Abstract
Purpose
Medulloblastomas are the most common malignant tumors of the central nervous system in childhood. The incidence is about 19–20% between children younger than 16 years old with peak incidence between 4 and 7 years. Despite its sensibility to no specific therapeutic means like chemotherapy and radiotherapy, the treatment is very aggressive and frequently results in regression, growth deficit, and endocrine dysfunction. From this point of view, new treatment approaches are needed such as molecular targeted therapies. Studies in glioblastoma demonstrated that ASPM gene was overexpressed when compared to normal brain and ASPM inhibition by siRNA-mediated inhibits tumor cell proliferation and neural stem cell proliferation, supporting ASPM gene as a potential molecular target in glioblastoma. The aim of this work was to evaluate ASPM expression in medulloblastoma fragment samples, and to compare the results with the patient clinical features.
Methods
Analysis of gene expression was performed by quantitative PCR real time using SYBR Green system in tumor samples from 37 children. The t test was used to analyze the gene expression, and Mann–Whitney test was performed to analyze the relationship between gene expressions and clinical characteristics. Kaplan–Meier test evaluated curve survival.
Results
All samples overexpressed ASPM gene more than 40-fold. However, we did not find any association between the overexpressed samples and the clinical parameters.
Conclusion
ASPM overexpression may modify the ability of stem cells to differentiate during the development of the central nervous system, contributing to the development of medulloblastoma, a tumor of embryonic origin from cerebellar progenitor cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) is an embryonal brain tumor that arises in the cerebellum and comprises the most common malignant brain tumor in children [5, 8, 13], accounting for 15–20% of central nervous system tumors [11, 13]. This tumor occurs predominantly in the first decade of life, with a peak of incidence between 4 and 7 years of age [13]. Currently, therapeutic regimens of MB involving surgery, radiotherapy, and chemotherapy [5] can cure 60% of the patients with an average 5-year progression-free survival, even so, about 10–15% of the patients die from disease within 2 years after diagnosis [5, 8]. Despite of considerable advances, the treatment still is very aggressive, and a lot of patients suffer severe lifelong neurocognitive deficiencies due to radiation-induced damage of the developing brain and endocrine dysfunction [5, 12, 13].
Looking for molecular markers, recent work in a different malignant brain tumor, glioblastoma, showed abnormal spindle-like microcephaly-associated (ASPM) gene as a potential molecular target. This study showed that ASPM expression is increased in glioblastoma when compared with normal brain, and its inhibition by siRNA-mediated knockdown inhibits tumor cell proliferation and neural stem cell proliferation [7]. ASPM gene maps to chromosome 1q31 and is essential for normal mitotic spindle function in embryonic neuroblasts [1]. The product of ASPM gene is a spindle pole/centrosome protein which promotes neuroblast proliferation and manages the orientation of mitotic cleavage, allowing symmetric and proliferative division of neuropithelial cells during brain development, as well as brain size expansion [4]. Hence, centrosome defects cause chromosome failure of segregation and, accordingly, lead to aneuploidy [16] and genetic instability, and modifications within this gene as mutation produces cause primary microcephaly [1, 2]. Moreover, due to high expression of ASPM in many tumor cells, studies have been suggested an important involvement of this gene in malignant transformation and tumor progression [9, 10].
Since ASPM gene fulfills an important role in brain size and is actively involved in cell proliferation in embryonic development and tumorigenesis, the purpose of the present study was to evaluate ASPM expression in MB samples and related the molecular results with the patient clinical features.
Material and methods
Patients
We selected 37 MB samples from patients attending the Pediatric Oncology Institute (IOP)/Grupo de Apoio ao Adolescente e a Criança com Câncer (GRAACC)—Federal University of São Paulo (UNIFESP)—Brazil. Informed consent was obtained from all patients/guardians according to the University’s IRB (CEP/UNIFESP Nº1120/01). All MB patients were classified according to Chang et al. (1969) [3], as follows: M stage include include M0 (no evidence of disseminated disease), M1 (tumor cells identified by cerebrospinal fluid cytology only), M2 (intracranial metastatic tumor detectable by computed tomography (CT) or magnetic resonance imaging (MRI), and M3 (spinal metastatic tumor detectable by CT myelography or spine MRI).
RNA extraction and cDNA synthesis
Total RNA was isolated from frozen samples pulverized under nitrogen liquid and extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). To reduce the risk of genomic DNA contamination, DNase treatment was performed using deoxyribonuclease I Amplification Grade (Invitrogen, Carlsbad, CA, USA). The concentration of RNA was determined by spectrophotometry, and total RNA integrity was monitored by visualization of ribosomal RNAs (28S and 18S) on 1.0% agarose gel. One microgram of total RNA derived from 37 MB samples was reverse-transcribed using the SuperScript III Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Quantitative real-time RT-PCR
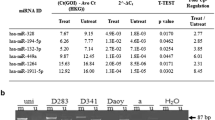
Oligonucleotides for ASPM and hypoxanthine-guanine phosphoribosyl transferase (HPRT) were designed using Primer Express Software from Applied Biosystems (Foster City, CA, USA) warranting that forward and reverse sequences were in different exons. We conducted a BLAST search to confirm the total gene specificity of the nucleotide sequences chosen for primers. Real-time polymerase chain reaction (PCR) amplification and data analysis were performed using the ABI Prism 7500 Sequence Detector System Applied Biosystems (Foster City, CA, USA). Each cDNA sample was mixed with 12 μl of Mastermix (SYBR® Green PCR Master Mix, Applied Biosystems). Cycling conditions consisted of two singles steps at 50°C and 95°C for 2 min and 10 min, respectively, followed by 40 cycles of amplification at 95°C for 15 s and at 60°C for 1 min for annealing and elongation. Experiments were performed in triplicate for both target genes and the endogenous gene. For each sequence, a standard curve was constructed to determine the assay sensitivity. The data were averaged from the values obtained in each reaction. Relative expression was automatically calculated and analyzed according to 2−∆∆Ct method described previously [10]. HPRT was used as a housekeeping gene in order to standardize its relative expression levels with relative expression levels of two genes studied. A no-template control was included in each amplification reaction, as well as a reference RNA. The PCR efficiencies of the two genes were comparable (≥95%).
Statistical analysis
Statistical analysis was performed using Graph Pad Prism 4 Program Software (San Diego, CA, USA). The clinical variables correlated with gene expression were: histological MB subtype, metastatic status at diagnosis, risk, surgical resection, follow-up, and relapse. Overall survival was defined as the time from diagnosis until date of either the more recent follow-up or death. Overall survival was generated by applying the Kaplan–Meier method. The t test was used to analyze the gene expression of each gene studied. The Kruskal–Wallis and Mann–Whitney tests were used to analyze the relationship between the gene expressions and the clinical characteristics. Categorical data were studied using chi-square and Fisher’s exact test applying software VassarStats (Website for Statistical Computation). In all cases, p < 0.05 was considered statistically significant.
Results
All clinical and pathological variables of patients with tumors used in this study are summarized in Table 1. The median age of patients was 8 years (range 5 months to 18 years old). In our samples we found: stage M0, 67% (n = 25), stage M1, 6% (n = 2), stage M2, 13% (n = 5), and stage M3, 13% (n = 5). Stages M1, M2, and M3 were included in just one group named M+, 32,4% (n = 12). The median expression value of each M stage is summarized in Table 2. Twenty-one of 37 patients died from disease, and 16 patients were alive disease-free or in treatment. Most of our samples had classic MB (92%).
The ASPM gene was overexpressed (cut off ≥ 2.0) in all 37 samples (p < 0.0001; Fig. 1). By analyzing the clinical characteristics of these samples, we observed that 21 of 37 patients (57%) were high risk, three (0.08) belonged to the anaplastic histology or anaplastic/large cell, 21 (57%) died due to illness or from complications of treatment, 17 (46%) had partial resection of the tumor, and nine of 37 patients (24%) relapsed. Additionally, 12 of 37 patients (32%) were metastatic (Table 1). There was no significant correlation between gene expressions and clinical and survival parameters.
Discussion
Recent studies have shown that ASPM plays an important role in cytokinesis and cell proliferation in transformed cell lines, fetal tissue, and tumor cells [1, 15]. Despite these characteristics, few studies have been conducted to investigate its role in human cancers. During embryonic development of mice, ASPM is expressed in the cerebral cortical ventricular zone, and its expression is very low close to birth when neurogenesis in the cortical ventricular zone is complete. This suggests that ASPM is preferentially expressed in progenitor cells that produce neurons [1].
The reduced expression of ASPM inhibits proliferation of neural progenitor cells, thereby reducing the number of neurons formed, resulting in a small brain that is characteristic of autosomal recessive primary microcephaly (MCPH) [4]. In adults, ASPM mRNA is expressed in breast, lung, pancreas, uterus, colon, thyroid, liver, bladder, kidney, ovary, testis, stomach, lymph node, cervix, and esophagus, but is not found in the brain and skeletal muscle [9, 14]. Its expression is also increased in transformed cells and some types of cancers such as uterus, ovary, breast, colon, thyroid, testis, lymph node, stomach, and more recently, was found in glioblastoma and hepatocellular carcinoma [7, 9, 10]. Finally, besides ASPM overexpression as an important factor associated with hepatocellular carcinoma progression, it is a determinant for vascular invasion, lowest 5-year survival, and hence, poorer prognosis [10].
In our study, all 37 samples analyzed MBs showed high expression of ASPM (p < 0.0001). Although some studies have found an association between the expression of ASPM and the degree of malignancy of some tumors, we found no correlation between the increased expression of this gene and clinical parameters studied here. This suggests that ASPM may be involved only in the origin of MB as part of abnormal neurogenesis during embryonic development. Our suggestion is that overexpression of this gene may modify the ability of stem cells to differentiate during the development of the central nervous system, contributing to the development of MB, a tumor of embryonic origin from cerebellar progenitor cells. However, this is a pilot study and we suggest that further investigation is warranted. More studies, especially functional ones, should be performed in order to confirm this hypothesis once investigation on glioma confirms that ASPM can be regulated at a translational level [6].
References
Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF, Walsh CA, Woods CG (2002) ASPM is a major determinant of cerebral cortical size. Nat Genet 32:316–320
Brinkley BR (2001) Managing the centrosome numbers game: from chaos to stability in cancer cell division. Trends Cell Biol 11:18–21
Chang CH, Housepian EM, Herbert C Jr (1969) An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology 93:1351–1359
Fish JL, Kosodo Y, Enard W, Paabo S, Huttner WB (2006) Aspm specifically maintains symmetric proliferative divisions of neuroepithelial cells. Proc Natl Acad Sci USA 103:10438–10443
Guessous F, Li Y, Abounader R (2008) Signaling pathways in medulloblastoma. J Cell Physiol 217:577–583
Horvath S, Zhang B, Carlson M, Lu KV, Zhu S, Felciano RM, Laurance MF, Zhao W, Qi S, Chen Z, Lee Y, Scheck AC, Liau LM, Wu H, Geschwind DH, Febbo PG, Kornblum HI, Cloughesy TF, Nelson SF, Mischel PS (2006) Analysis of oncogenic signaling networks in glioblastoma identifies ASPM as a molecular target. Proc Natl Acad Sci USA 103:17402–17407
Korshunov A, Benner A, Remke M, Lichter P, von Deimling A, Pfister S (2008) Accumulation of genomic aberrations during clinical progression of medulloblastoma. Acta Neuropathol 116:383–390
Kouprina N, Pavlicek A, Collins NK, Nakano M, Noskov VN, Ohzeki J, Mochida GH, Risinger JI, Goldsmith P, Gunsior M, Solomon G, Gersch W, Kim JH, Barrett JC, Walsh CA, Jurka J, Masumoto H, Larionov V (2005) The microcephaly ASPM gene is expressed in proliferating tissues and encodes for a mitotic spindle protein. Hum Mol Genet 14:2155–2165
Lin SY, Pan HW, Liu SH, Jeng YM, Hu FC, Peng SY, Lai PL, Hsu HC (2008) ASPM is a novel marker for vascular invasion, early recurrence, and poor prognosis of hepatocellular carcinoma. Clin Cancer Res 14:4814–4820
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
MacDonald TJ (2008) Aggressive infantile embryonal tumors. J Child Neurol 23:1195–1204
Pizer B, Clifford S (2008) Medulloblastoma: new insights into biology and treatment. Arch Dis Child Educ Pract Ed 93:137–144
Rhoads A, Kenguele H (2005) Expression of IQ-motif genes in human cells and ASPM domain structure. Ethn Dis 15: S5-88-91
Riparbelli MG, Callaini G, Glover DM, Avides Mdo C (2002) A requirement for the abnormal spindle protein to organise microtubules of the central spindle for cytokinesis in Drosophila. J Cell Sci 115:913–922
Shimada M, Komatsu K (2009) Emerging connection between centrosome and DNA repair machinery. J Radiat Res (Tokyo) 50:295–301
Wakefield JG, Bonaccorsi S, Gatti M (2001) The drosophila protein asp is involved in microtubule organization during spindle formation and cytokinesis. J Cell Biol 153:637–648
Ackowledgments
This work was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Grupo de Apoio ao Adolescente e à Criança com Câncer (GRAACC/UNIFESP).
It is declared in this work that there is no conflict of interest between the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial and material support
GRAACC/CAPES/FAPESP N° 04/12133-6.
Rights and permissions
About this article
Cite this article
Vulcani-Freitas, T.M., Saba-Silva, N., Cappellano, A. et al. ASPM gene expression in medulloblastoma. Childs Nerv Syst 27, 71–74 (2011). https://doi.org/10.1007/s00381-010-1252-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-010-1252-5