Abstract
Material and methods
The pathogenesis of cerebral arteriovenous malformations (cAVMs) is still not well understood. Generally, cAVMs are thought to be congenital lesions originating prenatally. We report a 7-year-old boy diagnosed with a de novo cAVM after 3 years of recurrent epileptic seizures.
Results
MR imaging at 4 years of age was normal. Follow-up MR imaging 3 years later demonstrated a de novo 2-cm cAVM in the right occipital lobe, confirmed by conventional angiography. We reviewed five previously reported cases of de novo cAVMs who did not have a previous neurovascular abnormality. Including our case, recurrent epileptic seizures are the major presentation (83.3 %) before de novo cAVM occurrence.
Conclusion
We suggest that epileptic seizure is a potential trigger of de novo cAVMs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral arteriovenous malformations (cAVMs) are vascular malformations in the brain characterized by arteriovenous shunt through a collection of tortuous vessels (nidus) without an intervening capillary bed [28]. cAVMs were traditionally thought to be congenital in origin [15]. However, cases of de novo cAVM have been reported which challenge the traditional viewpoint [2–4, 6, 10, 11, 14, 16, 23–25, 27]. Here, we present a case of de novo cAVM in a child after 3 years of recurrent epilepsy and discuss the pathogenesis of de novo cAVMs and the influence of epileptic seizures on the cAVMs.
Case report
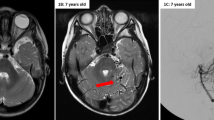
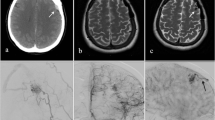
A 7-year-old boy (born in 2006) developed a fever when he was 4 years old (2010). During the fever, he suffered from sudden convulsions with teeth, fists clenched and eyes turned up, which lasted about 30 s. He was sent to the emergency department for symptomatic treatment. The first MR imaging revealed no abnormalities, or any sign of cAVM (Fig. 1a). The tentative diagnosis was febrile seizure, and the doctor suggested a clinical observation with no antiepileptic drugs. No neurological deficit was found after he recovered. In the following 3 years, the aforementioned seizures occurred annually. The symptoms recurred again on August 2013. A second MR imaging revealed an abnormal signal in the right occipital lobe of the T2-weighted scan (Fig. 1b). Visual fields examination showed scotoma in the left visual field (Fig. 2a, b). The boy was then admitted to our hospital, and a right occipital lobe cAVM was confirmed by conventional angiography. The feeding artery of the cAVM came from the branches of the right posterior cerebral artery, and the cAVM drained to the sigmoid sinus and superior sagittal sinus via the superficial veins (Fig. 3a, b). A right occipital craniotomy was successfully performed to remove the 2-cm cAVM (Fig. 4a). Postoperative pathology confirmed the diagnosis of cAVM (Fig. 4b). Postoperative angiography revealed no cAVM residue (Fig. 3c, d). He recovered well after the operation except for the visual defect (Fig. 2c, d).
Literature review
We reviewed the published cases of de novo cAVMs using the search term “de novo” and “cerebral arteriovenous malformations” in PUBMED. A total of 12 cases of de novo cAVMs were found [2–4, 6, 10, 11, 14, 16, 23–25, 27]. Among the total 13 cases of de novo cAVMs, six had accompanying cerebral vascular diseases and one had radiotherapy for medulloblastoma (Table 1). The remaining six cases including ours had no history of cerebral vascular disease or brain tumor. Of these six cases, five (83.3 %) had recurrent epileptic seizures, half were children, and only one patient had a prior history of hemorrhage. The other five only had a history of epilepsy seizures before the de novo cAVMs were found. In these five patients, one girl had a history of traumatic brain injury 4 years ago and another girl showed developmental delay (Table 2).
Discussion
We present a rare case of de novo cAVM formation in a child with a long history of epilepsy before the lesion was found. We also reviewed five previously reported cases of de novo cAVMs with no previous neurovascular abnormalities. Including our case, epileptic seizures are the major presentation (83.3 %) before de novo cAVM occurrence, whereas in the ordinary cAVMs patients, seizures as initial presentation occur in less than 30 % of patients [7].
The pathogenesis of cAVMs is not completely understood. It has been suggested that cAVMs are primarily congenital, originating at or before the 40 to 80-mm embryo length stage and may be related to a primary abnormality of primordial capillary or venous formation [19]. However, our case and a few previous reports of de novo cAVMs challenge the concept that cAVMs are purely congenital lesions. These reports suggest that actually cAVMs are not static lesions; growth, shrinkage, and spontaneous resolution of cAVMs with time have been documented [1, 5, 17, 18]. From the dynamic nature of cAVMs, it is possible that acquired inciting events might be the catalyst for the formation of cAVMs later in life. Desal et al. reported a case of the development of multiple de novo vascular malformations (transverse sinus, dural fistula, and posterior fossa cavernomas) following acoustic neuroma surgery. They speculated that venous occlusive disease and ischemia may be powerful revealing triggers and support the capillary venous origin of some vascular malformations [8].
Seizures contribute to hypoxic-ischemic brain injury, particularly in childhood. In ischemic brain regions, abundant potent angiogenic factors like vascular endothelial growth factor (VEGF) can be produced [22, 26]. Studies of seizure-prone patients with cAVMs reveal impaired peri-nidal cerebral reserve and concomitant venous congestion [9]. Additionally, research has shown that VEGF is upregulated in neurons and glial cells after epileptic seizures and counteracts seizure-induced neurodegeneration [21].
It has been proposed that overstimulated angiogenesis may lead to cAVMs. VEGF may be a key link between insult and cAVM formation. In animal models, deletion of the ALK1 gene can induce cAVM formation [29]. Gene microarray analysis of human cAVMs demonstrated increased VEGF gene expression levels for angiogenesis, accompanied by increased protein product [12]. It has been proven that the notch signaling pathway is an important molecular candidate in cAVM pathogenesis, which seems to depend on local levels of VEGF [20].
William L. Young. et al. proposed a “response-to-injury” model of cAVM pathogenesis [13]. An inciting event turns on the cAVM pathogenesis pathway which can involve angiogenesis, endothelial mitogenesis, and vascular stabilization. When this response is superimposed on an underlying structural defect or genetic background, the normal injury response may shift towards an abnormal dysplastic response. It is obvious that recurrent epileptic seizures were more common in the group of patients who had no other cerebral vascular disease with de novo cAVMs. Epileptic seizures may be an initial clinical manifestation in unruptured cAVM and could also be a consequence of a complication. Additionally, the recurrent epileptic seizures might be the inciting event in the angiogenesis pathway of the de novo cAVMs by upregulating VEGF expression.
Conclusion
cAVMs are a dynamic disease which can grow postnatally. Recurrent epileptic seizures may be a possible trigger for de novo cAVMs by upregulating the VEGF expression. The effect of the epileptic seizures on the angiogenesis of cAVMs needs to be further studied.
References
Abdulrauf SI, Malik GM, Awad IA (1999) Spontaneous angiographic obliteration of cerebral arteriovenous malformations. Neurosurgery 44(280–287):287–288
Akimoto H, Komatsu K, Kubota Y (2003) Symptomatic de novo arteriovenous malformation appearing 17 years after the resection of two other arteriovenous malformations in childhood: case report. Neurosurgery 52(228–231):231–232
Alvarez H, Perry V, Solle M, Castillo M (2012) De novo cerebral arteriovenous malformation in a child with previous cavernous malformation and developmental venous anomaly. J Neurosurg Pediatr 9:327–330
Bai Y, He C, Zhang H, Ling F (2012) De novo multiple dural arteriovenous fistulas and arteriovenous malformation after embolization of cerebral arteriovenous fistula: case report. Childs Nerv Syst 28:1981–1983
Bendok BR, Getch CC, Ali MJ, Parish T, Batjer HH (2002) Spontaneous thrombosis of a residual arteriovenous malformation in eloquent cortex after surgery: case report. Neurosurgery 50(1142–1145):1145–1146
Bulsara KR, Alexander MJ, Villavicencio AT, Graffagnino C (2002) De novo cerebral arteriovenous malformation: case report. Neurosurgery 50(1137–1140):1140–1141
Choi JH, Mohr JP (2005) Brain arteriovenous malformations in adults. Lancet Neurol 4:299–308
Desal HA, Lee SK, Kim BS, Raoul S, Tymianski M, TerBrugge KG (2005) Multiple de novo vascular malformations in relation to diffuse venous occlusive disease: a case report. Neuroradiology 47:38–42
Fierstra J, Conklin J, Krings T, Slessarev M, Han JS, Fisher JA, Terbrugge K, Wallace MC, Tymianski M, Mikulis DJ (2011) Impaired peri-nidal cerebrovascular reserve in seizure patients with brain arteriovenous malformations. Brain 134:100–109
Friedman JA, Pollock BE, Nichols DA (2000) Development of a cerebral arteriovenous malformation documented in an adult by serial angiography. Case report. J Neurosurg 93:1058–1061
Gonzalez LF, Bristol RE, Porter RW, Spetzler RF (2005) De novo presentation of an arteriovenous malformation. Case report and review of the literature. J Neurosurg 102:726–729
Hashimoto T, Lawton MT, Wen G, Yang GY, Chaly TJ, Stewart CL, Dressman HK, Barbaro NM, Marchuk DA, Young WL (2004) Gene microarray analysis of human brain arteriovenous malformations. Neurosurgery 54(410–423):423–425
Kim H, Su H, Weinsheimer S, Pawlikowska L, Young WL (2011) Brain arteriovenous malformation pathogenesis: a response-to-injury paradigm. Acta Neurochir Suppl 111:83–92
Mahajan A, Manchandia TC, Gould G, Bulsara KR (2010) De novo arteriovenous malformations: case report and review of the literature. Neurosurg Rev 33:115–119
Mast H, Koennecke HC, Meisel J, Osipov A, Hartmann A, Lasjaunias P, Pile-Spellman J, Hacein-Bey L, Young WL, Mohr JP (1998) Therapy of cerebral arteriovenous malformations. Nervenarzt 69:287–295
Mathon B, Blauwblomme T, Bolle S, Dufour C, Nagarra O, Brunelle F, Puget S (2013) De novo arteriovenous malformation after brain radiotherapy for medulloblastoma in a child. Neurology 81:398–399
Mendelow AD, Erfurth A, Grossart K, Macpherson P (1987) Do cerebral arteriovenous malformations increase in size? J Neurol Neurosurg Psychiatry 50:980–987
Morioka T, Nishio S, Hikita T, Chung LH, Soejima T (1988) Marked growth of an angiographically occult arteriovenous malformation: case report. Neurosurgery 23:101–103
Mullan S, Mojtahedi S, Johnson DL, Macdonald RL (1996) Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg 85:1–8
Murphy PA, Lu G, Shiah S, Bollen AW, Wang RA (2009) Endothelial Notch signaling is upregulated in human brain arteriovenous malformations and a mouse model of the disease. Lab Investig 89:971–982
Nikitidou L, Kanter-Schlifke I, Dhondt J, Carmeliet P, Lambrechts D, Kokaia M (2012) VEGF receptor-2 (Flk-1) overexpression in mice counteracts focal epileptic seizures. PLoS One 7:e40535
Nilsson I, Rolny C, Wu Y, Pytowski B, Hicklin D, Alitalo K, Claesson-Welsh L, Wennstrom S (2004) Vascular endothelial growth factor receptor-3 in hypoxia-induced vascular development. FASEB J 18:1507–1515
O’Shaughnessy BA, DiPatri AJ, Parkinson RJ, Batjer HH (2005) Development of a de novo cerebral arteriovenous malformation in a child with sickle cell disease and moyamoya arteriopathy. Case report. J Neurosurg 102:238–243
Ozsarac M, Aksay E, Kiyan S, Unek O, Gulec FF (2012) De novo cerebral arteriovenous malformation: Pink Floyd’s song “Brick in the Wall” as a warning sign. J Emerg Med 43:e17–e20
Schmit BP, Burrows PE, Kuban K, Goumnerova L, Scott RM (1996) Acquired cerebral arteriovenous malformation in a child with moyamoya disease. Case report. J Neurosurg 84:677–680
Shweiki D, Itin A, Soffer D, Keshet E (1992) Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 359:843–845
Stevens J, Leach JL, Abruzzo T, Jones BV (2009) De novo cerebral arteriovenous malformation: case report and literature review. AJNR Am J Neuroradiol 30:111–112
van Beijnum J, van der Worp HB, Buis DR, Al-Shahi SR, Kappelle LJ, Rinkel GJ, van der Sprenkel JW, Vandertop WP, Algra A, Klijn CJ (2011) Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA 306:2011–2019
Walker EJ, Su H, Shen F, Choi EJ, Oh SP, Chen G, Lawton MT, Kim H, Chen Y, Chen W, Young WL (2011) Arteriovenous malformation in the adult mouse brain resembling the human disease. Ann Neurol 69:954–962
Acknowledgments
This study was supported by a “National science and technology Support Plan” (No.2011BAI08B08, Principle investigator: Professor Shuo Wang) grant from the Ministry of Health of China
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, J., Li, Y., Cao, Y. et al. De novo cerebral arteriovenous malformations: is epileptic seizure a potential trigger?. Childs Nerv Syst 30, 1277–1281 (2014). https://doi.org/10.1007/s00381-014-2413-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-014-2413-8