Pressure after Traumatic Brain Injury trial randomly assigned 60 patients to either a standard-of-care CPP target of 60–70 mm Hg or to the personalized autoregulation-guided CPP targets. In the intervention group, patients spent more time concordant with their CPPopt and significantly less time below the CPPopt trendline than patients in the control group (19.1 vs. 34.6%; p < 0.001). The study was not powered for efficacy; however, there was a trend toward better functional outcomes as measured by GOS-E at 6 months among patients treated with autoregulation-guided CPP management (p = 0.16). Whether it is best to aim for CPPopt directly, a fixed range around it (e.g., ± 5 mm Hg) as used in the Targeting Autoregulation-Guided Cerebral Perfusion Pressure after Traumatic Brain Injury trial, or a continuously estimated LA, remains to be determined.
Our study has several limitations. First, the number of patients and brain tissue hypoxia episodes included in the analysis was limited. While the high-quality data collection and inclusion of patients from multiple centers may alleviate some concerns, the results need to be confirmed in a larger cohort. Second, although the interventions to augment CPP were guided by the BOOST-II study protocol, there was significant heterogeneity of treatment (intravenous fluids vs. vasoactive medication, type vasopressor, dose). Third, while our analysis primarily focused on hemodynamic augmentation, it is important to recognize that other mechanisms also play a significant role in maintaining CBF and metabolism. CO2 reactivity, which refers to the change in blood flow due to variations in the partial pressure of CO2, as well as neurovascular coupling, which involves the adjustment in regional blood flow to meet the immediate metabolic demands of underlying neuronal activity, can also affect CBF. Both mechanisms operate independently of changes in CPP and could have influenced PbtO2 measurements. Additionally, hyperoxia may have impacted PbtO2 levels, even though no changes should have been made to the fraction of inspired oxygen. Unfortunately, no measurements were available for the partial pressure of oxygen in the arterial blood during the intervention. Fourth, the gold standard for measuring cerebral autoregulation remains to be defined. While time-correlation analysis is the most widely accepted technique for continuously measuring cerebral autoregulation in patients with TBI, various CBF surrogates, such as ICP, PbtO2, near-infrared spectroscopy–based tissue oximetry, or transcranial Doppler mean flow velocity, have been used. PRx has been the most explored and validated marker of cerebral autoregulation in TBI and correlates strongly with unfavorable neurological outcomes [22, 23]. The ORx has shown a good correlation with the more established PRx, especially when averaged over longer intervals [24, 25]. The same directional effect, albeit not statistically significant, was observed when using PRx to compute LA (Table S6). Although our analysis specifically targeted instances of brain tissue hypoxia treated solely with hemodynamic augmentation, the study protocol encompassed other interventions that could have affected ICP and potentially complicated the correlation between ICP and systemic hemodynamic parameters. Moreover, PRx provides global estimations of CBF control that may not fully capture regional variations in cerebrovascular control mechanisms.
Conclusions
Episodes of brain tissue hypoxia correlate with cerebral hypoperfusion characterized by an increased time with CPP below the LLA that can be improved with CPP augmentation. Thus, maintaining CPP in the range in which an individual patient autoregulates most efficiently is a promising strategy for optimizing cerebral physiology and potentially limiting secondary brain injury. However, prospective trials are needed to test whether an autoregulation guided CPP treatment protocol will improve relevant clinical biomarkers and functional outcomes.
Source of support
This project received no specific funding.
References
Wang A, Ortega-Gutierrez S, Petersen NH. Autoregulation in the neuro ICU. Curr Treat Option Ne. 2018;20:20.
Czosnyka M, Miller C. Monitoring P in the IMCC on M. Monitoring of cerebral autoregulation. Neurocrit Care. 2014;21(Suppl 2):S95-102.
Roux PL, Menon DK, Citerio G, Vespa P, Bader MK, Brophy GM, et al. Consensus summary statement of the international multidisciplinary consensus conference on multimodality monitoring in neurocritical care: a statement for healthcare professionals from the neurocritical care society and the European society of intensive care medicine. Neurocrit Care. 2014;21(Suppl 2):S1-26.
Aries MJH, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. 2012;40:2456–63.
Donnelly J, Czosnyka M, Adams H, Robba C, Steiner LA, Cardim D, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45:1464–71.
Okonkwo DO, Shutter LA, Moore C, Temkin NR, Puccio AM, Madden CJ, et al. Brain oxygen optimization in severe traumatic brain injury phase-II. Crit Care Med. 2017;45:1907–14.
Beqiri E, Smielewski P, Robba C, Czosnyka M, Cabeleira MT, Tas J, et al. Feasibility of individualised severe traumatic brain injury management using an automated assessment of optimal cerebral perfusion pressure: the COGiTATE phase II study protocol. BMJ Open. 2019;9:e030727.
Liu X, Maurits NM, Aries MJH, Czosnyka M, Ercole A, Donnelly J, et al. Monitoring of optimal cerebral perfusion pressure in traumatic brain injured patients using a multi-window weighting algorithm. J Neurotraum. 2017;34:3081–8.
Depreitere B, Güiza F, den Berghe GV, Schuhmann MU, Maier G, Piper I, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg. 2014;120:1451–7.
Bagiella E, Novack TA, Ansel B, Diaz-Arrastia R, Dikmen S, Hart T, et al. Measuring outcome in traumatic brain injury treatment trials. J Head Trauma Rehab. 2010;25:375–82.
Marín-Caballos AJ, Murillo-Cabezas F, Cayuela-Domínguez A, Domínguez-Roldán JM, Rincón-Ferrari MD, Valencia-Anguita J, et al. Cerebral perfusion pressure and risk of brain hypoxia in severe head injury: a prospective observational study. Crit Care. 2005;9:R670.
Schmidt JM, Ko S-B, Helbok R, Kurtz P, Stuart RM, Presciutti M, et al. Cerebral perfusion pressure thresholds for brain tissue hypoxia and metabolic crisis after poor-grade subarachnoid hemorrhage. Stroke. 2011;42:1351–6.
Megjhani M, Weiss M, Ford J, Terilli K, Kastenholz N, Nametz D, et al. Optimal cerebral perfusion pressure and brain tissue oxygen in aneurysmal subarachnoid hemorrhage. Stroke. 2022.
Chang JJJ, Youn TS, Benson D, Mattick H, Andrade N, Harper CR, et al. Physiologic and functional outcome correlates of brain tissue hypoxia in traumatic brain injury. Crit Care Med. 2009;37:283–90.
Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury fourth edition. Neurosurgery. 2017;80:6–15.
Chesnut R, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46:919–29.
Hawryluk GWJ, Aguilera S, Buki A, Bulger E, Citerio G, Cooper DJ, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019;45:1783–94.
Aries MJH, Wesselink R, Elting JWJ, Donnelly J, Czosnyka M, Ercole A, et al. Enhanced visualization of optimal cerebral perfusion pressure over time to support clinical decision making. Crit Care Med. 2016;44:e996–9.
Silverman A, Kodali S, Strander S, Gilmore EJ, Kimmel A, Wang A, et al. Deviation from personalized blood pressure targets is associated with worse outcome after subarachnoid hemorrhage. Stroke. 2019;50:2729–37.
Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51:914–21.
Tas J, Beqiri E, van Kaam RC, Czosnyka M, Donnelly J, Haeren RH, et al. Targeting autoregulation-guided cerebral perfusion pressure after traumatic brain injury (COGiTATE): a feasibility randomized controlled clinical trial. J Neurotraum. 2021;38:2790–800.
Sorrentino E, Diedler J, Kasprowicz M, Budohoski KP, Haubrich C, Smielewski P, et al. Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care. 2012;16:258–66.
Czosnyka M, Czosnyka Z, Smielewski P. Pressure reactivity index: journey through the past 20 years. Acta Neurochir. 2017;159:2063–5.
Jaeger M, Schuhmann MU, Soehle M, Meixensberger J. Continuous assessment of cerebrovascular autoregulation after traumatic brain injury using brain tissue oxygen pressure reactivity&ast. Crit Care Med. 2006;34:1783–8.
Owen B, Vangala A, Fritch C, Alsarah AA, Jones T, Davis H, et al. Cerebral autoregulation correlation with outcomes and spreading depolarization in aneurysmal subarachnoid hemorrhage. Stroke. 2022;53:1975–83.
Author information
Authors and Affiliations
Contributions
AP, AdH, and NHP contributed to the study concept and design. NRT, JB, and RDA contributed to data acquisition. AP, EJG, SOG, AdH, and NHP contributed to the analyses and interpretation of data. AP, EJG, JAK, LB, MO, RB, GJF, CM, SOG, NRT, JB, RDA, AdH, and NHP contributed to the drafting and critical revisions of the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
NHP is supported by the National Institutes of Health - National Institute of Neurological Disorders and Stroke (NIH-NINDS) (K23NS110980) and has received clinical research funding from Liminal Sciences. ADH is supported by NIH-NINDS (K23NS105924), has received investigator-initiated clinical research funding from the AAN, has received consultant fees from Integra and Novo Nordisk, has equity in TitinKM and Certus, and receives author fees from UpToDate. EJG is supported by NIH-NINDS (R01NS117904) and receives consulting fees from UCB. SOG is supported by NIH-NINDS (R01NS127114, R03NS1202228), industry investigator-initiated grants from Stryker Neurovascular, Medtronic, Microvention, Methinks, and VizAi, and Society of Vascular and Interventional Neurology, and consulting fees from Medtronic, Stryker, and Microvention. CCM is supported by NIH-NINDS R21NS119992, receives consulting fees from Microvention-Terumo and Stryker, and speaker fees from Penumbra and Silk Road Medical. NRT and JB are supported by NIH-NINDS (U01NS099084).
Ethical Approval/Informed Consent
The Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II trial was approved by the local institutional review board at each participating center, and proxy informed consent was obtained from each study participant. This secondary analysis was performed on deidentified data obtained from the Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase II investigators under a data use agreement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prasad, A., Gilmore, E.J., Kim, J.A. et al. Impact of Therapeutic Interventions on Cerebral Autoregulatory Function Following Severe Traumatic Brain Injury: A Secondary Analysis of the BOOST-II Study. Neurocrit Care 41, 91–99 (2024). https://doi.org/10.1007/s12028-023-01896-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01896-x