Abstract
Background
Ventriculoperitoneal shunt (VPS) surgery is the most common procedure performed for the treatment of hydrocephalus. Erosive bladder perforation by a peritoneal catheter is an extremely rare complication of VPS. Only ten cases involving the normal (non-augmented) urinary bladder have been reported so far.
Case
We report a case of erosive bladder perforation, intra-corporeal knot formation, and perurethral extrusion of the distal end of VPS. This is the second only case report so far in the world literature showing triad of uncommon VPS complications in a single patient.
Conclusion
Prompt management could avoid further complications. Patient’s parents should be aware about this rare complication, so that they can seek timely medical help.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventriculoperitoneal shunt (VPS) surgery for the treatment of hydrocephalus is one of the most commonly performed single procedure in any neurosurgical institution. Infection and blockage of shunt are its common known complications. However, migration and knot formation are relatively uncommon. Hollow organ perforation is very uncommon. Perforation of the gut, scrotum, umbilicus, and vagina has been reported previously. Bladder perforation by the distal end of VPS is very rare; only ten cases involving the normal (non-augmented) urinary bladder have been reported so far. We are reporting a case of uncommon VPS complications with bladder perforation, intra-corporeal knotting, and extrusion through the penile urethra in a 7-year-old male child. Only one case has been reported previously with this triad [14].
Case report
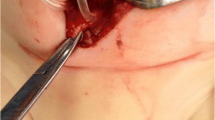
A 7-year-old male child presented with complaints of extrusion of shunt tube from the penis (Fig. 1). He gives a history of operation for right-sided VPS at the age of 9 months for congenital hydrocephalus. At the age of 5 years, right-sided VPS got blocked; for which de novo shunt was put in the left lateral ventricle and connected with another peritoneal opening on the left side.
On examination, the child was afebrile, conscious, and coherent. No focal neurological deficit or any meningeal signs were evident. The abdomen was soft, and bowel sounds were present. Peritoneal catheter was coming out of the urethra with drops of cerebrospinal fluid (CSF) coming out from the distal end (Fig. 1a). A written consent from the parents of the patient has been taken for publication of images of his genitals.
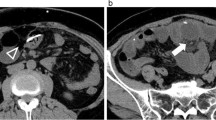
Non-contrast computerized tomography scan of the head revealed biventricular shunts in situ with no evidence of hydrocephalus. A plain radiograph of the abdomen including the chest and pelvis (Fig. 2) revealed radiopaque catheters (Chhabra shunt) on both sides of the chest and abdomen, with intra-corporeal knotting of both catheters without the evidence of any calculus around them. On ultrasound examination, right sided catheter was coiled, and there was a knot formation in the urinary bladder, and the distal end was coming out through penile urethra. There was no intravesical calculus. The left-sided shunt was coiled and lying in abdominal cavity.
Right-sided shunt was explored in the abdomen and cut, and the peritoneal end was pulled with a knot through the urethra (Fig. 1b), and the cranial end and rest of the shunt were removed by exploring for cranial wound. A Foley catheter was left in the bladder. A CSF examination from the ventricular end including culture was normal. Treatment included intravenous administration of antibiotics and anticonvulsants for 5 days. The patient was discharged on the seventh day of removal of the shunt. Indwelling Foley catheter was removed after 15 days of shunt removal. On 6-month follow-up, the patient had no complains.
Discussion
Spontaneous knotting of the peritoneal end of shunt is rare. Possible causes of knotting may be memory effect of VPS, gut pulsations, pulsations from the nearby blood vessels, etc. Memory effect of peritoneal catheter means that the shunt tubing can twist in the body, and it appears very similar to that of the pre-insertion shunt in the packaging when it is supplied, due to its retained memory [9].
Visceral perforation by the shunt apparatus is relatively unusual. Perforation into different organs or through natural or artificial orifices has been described. The most common site of catheter migration is through the anus (68.6 % ), followed by the scrotum (22 % ), umbilicus (6.9 % ), and vagina (3.2 % ) [7]. Bladder perforation is an extremely rare complication of VPS surgery [15]. The location of the bladder makes it a highly unlikely site of peritoneal catheter penetration, as the catheter must pass through the peritoneum into the extraperitoneal space and subsequently perforate the bladder [15]. The distal end of the VPS can perforate through a viscus with ventricular end in situ. It is common in cases of long peritoneal ends. In the present case, ventricular end was in place, while distal end perforated the bladder.
Bladder perforation by ventriculoperitoneal shunt has been reported in the normal bladder wall as well as in the augmented bladder as shown in Table 1. In the present case, ventriculoperitoneal shunt perforated a normal (non-augmented) bladder wall and got exteriorized through the urethral orifice.
Grosfeld et al. reported, for the first time, the urinary bladder perforation by ventriculoperitoneal shunt in two patients, aged 3 months and 1 year, in 1974 [11]. Both had fever, abdominal pain, distension, and erythema of the abdominal wall between the umbilicus and the pubis. Abdominal x-ray films demonstrated catheter in the bladder. Catheter removal, bladder repair, supra-pubic cystostomy, and antibiotic therapy resulted in recovery in each case.
Pohlman et al. reported the first case of erosive urinary bladder perforation by ventriculoperitoneal shunt with intravesical knotting and urethral extrusion of catheter in 14-year-old male patient with cerebral palsy with hydrocephalus in 2011 [15].
The present case is the second case of bladder perforation with intravesical knotting and per-urethral extrusion of catheter.
The mechanisms by which peritoneal catheters may perforate hollow organs are not fully understood yet. Prasad et al. suggested that perforation occurs via a series of stepwise events, namely catheter fixation or anchoring, penetration, perforation, entry into the urethra, and finally extrusion [16]. Bowel peristaltic movements, increased intra-abdominal pressure, and CSF pulsations are believed to provide a continuous source of mechanical pressure that ultimately lead to necrosis at the anchoring site, which allow the occurrence of viscus perforation. Generally, anchoring to the organ serosa appears to be initiated by a local inflammatory reaction around the distal catheter that facilitates erosion and perforation.
Various shunt factors had been suggested by various authors to perforation such as sharp abdominal tip, long abdominal end of catheter, bioreactivity of shunt components, relatively stiff consistency, barium coating of shunt, silicon allergy, etc. Despite initial reports that seemed to demonstrate a higher tendency of perforation with spring-coiled catheters, multiple peritoneal catheter types have been associated with perforation. In the present case, Chabra shunt® was used. It has a “Slit n Spring” valve with 75-cm distal part, distal end closed with silicone plug, and has two pairs of slit valve. One ventricular part 15 cm long closed at the end with black silicone tantalum-impregnated plug. VPS is radiopaque, and its outer diameter is 2.5 mm and inner diameter, 1.3 mm. Catheter is made up of silicon and contains barium sulfate for x-ray detectability. The most important precaution before the implantation of the shunt is that the whole system must invariably be flushed with distilled water or saline. This is done to ensure that the slit valves open up to their normal size again before the shunt is implanted. Silicone rubber has a slight tendency to stick when it is in a dry state. The flushing process brings the valves back to their normal operational condition. After implantation, the moisture of the CSF itself keeps the valve in normal condition. In present case, the shunt got blocked when the patient was 5 years old, leading to local dryness and impaction in the bladder wall, although the shunt was functioning when the patient presented to us with per-urethral extrusion.
The tip of the distal catheter has silicon balls. Although rarely there is peritoneal fluid in peritoneal cavity, in adverse circumstances, it may be possible that the slightly adhesive property of silicon in dry state may provoke anchoring to organ serosa. In a study, Boch et al. observed distal tubing calcifications in barium-impregnated catheter [3]. In their study, calcifications contained particles of silicon and barium sulfate signifying fragmentation of polymers. Usually, a 40-cm length of the peritoneal catheter is left inside [18]. Couldwell et al. reported in their experience with the primary insertion of an open-ended peritoneal tubing with extended length (120 cm) undertaken expressly to avoid the need for a lengthening procedure because of growth of the patient that there was no increase in the distal complication rate and specifically no complications were experienced that were directly related to the use of the extended length tubing [5].
Various presentations of patients with bladder perforation by VP shunt are reported in the literature: abdominal pain, distension, and erythema of the abdominal wall between the umbilicus and the pubis, urinary tract infection, bladder calculi, hematuria, and catheter coming out of urethra as shown Table 1. Peritonitis due to intra-peritoneal leakage of urine can impair CSF absorption in the peritoneum, and features of raised intracranial pressure may occur.
In the present case, the mother noticed a whitish catheter coming out of the urethra. There were no abdominal findings. The child had no sign of meningitis or raised intracranial pressure. Prompt management could avoid further complications.
Treatment of organ perforation or catheter extrusion is a surgical emergency; the optimal management is a continuous source of controversy in the literature. The limited clinical evidence provided by the small number of clinical reports precludes definitive formulation of formal recommendation for optimal management. There is a general consensus that the cornerstone of treatment is shunt removal. If the patient is clinically stable and there is no evidence of infection, it is reasonable to proceed with removal of the migrated shunt.
Tubing can be cut proximally and pulled out through the urethra, allowing the bladder to heal on its own. Leaving the Foley catheter in the bladder is an extra safeguard. This method was used in the present case. When the catheter was pulled, it came out easily with the knot, without any resistance. The shunt is made up of silicon (very soft material), even softer than latex, which is used for the Foley catheter. Chances of injury caused by the shunt knots are negligible. Alternatively, the bladder can be approached extraperitoneally, and cystotomy may be performed at the anterior aspect to identify the catheter within the bladder. This method was used by Pohlman et al. [15]. A urology consultation was done in the present case, and cystoscopic intervention/open cystotomy was kept as a reserve in case the catheter would not deliver with gentle pulling. The entire shunt system should be removed. In the present case, there was already a functioning left-sided shunt in situ. The left-sided shunt was left inside. In case if there is no other functioning shunt, then either external ventricular drainage can be done or opposite-side VP shunt can be inserted later on.
Conclusion
Adequate follow-up of patients with a VP shunt is important. Prompt management could avoid further complications. The patient’s parents should be aware about this rare complication, so that they can seek timely medical help.
References
Barker GM, Läckgren G, Stenberg A, Arnell K (2006) Distal shunt obstruction in children with myelomeningocele after bladder perforation. J Urol 176:1726–8
Binning MJ, Ragel BT, Walker ML, Kestle JRW (2006) Retained peritoneal shunt tubing causing hematuria: case illustration. J Neurosurg 104:434
Boch AL, Hermelin E, Sainte-Rose C, Sgouros S (1998) Mechanical dysfunction of ventriculoperitoneal shunts caused by calcification of the silicone rubber catheter. J Neurosurg 88:975–82
Chen TH, Lin MS, Kung WM, Hung KS, Chiang YH, Chen CH (2011) Combined ventriculoperitoneal shunt blockage, viscus perforation with migration into urethra, presenting with repeated UTI. Ann R Coll Surg Engl 93:151–153
Couldwell WT, LeMay DR, McComb JG (1996) Experience with use of extended length peritoneal shunt catheters. J Neurosurg 85:425–7
de Aguiar GB, Mizrahi C, Aquino JH, Tavares CM, Telles C, Nigri F, Acioly MA (2011) Urethral extrusion of a peritoneal catheter in a patient with a neobladder: a rare complication of shunt insertion. Neuropediatrics 42:124–127
de Aquino HB, Carelli EF, Borges Neto AG, Pereira CU et al (2006) Nonfunctional abdominal complications of the distal catheter on the treatment of hydrocephalus: an inflammatory hypothesis? Experience with six cases. Childs Nerv Syst 22:1225–1230
De Jong L, Van Der Aa F, De Ridder D, Van Calenbergh F (2011) Extrusion of a ventriculoperitoneal shunt catheter through an appendicovesicostomy. Br J Neurosurg 25:115–6
Dominguez CJ, Tyagi A, Hall G, Timothy J, Chumas PD (2000) Sub-galeal coiling of the proximal and distal components of a ventriculo-peritoneal shunt. An unusual complication and proposed mechanism. Childs Nerv Syst 16:493–5
Eichel L, Allende R, Mevorach RA, Hulbert WC, Rabinowitz R (2002) Bladder calculus formation and urinary retention secondary to perforation of a normal bladder by a ventriculoperitoneal shunt. Urology 60:344
Grosfeld JL, Cooney DR, Smith J, Campbell RL (1974) Intra-abdominal complications following ventriculoperitoneal shunt procedures. Pediatrics 54:791–796
Mevorach RA, Hulbert WC, Merguerian PA, Rabinowitz R (1992) Perforation and intravesical erosion of a ventriculoperitoneal shunt in a child with an augmentation cystoplasty. J Urol 147:433–434
Mihajlović M, Tasić G, Raičević M, Mrdak M, Petrović B, Radlović V (2012) Asymptomatic perforation of large bowel and urinary bladder as a complication of ventriculoperitoneal shunt: report of two cases. Srp Arh Celok Lek 140:211–215
Murthy KVR, Reddy SJ, Prasad DVSRK (2009) Perforation of the distal end of the ventriculoperitoneal shunt into the bladder with calculus formation. Pediatric Neurosurgery 45:53–55
Pohlman GD, Wilcox DT, Hankinson TC (2011) Erosive bladder perforation as a complication of ventriculoperitoneal shunt with extrusion from the urethral meatus: case report and literature review. Pediatr Neurosurg 47:223–226
Prasad VS, Krishna AM, Gupta PK (1995) Extrusion of peritoneal catheter of ventriculoperitoneal shunt through the urethra. Br J Neurosurg 9:209–210
Surchev J, Georgiev K, Enchev Y, Avramov R (2002) Extremely rare complications in cerebrospinal fluid shunt operations. J Neurosurg Sci 46:100–103
Trojanowski T (2009) Hydrocephalus in adults (including normal pressure hydrocephalus syndrome). In: Sindou M (ed) Practical handbook of neurosurgery: rom leading neurosurgeons. Springer, Vienna, pp 429–440
Yerkes EB, Rink RC, Cain MP, Leurssen TG, Casale AJ (2001) Shunt infection and malfunction after augmentation cystoplasty. J Urol 165:2262–2264
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kataria, R., Sinha, V.D., Chopra, S. et al. Urinary bladder perforation, intra-corporeal knotting, and per-urethral extrusion of ventriculoperitoneal shunt in a single patient: case report and review of literature. Childs Nerv Syst 29, 693–697 (2013). https://doi.org/10.1007/s00381-012-1995-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-012-1995-2