Abstract
Aim
Split cord malformations (SCMs) are rare congenital anomalies of the vertebrae and the spinal cord. Tethered cord syndrome (TCS) is a clinical condition of various origins that arises from tension on the spinal cord. Radiographic findings may include and/or associate split cord malformations and the other neural tube defects. However, the spinal cord can even be tethered by a filum terminale with normal appearance and normal level conus medullaris in magnetic resonance imaging (MRI). The aim of our study is to show whether SMC patients with normal or abnormal MRI findings had all histological abnormal filum terminale and also to show that the standard SCM repairing operation without cutting filum will not achieve total release.
Material and methods
We have reviewed 33 SCM patients between July 2005 and December 2013. They were operated by adding untethering procedure of filum terminale following standard surgical intervention, and a part of the filum was taken for histopathological examination even though MRI did not show the presence of abnormality of filum terminale.
Results
We found that abnormal filum terminale with a normal appearance may had dense collagen fibers, wide and numerous capillaries, and hyaline formation, while normal filum terminale is a mixture of collagen fibers and blood vessels. We did not obtain positive Verhoeff elastic fiber staining. The elastic fibers had disappeared in all fila terminalia, except control cadaver group.
Conclusion
Our results showed that all fila of SCM patients had loss of elastic fibers and increased of hyalinization, which means loss of elasticity of filum terminale. Less severe traction may remain asymptomatic in childhood and present with neurological dysfunction later in life. For this reason, surgical procedure of SCM patients including releasing of filum terminale seems more beneficial for the patients and be better for long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Split cord malformations (SCMs) are rare developmental abnormalities of the vertebrae and the spinal cord first defined by Bentley and Smith [1, 17]. Hertwig has reported double cord malformations in which the hemicords reside in separate dural sacs fixed by a midline osteocartilagenous spur, in 1892. Herren and Edwards used the term diplomyelia to describe complete duplication of spinal cord segment where the double cords reside in a single dural sac with no midline elements [18].
Tethered cord syndrome (TCS) is a clinical condition of various origins that arises from tension on the spinal cord. TCS is characterized by motor and sensorial changes in the legs, back and leg pain, foot deformities, urinary dysfunction, and spinal column deformity [4–6, 10, 22–24]. Radiographic findings may include and/or associate split cord malformations, lipomyelomeningocele, myelomeningocele, dermal sinus, and the others. However, the spinal cord can even be tethered by a filum terminale (FT) with normal appearance and normal level conus medullaris in magnetic resonance imaging (MRI) [21].
The aim of our study is to show whether SCM patients with normal or abnormal MRI findings had all histological abnormal filum terminale and also to show that the standard SCM repairing operation without cutting filum terminale will not achieve total release.
Material and methods
The 33 patients with SCMs who were surgically treated between July 2005 and December 2013 were reviewed. All surgical interventions were performed at the Celal Bayar University School of Medicine, Department of Neurosurgery. The study was approved by the hospital’s institutional review board committee. Patients were retrospectively studied for clinical profile (Table 1), symptoms, signs, and associated anomalies (Table 2). MRI findings, surgical procedures, histopathological examination of specimens, and clinical outcome were given in the results.
Surgical intervention
Before surgery, we identified level of SCM on the patient with using C arm fluoroscopy in the operating room for surgical incision. Total laminectomy performed both SCM I and SCM II (Figs. 1 and 2). In the patients who had SCM I malformation, laminectomy was limited around the attachment of the median septum. The septum was dissected subperiostally, and this osseocartilaginous median malformation removed surgically by using fine Kerrison Ronguer or by using diamond drills (Fig. 3a–b). After opening dura on the both sides of the dural cleft, fibrous bands and arachnoid adhesions were sectioned. Following releasing of hemi cords, neural tissue and dural sleeve were closed only on the posterior. The anterior dura was left open. In the patients who had SCM II malformation, laminectomy was extended caudal end of split segment. After midline dural sectioning, fibrous septum was excised. If there was dorsal root that terminated on the dura (nonfunctional) or any associated anomaly such as dermoid, epidermoid, lipoma, and neuroenteric cyst that might lead to tethering, they were also surgically corrected in both of SCMs. Additionally, the filum terminale was explored and sectioned in all cases even if normal appearance of filum was seen in the MRI (Fig. 4). Following the untethering procedure, a part of the filum was taken for histopathological examination. All the patients received antibiotics for 5 days postoperative, and they were discharged between 7 and 10 days following surgery. Patients were followed up ranging from 3 months to 9 years.
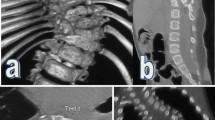
(Left) Type I SCM consists of two hemicords, each contained within its own dural tube and separated by a dura-sheathed rigid osseocartilaginous median septum (*), laminectomy limited around the attachment of the median septum, (right) septum removed surgically by using fine Kerrison Ronguer or by using diamond drills (*)
(Left) T2-weighted axial magnetic resonance image of thoracolumbar spine shows two hemicords housed in a single dural tube. (Middle) T2-weighted and (right) T1-weighted sagittal magnetic resonance images of thoracolomber spine shows normal level (L1-2) (left arrow) of conus medullaris and normal appearance of filum terminale
Tissue collection, fixation, and histological examination
The samples were divided into five groups. Group A1 was SCM I patients with abnormal FT. Group A2 was SCM I patients with normal appearance of FT. Group B1 was SCM II patients with abnormal FT. Group B2 was SCM II patients with normal appearance of FT. Group C was control group that fila obtain from normal human cadavers. All cadavers were fresh, and medical records of all of them were checked whether they had tethered cord symptoms or signs before.
All fila were fixed in a 10 % formalin solution less than 48 h. The samples were washed with tap water and were soaked in a series of ethanol 60, 70, 80, 90, and 100 %. Then, they were held in a solution of xylene for 90 min and were embedded in paraffin at 60 °C.
Serial transverse sections, 5 μm thickness, were taken by using the Rotary Microtome (RM 2135i Leica) from the blocks and prepared for both histological and histochemical and staining. Sections de-waxed at less than 60 °C overnight were immersed in xylene for 1 h and then rehydrated through a graded series of ethanol (95, 80, 70, and 60 %) for 2 min in each concentration, and they were then washed in tap water. Sections were stained with hematoxylin (01562E, Surgipath, Bretton, Peter Borough, Cambridgeshire)–eosin (01602E, Surgipath, Bretton, Peter Borough, Cambridgeshire) (HE), Verhoeff Van Gieson (010308, Diapath, Martinengo, Italy), and Masson Trichrome (04-010802, Bio-optica, Milano, İtaly).
Results
Patient demographics
Thirty-three patients were 23 (70 %) female and 10 (30 %) male, with mean age 11.8 (range 1 month–48 years); median was 6 years old. There was no remarkable gender predominance in type I SCMs than the type II SCMs (female patient’s rate in SCM I was 69.5 % and in the SCM II was 66.6 %). There was only one female patient who had type I SCM and type II SCM in both (Table 1).
Symptoms and signs
Most common symptoms and signs are summarized skin stigmata, neurological findings, and associated urinary and orthopedically problems (Table 2). Skin stigmata was present in 21 (63.6 %) patients (two patients had both sacral dimple and hypertrichosis). Hypertrichosis was the most common skin finding. Nine patients had myelomeningocele (MMC) or MMC manqué; two patients had lipomyelomeningocele; one patient had small meningocele, and three patients had a dermal sinus tract. The most common orthopedic problem was scoliosis. Seven patients had scoliosis and/or kyphoscoliosis, and six patients had foot deformity. Some patients had more than one orthopedic deformity (scoliosis and foot deformity in same time). Eight patients (above 3 years old) had urinary incontinence; two of these eight patients had bowel incontinence too. Neurological motor deficit was recorded in 30 %. Nine patients had low back pain and/or leg pain or both (five back pain, two leg pain, two both).
There were only three patients asymptomatic, in which two patients had prenatal ultrasonographic SCMs diagnosis and one incidentally diagnosed during the evaluation of the subcutaneous thoracolumbar mass. SCM’s associated spinal malformations were given also in Table 1.
Radiology
Plain X-rays of the spine were obtained in all patients. In order to avoid radiation in patients under age 6, computerized tomography (CT) scans were not performed if previously not carried out. MRI was performed in all the patients to find out the type of SCM and associated anomalies. Twenty-three patients had thick, fatty and/or tight filum terminale. Although in seven patients, the FT had normal appearance on MRI, it was found tight at surgery (Fig. 4). Scoliosis, congenital vertebrae anomalies, bony spur, bifid lamina and widened interpedicular distance at the level of SCM, and other associated neural developmental anomalies have been seen in plain X-rays, CT scan, and/or MRI. SCMs were most common in lumbar area (54.5 %) followed by thoracolumbar level (30.3 %), and in thoracal levels (15 %) (Table 1). In cervical levels, SCMs were not seen in our patients.
Associated lesions
All SCM patients had an associated spinal abnormality; in addition, some had more than one developmental anomaly (Table 1). Twenty-six patients (78.7 %) had a thick, tight, and/or fatty filum. In that case, the most common associated condition was tight filum terminale. Nine patients had MMC or MMC manqué, which all had tethered cord findings on MRI. Three patients had dermal sinus tract, and the others had lipomyelomeningocele, meningocele, and dermoid in two, one, and one patients, respectively.
Histology
“+” sign is used to mention the density of immunohistochemistry staining of the desired molecule, where as “+” is less dense and more “+” signs indicate more dense staining.
In group A1, moderate (++) collagen staining and dense (+++) hyalinization were observed, but weak (−) Verhoeff staining and very small number of elastic fibers were seen (Fig. 5a, b).
Moderate collagen and dense hyalinization in Masson Trichrome staining (a), weak Verhoeff staining and very small number of elastic fibers (b) in group A1. Moderate collagen and low hyalinization in Masson Trichrome staining (c), weak Verhoeff staining and small number of elastic fibers (d) seen in group A2. Collagen (➞), hyalin (⬇⬇), elastic fibers (➞)
In group A2, moderate (++) collagen staining and low (+) hyalinization were observed. Weak (−) Verhoeff staining and also small number of elastic fibers were seen (Fig. 5c, d).
In group B1, moderate (++) collagen staining and low (+) hyalinization were observed, but weak (−) Verhoeff staining and very small number of elastic fibers were seen (Fig. 6a, b).
Moderate collagen and low hyalinization in Masson Trichrome staining (a), weak Verhoeff staining and very small number of elastic fibers (b) in group B1. Moderate collagen and moderate hyalinization in Masson Trichrome staining (c), weak Verhoeff staining and small number of elastic fibers (d) seen in group B2. Collagen (➡), hyalin (⬇⬇), elastic fibers (➞)
In group B2, moderate (++) collagen staining and moderate (++) hyalinization were observed. Weak (−) Verhoeff staining and very small number of elastic fibers were seen like the other SCM groups (Fig. 6c, d).
In the control group (group C), there were moderate (++) collagen staining but weak (−) hyalinization. However, unlike the others, dense (+++) Verhoeff staining and also numerous of elastic fibers were seen (Fig. 7; Table 3).
Outcome
There was one death in our series. In this 48-year-old patient, massive pulmonary embolism developed unexpectedly in the first postoperative day, and CPR did not help, and cardiac arrest occurred.
In two patients, cerebrospinal fluid (CSF) leak occurred but surgical repairing is not required. For one patient, transient unilateral leg paresis occurred but clinical improvement was detected in 6 weeks. All patients were followed up ranging from 3 months to 9 years, and there was no permanent complication detected. None of the patients showed worsening of scoliosis or other orthopedically complaint or neurological status.
First of all, while these surgical interventions for these pediatric neurosurgical cases are essentially prophylactic interventions, we do not expect worsening of neurological status of our patients. Hopefully, this came through as we expected. Furthermore, pain in all patients relieved, scolisosis angle stopped at its level at the time of operation (this is considered as improvement by us, because they did not need major orthopedically corrective scoliosis surgery anymore)
Discussion
There is a decreasing trend in neural tube defect (NTD) in the world literature. Since the second half of twentieth century, the incidence of NTD and associated spinal developmental abnormalities had decreased from 40–50/10,000 live births to 3–4/10,000 live births [11]. Many hypotheses were reported to explain the etiology of SCM. Lichtenstein advocated a primary abnormality in the mesoderm while Herren and Edwards were proponents of the overgrowth hypothesis. It was not until 1968 that both hypotheses were challenged with Gardner’s proposition of the hydromyelic theory followed by Hendrick’s hypotheses of an accessory neuroenteric canal in 1971 [17]. All SCMs originate from one basic error that occurs at approximately which the notocord progresses cranially and meets an adhesion (presumably developed by the remnants of accessory neuroenteric canals as stated by Kovalevski) that divides the notocord into two lanes. Since the development of the neural tissue is closely related to the signals coming from the notocord as sonic hedgehodge (shh) gene, two lanes of notocord cause to develop two different lanes of neural tissue at the side of the ectoderm. This basic error is the formation of an accessory neurenteric canal between the yolk sac and amnion, which is subsequently invested with mesenchyme to form an endomesenchymal tract that splits the notochord and neural plate [2, 10]. In 1992, Pang et al. presented the unifying theory of split cord malformation proposing that SCMs originate from one basic ontogenic error occurring around the time when the primitive canal closes [14, 17]. Despite the unified theory of embryogenesis proposed by Pang et al., much confusion still remains as to the classification and development of this malformation [8].
Skin stigmata are a well-known sign of occult spinal dysraphism. Izci et al. highlighted the diagnostic value of skin marker [7]. In our study, two thirds of patients had cutaneous marker, in addition to orthopedical deformities, which are both the evidence of SCM. Incidence of skin lesions in patients with spina bifida aperta (MMC, myeloschisis, etc.) was significantly less than that in those without them. Although, skin lesions strongly suggest a developmental abnormality, but none of these are diagnostic alone.
The common site of this developmental anomaly is thoracolumbar area and lumbar area, as seen in our study and previously reported by others. In our study, 28 patients (85 %) had SCM at lumbar (18 patients) or thoracolumbar (10 patients) area. Although, SCM is reported in cervical and sacral areas rarely [2, 3, 9, 11], we did not see SCM in these areas.
A low-lying conus and thick FT are related to abnormal retrogressive differentiation of the secondary neural tube with failure of terminal cord involution [9]. Following neurulation, the distal neural tube undergoes canalization [2–4, 10]. The filum terminale (nervus impar) is created when the caudal neural tube regresses between the ventriculus terminalis and the caudal cell mass [12]. It is a fibrous band consisting of vascular layers that extends from the tip of the conus medullaris to blend with the periosteum of the posterior surface of the coccyx. However, other types of tissue are scattered within this band and play significant roles in many of its pathologies [17].
Pang proposed a new classification of SCM for all double spinal cords [14]. They also have proposed a unified theory that explains the embryo-genetic mechanisms of all variants of split cord malformations [2, 9, 11, 14]. Type I SCM consists of two hemicords, each contained within its own dural tube and separated by a dura-sheathed rigid osseocartilaginous median septum (Fig. 1). Type II SCM consists of two hemicords housed in a single dural tube separated by a nonrigid, fibrous median septum (Fig. 2) [2, 14, 18]. These two essential features necessary for typing, the state of the dural tube and the nature of the median septum, do not ever overlap between the two main forms and can always be demonstrated by imaging studies so that accurate preoperative typing is always possible [14]. CT scan may be useful in SCM type I patients and three dimensional CT especially in scoliosis patients. However, MRI is the most important investigation in these patients. In our series, all patients had spinal and cranial MRI at the suspected site of the lesion and to exclude Chiari malformation. In fact, MRI is useful to find associated congenital developmental anomalies of the spinal cord and vertebral column. MRI is also useful to find out thick or fatty filum or low level of conus medullaris. On the other hand, Selcuki et al. showed that normal appearance of the filum terminale on MRI does not mean that it really is totally normal. They reported that abnormal FT with a normal radiological appearance may have dense collagen fibers, wide and numerous capillaries, and hyaline formation, while normal FT is a mixture of collagen fibers and blood vessels [19, 21]. Furthermore, in their paper, Selcuki et al. reported a case which was deteriorated after previous operation for SCM, because thick and abnormal filum terminale was not cut at first procedure. This case report points to the importance of FT untethering in order to achieve total release [20].
Twenty six of 33 patients had thick and fatty filum terminale that mean tethering of spinal cord on MRI in our study. Fifteen of these 26 patients had associated spinal dysraphysm as MMC, lipomyelomeningocele, etc. Six spinal dysraphism patients had been operated previously in other medical centers. Four of them had an operation history of both of SCM and MMC simultaneously. In these six patients, there was progressive neurological deterioration like others, but it is interesting that only two patients showed an adhesion in the operative field. They had a common point which was none of them had unthethering procedure with releasing and cutting filum terminale. We performed second operation on these patients, and we released neural adhesions in two and filum sectioning in all. Hertzler et al. reported that all patients with MMC are born with a tethered cord and at birth, or shortly thereafter [4]. Phuong et al. retrospectively reviewed 45 cases of repaired MMC with TCS that did not undergo a detethering procedure and found that 60 % of the patients progressed with TCS and 89 % needed other surgical procedures [16].
In our series, symptoms related to congenital anomalies occurred most commonly in childhood, but many patients did not show symptoms until adulthood. There were six adult patients (range 24–48 years old), and some of them did not show an annoying symptom previously. Why do patients develop signs and symptoms in adulthood when the origin of these tethering lesions is often similar to those noted in the younger age groups? It is known that less common tethering lesions in the adult includes SCMs and a dermal sinus. SCMs were initially regarded as a pediatric trouble, but in many patients, the diagnosis is not established until symptoms manifest in adulthood. In our study, seven patients were above 16 years old and range of age was 16–48; median value was 30 years. Pang and Wilberger reported that patients with less severe traction remain asymptomatic in childhood and present with neurological dysfunction later in life. They described two categories: (1) children that were healthy in childhood with symptoms developing later in life and (2) those with stable deficits and previous diagnoses that were well until the onset of new or progressive deficits in adulthood [13, 15]. Yamada et al. identified several factors contributing to the onset of symptoms over time in patients with less severe traction, including (1) increasing fibrosis of the filum leading to progressive loss of viscoelasticity, which results in progressively increased traction in the lumbosacral cord; (2) a growth spurt that could cause a rapid increase in spinal cord tension; (3) an increase in physical activity (sports, exercise); and (4) development of spinal stenosis that can restrict movement and may accentuate tension [24, 25].
The bulk of the filum terminale is made of connective tissue layers, consists mainly of collagen fibers, and, to a less extent, elastin and elaunin fibers. These fibers are organized as longitudinal bundles and are lined by transverse, delicate fibers [17]. Elastic tissue fibers are shown with Verhoff’s Van Gieson staining. This stain is useful in demonstrating the presence of elastic tissue. Elastin has a strong affinity for this stain, and elastic fibers and cell nuclei are stained black, collagen fibers are stained red, and other tissue elements including cytoplasm are stained yellow. We did not obtain positive Verhoeff elastic fibers staining (Figs. 5 and 6) in both normal appearance of fila terminalia and the others. On the other hand, compared with the control group (group C), in all histological sections had increased hyalization and disappeared of elastic fibers. These histological results may explain the stretching of normal level conus medullaris with filum terminale consisting of less elastic and dense collagen fibers with hyalinization caused by simply loss of elasticity and also with normal appearance of filum terminale on MRI.
Conclusion and suggestions
Split cord malformations are rare congenital anomalies that may include different associated developmental abnormalities that most likely predispose spinal cord to tethering. Our results showed that all fila of SCM patients had loss of elastic fibers and increased of hyalinization, which means loss of elasticity of filum terminale even though MRI did not show abnormality of filum terminale.
Progressively increased traction in the lumbosacral cord may cause irreversible neurological deteriorations or orthopedic deformity. Less severe traction may remain asymptomatic in childhood and present with neurological dysfunction later in life. For this reason, surgical procedure of SCM patients including releasing of filum terminale seems more beneficial for the patients and be better for long term.
References
Bentley JF, Smith JR (1960) Developmental posterior enteric remnants and spinal malformations: the split notochord syndrome. Arch Dis Child 35:76–86
Ersahin Y (2013) Split cord malformation types I and II: a personal series of 131 patients. Childs Nerv Syst 29(9):1515–1526
Ersahin Y, Mutluer S, Kocaman S, Demirtas E (1998) Split spinal cord malformation in children. J Neurosurg 88:57–65
Hertzler DA 2nd, DePowell JJ, Stevenson CB, Mangano FT (2010) Tethered cord syndrome: a review of the literature from embryology to adult presentation. Neurosurg Focus 29(1):E1. doi:10.3171/2010.3.FOCUS1079, Review
Hoffman HJ, Hendrick EB, Humphreys RP (1976) The tethered spinal cord: its protean manifestations, diagnosis and surgical correction. Childs Brain 2(3):145–155
Hui H, Zhang ZX, Yang TM, He BR, Hao DJ (2013) Vertebral column resection for complex congenital kyphoscoliosis and type I split spinal cord malformation. Eur Spine J 14 doi:10.1007/s00586-013-3044-6
Izci V, Gonul M, Gonul E (2007) The diagnostic value of skin lesions in split cord malformation. J Clin Neurosci 14:860–863
Izci Y, Kural C (2011) Composite type of split cord malformation: rare and difficult to explain. Pediatr Neurosurg 47:461
Kim YD, Sung JH, Hong JT, Lee SW (2013) Split cord malformation combined with tethered cord syndrome in an adult. Korean Neurosurg Soc 54:363–365
Lew SM, Kothbauer KF (2007) Tethered cord syndrome: an updated review. Pediatr Neurosurg 43:236–248
Mahapatra AK (2011) Split cord malformation—a study of 300 cases at AIIMS 1990–2006. J Pediatr Neurosci 6(Suppl 1):41–45
Nievelstein RA, Hartwig NG, Vermeij-Keers C, Valk J (1993) Embryonic development of the mammalian caudal neural tube. Teratology 48:21–31
Pang D (1992) Split cord malformation. Part II: clinical syndrome. Neurosurgery 31:481–500
Pang D, Dias MS, Ahab Barmada M (1992) Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformation. Neurosurgery 31:451–480
Pang D, Wilberger JE Jr (1982) Tethered cord syndrome in adults. J Neurosurg 57(1):32–47
Phuong LK, Schoeberl KA, Raffel C (2002) Natural history of tethered cord in patients with meningomyelocele. Neurosurgery 50:989–995
Rizk E, Adeeb N, Hussein AE, Tubbs RS, Rozzelle CJ, Oakes WJ (2014) Duplicated filum terminale in the absence of split cord malformation: a potential cause of failed detethering procedures. Childs Nerv Syst 30(4):709–711
Salunke P, Kovai P, Malik V, Sharma M (2011) Mixed split cord malformation: are we missing something? Clin Neurol Neurosurg 113(9):774–778
Selcuki M, Coskun K (1998) Management of tight filum terminale syndrome with special emphasis on normal level conus medullaris (NLCM). Surg Neurol 50:318–322
Selcuki M, Umur AS, Duransoy YK, Ozdemir S, Selcuki D (2012) Inappropriate surgical interventions for midline fusion defects cause secondary tethered cord symptoms: implications for natural history report of four cases. Childs Nerv Syst 28(10):1755–1760
Selcuki M, Vatansever S, Inan S, Erdemli E, Bagdatoglu C, Polat A (2003) Is a filum terminale with a normal appearance really normal? Child’s Nerv Syst 19:3–10
Umur AS, Selcuki M, Selcuki D, Bedük A, Doganay L (2007) Adult tethered cord syndrome mimicking lumbar disc disease. Child’s Nerv Syst 24:841–844
Yamada S, Colohan AR, Won DJ (2009) Tethered cord syndrome. J Neurosurg Spine 10:79–80, author reply 80–81
Yamada S, Won DJ, Yamada SM (2004) Pathophysiology of tethered cord syndrome: correlation with symptomatology. Neurosurg Focus 16(2):E6
Yamada S, Zinke DE, Sanders D (1981) Pathophysiology of “tethered cord syndrome”. J Neurosurg 54:494–503
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barutcuoglu, M., Selcuki, M., Selcuki, D. et al. Cutting filum terminale is very important in split cord malformation cases to achieve total release. Childs Nerv Syst 31, 425–432 (2015). https://doi.org/10.1007/s00381-014-2586-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2586-1