Abstract
Background
Endoscopic cystocisternotomy is one of three surgical methods used to treat middle cranial fossa arachnoid cysts. There is debate about which method is the best.
Objective
The aim of this study is to evaluate the effectiveness and safety of endoscopic cystocisternotomy for treatment of arachnoid cysts of the middle cranial fossa.
Methods
Thirty-two patients with arachnoid cysts of the middle cranial fossa who had undergone endoscopic cystocisternal fenestration between 2004 and 2009 were studied retrospectively. Data were obtained on clinical and neuroradiological presentation, indications to treat, surgical technique, complications, and the results of clinical and neuroradiological follow-up.
Results
Among the 27 patients with symptoms before surgery, 8 had disappearance of symptoms and 17 had improvement of symptoms. The cyst was reduced in size or it completely disappeared in 24 (75%) patients. The incidence rate of complications was 18.8%.
Conclusions
Endoscopic cystocisternal fenestration is an effective treatment for symptomatic arachnoid cysts of the middle cranial fossa and should be the initial surgical procedure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachnoid cysts account for 1% of all intracranial space-occupying lesions [1]. The most common site of arachnoid cysts is the middle cranial fossa [2]. The pathogenesis of middle cranial fossa arachnoid cysts is controversial. It is believed that formation of these cysts is caused by abnormal growth of arachnoid during embryonic development, i.e., splitting of the arachnoid into two layers or repeated growth of the arachnoid. The cysts are then formed by cerebrospinal fluid (CSF) gradually accumulating between the layers. Expansion of the cysts compresses the temporal lobe, causing secondary agenesis of the temporal lobe [3–6].
Whereas it is generally agreed that surgical treatment is warranted for middle fossa arachnoid cysts (MFACs) when there are specific cyst-related clinical symptoms,[7–9] there is considerable controversy about whether surgery is warranted for asymptomatic arachnoid cysts. On the other hand, some authors have argued that surgery is necessary for large asymptomatic arachnoid cysts because minor head trauma may cause cyst rupture, subdural effusion, or subdural hemorrhage, resulting in acute intracranial hypertension that requires urgent surgical treatment [10, 11]. They reason that to avoid such serious consequences, surgery is required for even asymptomatic patients. Another concern is that persistent compression due to a cyst in infants and children may lead to decreased cerebral blood flow, which may affect the development of cerebral cortex. Some authors contend that timely treatment can improve metabolism of the compressed brain tissue and prevent occurrence of metabolism-related complications such as epilepsy in the future [12–14]. On the other hand, some authors have argued that surgical treatment is not necessary for asymptomatic MFACs, unless progressive enlargement of cysts is definite or the space-occupying effect is obvious due to the following three reasons: (1) Spacca et al. studied 40 patients with MFACs who underwent endoscopic cystocisternotomy. There were four patients whose symptoms disappeared completely postoperatively. .Although the cyst disappeared in two patients and was reduced in size in one patient, subdural hematoma still occurred in these four patients after head trauma in the postoperative period [15]. Therefore, it was concluded that the presumption that surgery used for asymptomatic cysts to prevent post-traumatic intracranial hemorrhage is no longer valid. (2) Because the pathogenesis of cysts is still not clear, whether the presence of cysts may affect brain development remains unclear [15]. (3) MFACs may disappear spontaneously due to minor trauma, forced breathing, holding breath, valsalva manoeuvre, severe coughing, loud crying, vigorous exercise, purulent meningitis, or no obvious predisposing cause. [16]
Currently, there are three types of surgical methods for MFACs: cyst-peritoneal shunting, microscopic craniotomy for cystocisternotomy and partial cyst excision, and endoscopic cystocisternotomy. Which is the best surgical procedure is still a subject of debate. For the treatment of intracranial arachnoid cysts, many authors have stated that endoscopic therapy should be the first choice. [17–19].
Endoscopic surgery has been performed on 32 patients with MFACs in our department. To obtain more information about this technique in order to further assess its role as a surgical method for treating patients with MFACs, we obtained and analyzed the clinical data of these 32 patients.
Patients and methods
Patients
This study included 32 patients with MFACs who underwent endoscopic surgery at the Department of Neurosurgery, Beijing Tiantan Hospital from January 2004 to June 2009. There were 20 male and 12 female patients. Their ages ranged from 6 months to 37 years (average, 7.8 years). Patient characteristics are listed in Table 1. This study was approved by the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University, Beijing.
According to the Galassi classification system,[20] MFACs are divided into three categories: type I, small spindle-shaped cysts limited to the anterior middle cranial fossa; type II, medium-size lesions usually triangular or quadrilateral in shape that occupy the anterior and central part of the middle cranial fossa and extend along the Sylvian fissure; type III, huge cysts, oval or round in shape, that almost fill the entire middle cranial fossa and extend toward the hemisphere through extensive areas. In this study, 15 patients had Galassi type II cysts (46.9%) and 17 had Galassi type III cysts (53.1%).
Imaging
All patients underwent preoperative head MRI and CT. The cyst was located in the right middle fossa in 11 patients and in the left middle fossa in 21 patients. CT showed space-occupying lesions with a clear boundary and the same density as CSF. MRI showed long T1 and long T2 signals, and the lesions had CSF equivalent signals. Head CT or MRI was performed postoperatively.
Surgical indications
Patients were treated surgically if they had any of the following indications: (1) Clear intracranial hypertension, which may be accompanied by headache, vomiting, papilloedema, unclosed anterior fontanel, or increased head circumference; (2) combined intracapsular hemorrhage, subdural hemorrhage, or hydrocephalus; (3) clear focal neurological deficits caused by a MFAC; (4) epilepsy; (5) although asymptomatic, an increasing trend in cyst volume; (6) cyst having manifestations of expansive growth such as local skull eminence or compression of surrounding brain tissue. In addition, five asymptomatic infants and children with large arachnoid cysts were also treated surgically. In such asymptomatic patients, cysts compress the brain tissue and cause a low metabolic state of brain tissue, which may affect brain development and function of adjacent brain tissue and also cause epilepsy.
Surgical methods
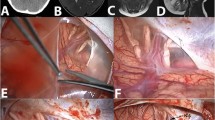
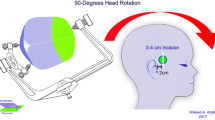
The endoscopic cystocisternotomy via transtemporal approach was chosen for all patients. The patients were placed in a supine position with their heads leaning toward the unaffected side. A 3-cm straight temporal incision was made according to the cyst position, and a 1.5-cm hole was drilled in the skull. A cruciate incision of the dura was made, and the bleeding was stanched completely. The cyst wall was cut open, and an endoscope was inserted into the cyst. The surgery was carried out under TV monitoring. After exploring the cyst cavity, the fenestration sites in the inner wall were determined according to the specific anatomical situation within the cyst. The endoscope was moved forward along the sphenoid ridge to observe bifurcation of the internal carotid artery, ipsilateral optic nerve, and oculomotor nerve. Usually, there are three optional sites for fenestration (Fig. 1) between the cyst and the basal cistern: (1) between the oculomotor nerve and the tentorial edge (Fig. 2); (2) between the oculomotor nerve and the internal carotid artery (Fig. 3); and (3) between the internal carotid artery and the optic nerve (Fig. 4). Multidirectional fenestration was performed to create communication between the arachnoid cyst and cisterns, including the basal cistern and Sylvian cistern. Initially, blunt fenestration was performed. If the cyst was tough and blunt, fenestration was not feasible, and scissors were used to create fenestration. The fenestration was subsequently expanded using a variety of instruments until it was greater than 0.5 cm. The basilar artery in the interpeduncular cistern could be seen clearly after fenestration (Fig. 5).
Results
Follow-up
Follow-up duration ranged from 8 to 73 months (mean, 26 months). Among the 27 patients with preoperative symptoms, 15 had disappearance of symptoms and 10 had improvement; in 2 patients symptoms remained unchanged. The improvement rate of clinical symptoms (symptoms disappeared or improved) was 92.6% (25/27). The percentage of patients who had headache that completely disappeared or was markedly reduced was 90.9% (10/11). Progression of skull deformation and head circumference increase was stopped in all patients. Symptoms of focal neurological deficit caused by cysts compressing adjacent structures were improved in all four affected patients (100%). Two patients experienced episodes of loss of consciousness before surgery, but no such episodes occurred after surgery. Three patients had preoperative dizziness. After surgery, dizziness disappeared in two of the patients and was improved in the other patient. Six patients had epilepsy before surgery, and seizures in five of them were reduced or disappeared while they were taking oral anti-epileptic drugs. Symptoms were not alleviated in two patients (7.4%, 2/27). In these two patients, cyst-cistern fenestrations were performed again, and it was found during surgery that the previous fenestration was closed. The symptoms were alleviated in one patient after surgery; however, the symptoms remained unchanged in the second patient after surgery. The symptoms were alleviated in this patient after cyst-peritoneal shunting was performed.
All patients underwent postoperative head CT on the day of surgery, and head CT or MRI was repeated at 3 and 9 months after surgery. Subsequently, head CT was carried out annually. The imaging results during follow-up showed that cysts disappeared in 5 patients (15.6%, Fig. 6) and were reduced in 19 patients (59.4%). No significant changes in cysts were observed in eight patients (25%). In two patients with hydrocephalus before surgery, the ventricles were significantly reduced after surgery.
a Preoperative MRI. The cyst almost occupied the entire middle cranial fossa; the temporal lobe was compressed. b. CT images 7 days after surgery. The size of the cyst was reduced substantially; the temporal lobe was partially restored back to the former position. c. CT images 6 months after surgery. The cyst had completely disappeared; the temporal lobe was restored back to its former position. d. CT images 12 months after surgery. The cyst had completely disappeared; the temporal lobe was restored back to its former position
Surgical complications
There were no deaths or new neurological deficit symptoms after surgery. Subdural effusion occurred in four patients after surgery. One of these patients required a subdural-peritoneal shunt. Subdural effusion gradually disappeared in the other three patients during follow-up. Oculomotor nerve palsy occurred in two patients and the symptom gradually disappeared after a half-year follow-up.
Discussion
Indications for surgery
With regard to the indications for surgery, it has been recommended that these indications should be strict, and surgeons should confirm whether a patient has the indications for surgery [21]. For patients with symptoms, it is true that many symptoms such as headache, increased intracranial pressure, epilepsy, psychomotor retardation, intracranial bleeding, attention deficit, hyperactivity, maniac depression, schizophrenia-like symptoms, paranoia, psychoses anosmia, Menière's disease, and hearing loss may not be induced by a cyst. Therefore, it is important to obtain a medical history and perform a physical examination, and evaluate diseases and assess the relationship between the cyst and symptoms for each patient before surgery is performed. In our study, repeated evaluation was carried out before surgery. The size of the cyst is not a factor affecting the clinical determination of surgery. But obvious cyst-related symptoms are an indication for surgery. Once cyst-related symptoms are present, surgery can be considered. In certain patients, the symptoms are not characteristic symptoms of a cyst in the fossa cranii media, but other causes are not found. For example, two patients in our study had transient loss of consciousness. The symptoms resolved after surgery. In addition, three patients had preoperative dizziness. After surgery, dizziness disappeared in two of the patients and was improved in the other patient. Six patients had epilepsy before surgery. Seizures in five of them were reduced or disappeared while they were taking oral anti-epileptic drugs during postoperative period. Therefore, we think that the indications for surgery should be strict and the selection of surgery should be cautious, but for patients in whom the other causes of symptoms are not found and symptoms might be caused by a cyst, endoscopic surgery can be considered because this technique is minimally invasive and relatively safe.
For patients without symptoms, it has been speculated that these patients are not suitable candidates for surgery [21]. Researchers who support prophylactic surgery propose that the cyst may spontaneously rupture resulting in subdural effusion, which is an indication for surgery. In addition, they are concerned that an intracerebral cyst may cause a spontaneous intracranial hematoma [10, 11]. However, it has been noted that the incidence of subdural effusion after endoscopic or microscopic surgery that requires a second surgery is two times higher than that caused by spontaneous rupture, and treatment of cyst with surgery cannot abolish the possibility of a spontaneous intracranial hematoma, which may occur after surgery [21]. Thus, it has been argued that prophylactic surgery should not be used for these patients [21]. However, it has been proposed that follow-up imaging in children operated on before 1 year of age may show a progressive filling of the temporal fossa, thus demonstrating that the temporal parenchyma is secondarily compressed by large cysts rather than being primarily dysplastic [22]. We therefore believe that surgery is indicated in young children with large temporal fossa cysts (Galassi type III) even if the cyst is apparently well tolerated. However, surgical treatment should only be considered for older children and adults only if they are symptomatic. In our study, the five patients without symptoms were young children. Because a cyst may affect the development of the brain, surgery was considered. Of the two youngest children, one was 16 months old, and the other was 19 months old. The cyst resolved after surgery in one child and the temporal lobe rose completely and a reduced cyst was observed in the other child. In the remaining three patients (2–5 years old), the cyst volume was decreased in two of the patients after surgery; the other had no changes in the cyst. Therefore, we think that surgery can be recommended when the cyst is relatively large in children younger than 2 years.
Surgical methods
Endoscopic cystocisternotomy was used for all 32 patients. The improvement rate of clinical symptoms was 92.6%, which is consistent with the reported effects of endoscopic therapy for MFACs by Spacca et al. (92.5%) [15]. It is also consistent with the results reported by Di Rocco et al. [22], although in that study, 2 of the 17 children with a Sylvian arachnoid cyst who were treated endoscopically had headache recurrence at 1 year and 20 months, respectively, after surgery. These two patients required further surgery and were free of symptoms at last follow-up. In addition, Levy et al.[7] reported 50 cases of MFAC in which microneurosurgery was used, and symptoms were improved or disappeared in 95% of patients, which is consistent with our results, Spacca et al's [15], and Di Rocco et al's [22]. However, the complication rate in Levy et al's study was 32% (16/50), which was significantly higher than the rate of 18.8% in our study and 20% in Spacca et al's study [15]. Di Rocco et al. [22], however, reported a postoperative complication rate of 29% (6/17) with five patients having subdural effusion postoperatively and one having hemorrhage. Three of the patients with subdural effusion required a shunt, and in the other two patients, the effusion resolved without surgical intervention. Although the complication rate in the study of Di Rocco et al. was more similar to that of the study by Levy et al., it should be noted that only 17 patients were included in Di Rocco et al's study compared with 32 patients in our study and 40 patients in Spacca et al's study. Another advantage of endoscopic surgery is that it can be completed through one bone orifice so that the operative time is shortened compared with microscopic craniotomy and the greater trauma caused by microscopic craniotomy can be avoided. Arai et al. [23] completed cyst-peritoneal shunting in 77 patients with middle cranial fossa arachnoid cysts, and the symptom improvement rates for headache, mild hemiparesis, and diplopia were 100%. However, eight patients underwent reoperation because of shunt blockage, and shunt dependence occurred in four patients. Compared with the shunt procedure, endoscopic cystocisternotomy has similar therapeutic effects, but it may avoid a lot of complications caused by a shunt including infection, shunt dependence, and shunt blockage, as well as some inherent disadvantages, such as foreign body implantation, psychological effects, and life-disrupting inconvenience [24]. It should also be noted that endoscopy can be performed in patients of all ages.
Endoscopy has two other advantages over microsurgery. One is that in endoscopy, lesions can be observed at different angles. Therefore, surgery can be performed with a small incision and only one hole in the skull. The other advantage is that lesions can be observed at a short distance. This allows for clearer identification of the lesion anatomy, which is beneficial for fistulation.
Endoscopic surgical methods
Middle cranial fossa arachnoid cysts can be divided into two types according to the needs of endoscopic surgery: (1) the inner wall of the cyst that cannot communicate with the basal cistern and (2) the inner wall of the cyst that can communicate with the basal cistern.
-
1.
For middle cranial fossa arachnoid cysts that can communicate with the basal cistern, endoscopic neurosurgery via the temporal approach is often selected.
-
2.
For small symptomatic middle cranial fossa arachnoid cysts in the temporal pole that cannot communicate with the basal cistern, the superior orbital approach can be used [25]. This approach allows an endoscope to be placed between the frontal lobe and the anterior skull base after the tension of the frontal lobe has decreased, and to be inserted along the orbital roof and the lesser wing of the sphenoid bone until it reaches the middle fossa. The arachnoid cyst can then be visualized and cyst-suprasellar cistern and cyst-interpeduncular cistern fenestrations can be carried out.
-
3.
Some authors have proposed that neuronavigation-assisted endoscopic cyst-lateral ventricle fenestration can be performed for MFACs, and no stent placement is required [26].
-
4.
Cotton string can be used for hemostasis when bleeding occurs, and the cerebrospinal fluid is removed, which is followed by hemostasis with electrocautery.
-
5.
In fistulation of a cyst, the cyst wall should not be cut apart from the adjacent brain tissue. The arterial wall of basal artery should be seen clearly after the endoscopic cystocisternotomy. This procedure may assure that the fluid in the cyst enters the cerebrospinal fluid circulation to achieve the surgical goal.
-
6.
Electric coagulation should be avoided as far as possible around the oculomotor nerve, or the oculomotor nerve may be damaged.
Postoperative complications of endoscopic surgery and precautions
The main complications of endoscopic surgery are postoperative subdural effusion and chronic subdural hemorrhage. In our study, postoperative subdural effusion occurred in four patients. Three patients were continuously followed up because they did not have obvious symptoms, and their subdural effusion was gradually absorbed and completely disappeared. One patient underwent subdural-peritoneal shunting. Possible reasons for postoperative subdural effusion are: (1) A large amount of CSF cannot be absorbed within a short period of time, which means CSF will enter into the subdural space through the fenestrations in the inner wall; (2) Brain tissue dysplasia occurs after long-term compression, and the brain tissue re-expands slowly. Meanwhile, the cyst collapses, and then the lateral side of the cyst is peeled off from the dura mater, resulting in subdural effusion. It has been suggested that the cyst can be reached via the transtemporal approach during endoscopic surgery [27]. Since the endoscope does not enter into the cyst through the cyst wall directly, entrance of cyst fluid into the subdural space is avoided, thereby preventing the occurrence of postoperative subdural effusion. It should be noted that some authors do not include mild dural efflusion as a complication, only severe dural effusion. They therefore report lower postoperative complication rates than would have been reported had mild effusion been included. We included both mild and severe dural effusion as a complication. Only one of our patients with dural effusion required a shunt.
For successful surgery, several points should be noted: (1) During analysis of the imaging before surgery, the surgeons should pay attention to the relationship between the cyst and its adjacent cortex and cistern. The skull-entering point and the path to the target area (basal cistern) need to be carefully selected. The surgical approach and drilling should avoid major functional areas, and the position for drilling in the temporal area should allow the endoscope to point at the inner wall of the basal cistern perpendicularly, so that the fenestration of the cyst into the basal cistern is relatively easy and safe. If the inner wall of the cyst is covered by the temporal cortex on the surface, endoscopic cyst-basal cistern fenestration is performed via the cortex. (2) The aims of surgery are to eliminate the space-occupying effects of cysts; therefore, it is not necessary to peel off the cyst wall. Cyst-cistern fenestration can achieve satisfactory results. The cyst wall should not be peeled off forcibly because it may easily cause bleeding, affecting the operative field and the surgical operation. (3) The fenestration should be expanded as much as possible during surgery as long as safety is ensured. The basilar artery should be observed clearly through the fenestration. The postoperative recurrence rate of MFACs is high; therefore, multiple fenestrations should be performed during surgery to prevent postoperative fenestration closure and failure of surgery. (4) As surgery is performed under an endoscope, hemostasis is difficult once serious bleeding occurs. Therefore, vascular injury should be avoided after the endoscope enters into the cyst cavity, and close attention should be paid to hemostasis at each step of surgery.
There are no clear-cut criteria to define successful surgery [21]. In our study, among 27 patients with symptoms, 7 had unreduced cyst volume after surgery, and only 2 patients had no improvement of symptoms. We speculate that the main criterion for successful surgery should be improvement of clinical symptoms instead of cyst volume reduction.
To summarize, we believe that endoscopic treatment of MFACs has the following advantages: (1) Surgical trauma is minor; (2) Surgical safety is relatively high; (3) The operative time is significantly shortened, and patients recover rapidly; (4) The therapeutic effects are definite, and there are fewer postoperative complications; (5) The psychological burden on patients is light.
We therefore conclude that endoscopic surgery should be the first choice for all types of MFACs [15]. With endoscopic surgery, good therapeutic effects can be achieved in most patients. The use of neuronavigation can be combined when necessary, allowing better identification of the target area for endoscope placement and help in identifying and protecting deep neurovascular structures simultaneously. Shunting or microsurgical treatment should be considered only when symptoms are not changed after endoscopic treatment [28].
References
Albuquerque FC, Giannotta SL (1997) Arachnoid cyst rupture producing subdural hygroma and intracranial hypertension: case reports. Neurosurgery 41:951–955
Helland CA, Wester K (2006) A population based study of intracranial arachnoid cysts: clinical and neuroimaging outcomes following surgical cyst decompression in children. J Neurosurg Pediatr 105(5 Suppl):385–390
Balsubramaniam C, Laurent J, Rouah E et al (1989) Congenital arachnoid cysts in children. Pediatr Neurosci 15:223–228
Passero S, Filosomi G, Cioni R et al (1990) Arachnoid cysts of the middle cranial fossa: a clinical, radiological and followup study. Acta Neurol Scand 82:94–100
Santamarta D, Aguas J, Ferrer E (1995) The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg 38:133–137
Von Wild K, Gullotta F (1987) Arachnoid cyst of the middle cranial fossa—aplasia of temporal lobe? Childs Nerv Syst 3:232–234
Levy ML, Wang M, Aryan HE et al (2003) Microsurgical keyhole approach for middle fossa arachnoid cyst fenestration. Neurosurgery 53:1138–1144, discussion 1144–5
Arroyo S, Santamaria J (1997) What is the relationship between arachnoid cysts and seizure foci? Epilepsia 38:1098–1102
Choi JU, Kim DS, Huh R (1999) Endoscopic approach to arachnoid cyst. Childs Nerv Syst 15:285–291
Wester K, Helland CA (2008) How often do chronic extra-cerebral haematomas occur in patients with intracranial arachnoid cysts? J Neurol Neurosurg Psychiatr 79:72–75
Parsch CS, Krauss J, Hofmann E et al (1997) Arachnoid cysts associated with subdural hematomas and hygromas: analysis of 16 cases, long-term follow up, and review of the literature. Neurosurgery 40:483–490
Martinez-Lage JF, Valenti JA, Piqueras C et al (2006) Functional assessment of intracranial arachnoid cysts with TC99 m-HMPAO SPECT: a preliminary report. Childs Nerv Syst 22:1091–1097
Sgouros S, Chapman S (2001) Congenital middle fossa arachnoid cysts may cause global brain ischaemia: a study with 99Tc-hexamethylpropyleneamineoxime single photon emission computerised tomography scans. Pediatr Neurosurg 35:188–194
Wester K, Hugdahl K (2003) Verbal laterality and handedness in patients with intracranial arachnoid cysts. J Neurol 250:36–41
Spacca B, Kandasamy J, Mallucci CL et al (2010) Endoscopic treatment of middle fossa arachnoid cysts: a series of 40 patients treated endoscopically in two centres. Childs Nerv Syst 26:163–172
Moon KS, Lee JK, Kim JH et al (2007) Spontaneous disappearance of a suprasellar arachnoid cyst: case report and review of the literature. Childs Nerv Syst 23:99–104
Greenfield JP, Souweidane MM (2005) Endoscopic management of intracranial cysts. Neurosurg Focus 19:E7
Shim KW, Lee YH, Park EK et al (2009) Treatment option for arachnoid cysts. Childs Nerv Syst 25:1459–1466
Nowosławska E, Polis L, Kaniewska D et al (2006) Neuroendoscopic techniques in the treatment of arachnoid cysts in children and comparison with other operative methods. Childs Nerv Syst 22:599–604
Galassi E, Tognetti F, Gaist G et al (1982) CT scan and medtrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol 17:363–369
Di Rocco C (2010) Sylvian fissure arachnoid cysts: we do operate on them but should it be done? Childs Nerv Syst 26:173–175
Di Rocco F, James SR, Roujeau T et al (2010) Limits of endoscopic treatment of sylvian arachnoid cysts in children. Childs Nerv Syst 26:155–162
Arai H, Sato K, Wachi A et al (1996) Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery 39:1108–1112
Kamikawa S, Inui A, Tamaki N et al (2001) Application of flexible neuroendoscopes to intracerebroventricular arachnoid cysts in children: use of videoscopes. Minim Invasive Neurosurg 44:186–189
Kabil MS, Shahinian HK (2007) Fully endoscopic supraorbital resection of congenital middle cranial fossa arachnoid cysts: report of 2 cases. Pediatr Neurosurg 43:316–322
Oertel JM, Baldauf J, Schroeder HW et al (2009) Endoscopic cystoventriculostomy for treatment of paraxial arachnoid cysts. J Neurosurg 110(4):792–799
Elhammady MS, Bhatia S, Ragheb J (2007) Endoscopic fenestration of middle fossa arachnoid cysts: a technical description and case series. Pediatr Neurosurg 43:209–215
Karabatsou K, Hayhurst C, Buxton N et al (2007) Endoscopic management of arachnoid cysts: an advancing technique. J Neurosurg 106(6 Suppl):455–462
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gui, Sb., Wang, Xs., Zong, Xy. et al. Assessment of endoscopic treatment for middle cranial fossa arachnoid cysts. Childs Nerv Syst 27, 1121–1128 (2011). https://doi.org/10.1007/s00381-011-1399-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-011-1399-8