Abstract
Introduction
A duplicated filum terminale appears to be a rare finding in the absence of a split cord malformation. Herein, we discuss this finding and its potential dysembryology.
Case reports
We report two cases of duplicated filum terminale without split cord malformation. The first case presented as an incidental finding with thickened filum terminale. At time of surgery, a thickened filum was resected and a smaller size filum was transected and both were confirmed with pathologic examination. The second case presented with a lumbar skin hemangioma. Screening MRI showed a duplicated filum terminale with fat signal in both structures. Pathology also confirmed the diagnosis of two fila terminalia.
Conclusions
The neurosurgeon should consider the possibility of two fila terminalia during operation to transect a single filum for tethered spinal cord.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Split cord malformation (SCM) is a rare subset of neural tube defects (NTD) [1–7]. Furthermore, the incidence of NTDs has declined progressively in the past 50 years [4]. Duplication of filum terminale seen on imaging or documented intraoperatively is a rare presentation. Herein, we present two patients with split filum terminale without associated spinal cord anomalies.
Case reports
Case 1
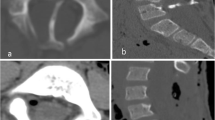
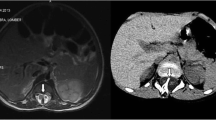
An 8-year-old patient was incidentally found to have a thickened fatty infiltrated filum on imaging performed following workup for acute pancreatitis. Urodynamic studies were performed without detection of abnormalities. The patient was taken to the operating room for sectioning of thickened filum terminale. The patient was noted to have a thickened filum with a secondary smaller filum terminale (Fig. 1). Section was performed and diagnosis was confirmed histologically for both structures.
Case 2
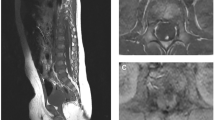
A 5-year-old patient was presented to the outpatient clinic with a lumbar level hemangioma. Imaging showed a duplicated filum terminale with fatty infiltration of both fila.
Discussion
The caudal cell mass gives rise to the filum terminale and ventriculus terminals. Specifically, at day 52 of gestation, the filum terminale is created when the caudal neural tube regresses between the ventriculus terminalis and the caudal cell mass [8]. Cells of secondary neuroectodermal origin will eventually differentiate into a fibrous layer representing the future filum terminale [8]. Incidental fat within the filum is found in up to 17 % of normal adults [9].
The filum terminale (nervus impar) is a fibrous band that extends from the tip of the conus medullaris to blend with the periosteum of the posterior surface of the coccyx. It was divided by Luschka into two components: intradural component (filum terminale internum) and extradural component (filum terminale externum or coccygeal ligament) [19, 22]. The limitation between these two parts is marked by the site of fusion between the intradural part and the dura mater of the dural sac. This fusion may occur at any level from L5 to S3, but usually at the level of S2 [22] (upper one-third). Although this fusion mostly occur in the midline, it may occur off of the midline dorsally, which makes it difficult to find during surgery [19]. According to Tarlov [20], the length of the intradural compartment was 4.2 cm in newborns and 15 cm in adults. Rauber and Kopsch [21] on the other hand, reported that the length of the internal filum was about 16 cm.
Early studies described the filum terminale simply as a fibrovascular band, and little attention was given to study this important structure until the beginning of the twelfth century. The filum terminale consists of fibrous connective tissue and vascular layers. However, other types of tissue are scattered within this band and play significant roles in many of its pathologies. The bulk of the filum terminale is made of connective tissue layers, consists mainly of collagen fibers, and, to a less extent, elastin and elaunin fibers. These fibers are organized as longitudinal bundles and are lined by transverse, delicate fibers. Tarlov [20] mentioned that this connective tissue represents a thickened pial sheath, which size increases caudally and is thinnest dorsally. He also added that the lumen of this connective tissue sheath forms a V shape, with the apex directed caudally. The limbs of the V represent the passage of the nerve fibers; and between the limbs of the V, which become increasingly thickened toward the apex, the nerve and interstitial structure of the filum is contained.
In 1892, Tourneux [23] mentioned that at the transition between the conus medullaris and the filum, the central canal flattens and continues within the filum as far as the vicinity of the dural cul-de-sac. He also mentioned the presence of medullary nerve cells, which also decreases in number until they disappear 1 mm below the dural sac. These findings were confirmed by Rauber and Kopsch [21] in 1909, who added that the medullary nerve cells were observed clinging to the sides of the filum. In 1933, Harmeier [24] described the microscopic characteristic of the intradural filum terminale. Large collections of ependymal cells were found in certain places without definite arrangement, and in other places, surrounding irregular cavities. Excessive numbers of corpora amylacea were also found beneath the pia mater and also scattered throughout the filum. Ganglion cells were also found at the periphery, and neuroblasts were scattered within the tissue. Axons were also described extending all the way down, and myelin was abundant in the proximal half of the filum. As the filum terminale internum progress downward, the amount of nerve tissue decreases gradually until it becomes confined as a peripheral thin layer [20].
Tarlov’s [20] description of the microscopic anatomy of the filum terminale was more detailed. He found that at the transition between the conus medullaris and the filum, the difference between anterior and posterior horns becomes gradually less pronounced with lack of demarcation of the gray and white matter. Nerve cells still can be seen, mainly within the first 3 cm of the intradural portion of the filum terminale. These nerve cells were occasionally large, multipolar cells. Some of these cells exhibit degenerative changes. Tracts of nerve fibers could also be seen continuing within the filum and gradually becoming less pronounced to attain a peripheral position in the filum. In the periphery, they become surrounded by Schwann cells and endoneurium and occasionally contain nerve cells. Many of these fibers were myelinated. The central canal may disappear and reappear again within the filum and it may also reach the dorsal border of the filum. In certain cases, the central canal bifurcates to form various numbers of outpouchings, which appears as multiple cavities in the cross section. Large numbers of glial cells are present within the intradural portion of the filum, and most of the time, these constitutes the main bulk of the filum. These glial cells are similar to those found in the central nervous system, and consist of ependymal cells, astrocytes, oligodendrocytes, and microglial cells. These cells are assumed to play their expected function, and are present mainly within the cranial part of the filum terminale and disappear caudally. The ependymal cells were the most abundant and the last to disappear of all the interstitial cells, as it extends as far as the first 2 cm of the extradural part of the filum. Ependymal cells assume many shapes, surround the cavities, and is scattered in many parts of the filum. Corpora amylacea were also described, and they represent the most common form of the degeneration products within the filum [20]. These structures are covered by the connective tissue sheath described above.
At the site of transition between the intradural and extradural portions, the filum becomes surrounded by arachnoid and dural layers. The arachnoid surrounds bundles of nerve fibers and the central portion of the filum. The cuboidal cells of the arachnoid at this level project into the fibrous stroma of the dural layer. Caudally, these become applied as a sheath with flattened lining cells that gradually disappear. The nerve fiber bundles become surrounded by the dural layer, which forms the epineurium. However, within the first few centimeters, all nerve fibers acquire a perineurium and an epineurium. Myelinated and non-myelinated nerve fiber bundles become less conspicuous gradually as they migrate laterally to their foramina of exit until they finally disappear. Small bundles of nerve fibers, however, were described along the entire course of the filum terminale. The filum finally blends with periosteum on the dorsum of the coccyx. Psammoma bodies were found at the junction between the two parts of the dura, mainly in older subjects [20]. According to Tubbs et al., no glial or ependymal cell rests were found in this part of the filum, and a variable number of smooth-muscle bundles were embedded within the fibrous stroma [18].
SCM is a rare developmental disorder first coined by Bentley and Smith [10]. Multiple hypotheses were presented to explain the etiology of SCM. In 1940, Lichtenstein advocated a primary abnormality in the mesoderm while Herren and Edwards were proponents of the overgrowth hypothesis [11, 12]. It was not until 1968 that both hypotheses were challenged with Gardner’s proposition of the hydromyelic theory followed by Hendrick’s hypotheses of an accessory neuroenteric canal in 1971 [13, 14]. In 1992, Pang et al. presented the unifying theory of split cord malformation proposing that SCMs originate from one basic ontogenic error occurring around the time when the primitive canal closes [6]. They classified SCM into two types. Type I consist of two hemicords housed in separate dural sacs separated by an osteocartilaginous septum, while type two consists of two hemicords in a single dural sac separated by a fibrous median septum. SCM is most commonly located in the thoracolumbar or lumbar areas [1, 3–7, 15, 16].
Masahito et al. reported a case of a 2-year-old with split conus and filum terminale without an intervening septum [17]. Herein, we present two patients with radiologic and pathologic documentation of a duplicated filum terminale without split cord malformation. Pang et al.’s unifying theory does not explain this isolated process since the development of the filum is separate from spinal cord embryogenesis. However, this could be a forme fruste manifestation of split cord malformation with an ontogenic error in the formation of the filum terminale. Finally, our findings add credence to a thorough intraoperative inspection of the thecal sac contents during the sectioning of the filum terminale in order to avoid missing a duplicated and less obvious filum, especially when the surgical opening is distal to the conus and the origin of the filum is not seen.
Conclusions
The neurosurgeon should consider the possibility of two fila terminale during operation to transect the filum terminale for a tethered spinal cord.
References
Ansari S et al (2007) Split cord malformation associated with myelomeningocele. J Neurosurg 107:281–285
Higashida T et al (2010) Myelomeningocele associated with split cord malformation type I–three case reports. Neurol Med Chir (Tokyo) 50:426–430
Jindal A, Mahapatra AK (2000) Split cord malformations—a clinical study of 48 cases. Indian Pediatr 37:603–607
Mahapatra AK (2011) Split cord malformation—a study of 300 cases at AIIMS 1990–2006. J Pediatr Neurosci 6:S41–S45
Mahapatra AK, Gupta DK (2005) Split cord malformations: a clinical study of 254 patients and a proposal for a new clinical-imaging classification. J Neurosurg 103:531–536
Pang D, Dias MS, Ahab-Barmada M (1992) Split cord malformation: part I: a unified theory of embryogenesis for double spinal cord malformations. Neurosurgery 31:451–480
Sinha S, Agarwal D, Mahapatra AK (2006) Split cord malformations: an experience of 203 cases. Childs Nerv Syst 22:3–7
Nievelstein RA et al (1993) Embryonic development of the mammalian caudal neural tube. Teratology 48:21–31
McLendon RE et al (1988) Adipose tissue in the filum terminale: a computed tomographic finding that may indicate tethering of the spinal cord. Neurosurgery 22:873–876
Bentley JF, Smith JR (1960) Developmental posterior enteric remnants and spinal malformations: the split notochord syndrome. Arch Dis Child 35:76–86
Herren R, Edwards J (1940) Diplomyelia (duplication of the spinal cord). Arch Pathol 30:1203–1214
Lichtenstein B (1940) "Spinal dysraphism"—spina bifida and myelodysplasia. Arch Neurol Psychiatr 44:794–810
Hendrick EB (1971) On diastematomyelia. Prog Neurol Surg 4:277–288
Gardner WJ (1968) Myelocele: rupture of the neural tube? Clin Neurosurg 15:57–79
Pang D (2001) Ventral tethering in split cord malformation. Neurosurg Focus 10(1):e6
Proctor MR, Scott RM (2001) Long-term outcome for patients with split cord malformation. Neurosurg Focus 10:e5
Masahito P, Ishikawa T, Sugano H (1988) Fish tumors and their importance in cancer research. Jpn J Cancer Res 79(5):545–555
Tubbs RS, Murphy RL, Kelly DR, Lott R, Salter EG, Oakes WJ (2005) The filum terminale externum. J Neurosurg Spine 3:149–152
Hansasuta A, Tubbs RS, Oakes WJ (1999) Filum terminale fusion and dural sac termination: study in 27 cadavers. Pediatr Neurosurg 30:176–179
Tarlov IM (1938) Structure of the filum terminale. Arch Neurol Psychiat 40:1–17
Rauber AA, Kopsch F (1909) Lehrbuch der Anatomie des Menschen. Georg Thieme, Leipzig
Fontes RB, Saad F, Soares MS, de Oliveira F, Pinto FC, Liberti EA (2006) Ultrastructural study of the filum terminale and its elastic fibers. Neurosurgery 58:978–984, discussion 978–984
Tourneux F (1892) Sur la structure et sur le développement du fil terminal de la moelle chez l'homme. Compt rend Soc de biol 44: 340
Harmeier JW (1933) The normal histology of the intradural filum terminale. Arch Neurol Psychiat 29:308–316
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rizk, E., Adeeb, N., Hussein, A.E. et al. Duplicated filum terminale in the absence of split cord malformation: a potential cause of failed detethering procedures. Childs Nerv Syst 30, 709–711 (2014). https://doi.org/10.1007/s00381-013-2190-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-013-2190-9