Abstract
Objects
Medulloblastoma (MB) is the most malignant primary brain tumor in early childhood that contains cellular and functional heterogeneity. Recent evidence has demonstrated that the tumor stem cells (TSC) may explain the radiochemoresistance of brain tumors, including MB. The aim of the present study is to investigate the possible role of TNF-related apoptosis-inducing ligand (TRAIL) in viability and tumorigenicity of MB cells and MB-derived TSC.
Methods
MB-associated TSC were isolated and cultured by serum-free medium with bFGF and EGF. The parental MB cells and MB-TSC cells were treated with TRAIL in different concentrations and assessed for cell viability, invasion ability, colony forming ability, and radiotherapy effect.
Results
We enrich a subpopulation of MB-TSC cells using tumor spheroid formation approach. MB-TSC display enhanced self-renewal and highly expressed “stemness” genes (CD133, Sox-2, Bmi1, Nestin). Additionally, MB-TSC showed significant resistance to TRAIL-induced apoptosis and radiosensitivity compared to the parental MB cells due antiapoptotic gene (c-FLIP, Caspase 8, Bcl-2, and Bax) upregulation.
Conclusions
Our data suggest that MB-TSC are resistant to TRAIL-induced apoptosis and tumorigenic properties. Understanding the molecular mechanisms by which to operate the physiological characteristics in MB-TSC cells offers attractive approach for MB treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) is an extremely malignant neoplasm in the central nervous system which occurs in infancy and childhood [5, 14]. As for remedy of MB, surgical excision alone is not curative [19, 36]. Virtually all tumors recur and patients die within 3 years without adjuvant treatment [36]. Local or disseminated whole neuroaxis relapse can be seen even after standard radiation treatment, including carniospinal axis irradiation and focal boost. Besides, radiotherapy has its own side-effects and may cause pituitary hormone dysfunction and behavior problems [5, 14]. Thus, to improve the therapeutic outcome and promote the life quality of the survivors, novel therapeutic targets and radiosensitizers are urgently needed.
TNF-related apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily, promotes apoptosis through receptor-mediated pathway [25]. TRAIL selectively kills transformed cancer cells without harming normal cells. Although TRAIL has been extensively studied as a therapeutic agent cancer, many cancer cells still developed resistance [15, 28]. One plausible possibility is the existence of tumor stem cells (TSC) within tumor cells [13, 31, 35]. It has been proposed that failure to effectively treat cancer may be relate to preferential resistance of these TSC to chemotherapeutic agents [22, 27]. Evidence for chemoresistance by stem-like cells in epithelial cell lines and xenogeneic tumor-derived cells has been presented [18, 34]. However, the TRAIL-mediated apoptotic and radio-sensitizing effects in the treatment of MB and MB-associated tumor stem cells were still undetermined.
We previously enriched the subpopulation of normal neural stem cell from hippocampal region and TSC derived from brain tumors through serum-free medium that contained bFGF and EGF growth factors [6, 7, 23]. In this present study, the specific aim is to investigate the possible role of TRAIL in proliferative and tumorigenicity effects in MB cells and MB-TSC cells and further elucidate the mechanisms.
Materials and methods
MB cell lines cultivation and enrichment of MB-TSC
The human medulloblastoma (Doay) cell line was purchased from ATCC Cell Bank (USA). The suspended cells were washed with phosphate-buffered saline (pH 7.2) and treated with 0.025% trypsin-EDTA (GIBCO) in HBSS solution (Sigma) for 15 min at 37°C and then passed through a 30-µm mesh nylon screen. The filtrate was centrifuged (800×g, 5 min), and the resulting cell pellet was seeded into a T75 flask. Cultures were grown in DMEM (GIBCO) containing 10% heat-inactivated fetal bovine serum, 2 mM glutamine, penicillin (100 U/ml), and streptomycin (100 µg/ml). Doay cells were then cultured in serum-free DMEM/F12 (GIBCO) medium supplemented with N2 supplement (R&D), 10 ng/ml human recombinant bFGF (R&D), and 10 ng/ml EGF [6, 8].
FACS analysis
For cell surface marker identification, the different passage cells were detached and stained with anti-CD133 with secondary phycoerythrin-coupled antibodies (Miltenyi Biotec). BMSCs were fixed with 2% paraformaldehyde until they were ready for analysis using FACSCalibur apparatus (Becton–Dickinson).
Cell viability determined by colorimetric assay
Parental MB and MB-derived TSC were cultured in a 96-well cell culture cluster (Costar, Acton, MA) at a density of 3 × 103 cells/well with 100 μl culture medium. After different time points of cultivation, the medium was discarded and replaced with an equal volume (100 μl) of fresh medium containing 0.2 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (Promega, Madison, WI) and 0.038 mg/ml of phenazine methosulfate (Promega, Madison, WI) and incubated for additional 1.5 h in a 37°C 5% CO2 incubation. Cell viability proportionate to optical density (OD) value was measured by colorimetric assay in terms of mitochondria activity to convert tetrazolium salt into a colored soluble formazan product in the medium. The OD values were measured at the wavelength of 490 nm with a 1420 multilabel counter VICTOR® from Wallac (Turku, Finland).
Real-time RT-PCR
Real-time RT-PCR was performed as previously described. Briefly, total RNA (1 μg) of each sample was reverse-transcribed in a 20-μl volume using 0.5 μg oligo dT and 200 U Superscript II RT (Invitrogen, Carlsbad, CA). The primer sequences used for real-time RT-PCR are shown in Table 1.
The amplification was carried out in a total volume of 20 µl containing 0.5 µM in each primer, 4 mM MgCl2, 2 µl LightCyclerTM–FastStart DNA Master SYBR green I (Roche Molecular Systems, Alameda, CA), and 2 µl of 1:10 diluted cDNA. PCRs were prepared in duplicate and heated to 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 55°C for 5 s, and extension at 72°C for 20 s. Standard curves (cycle threshold values versus template concentration) were prepared for each target gene and for the endogenous reference (GAPDH) in each sample. Quantification of unknown samples was performed using LightCycler Relative Quantification Software version 3.3 (Roche Molecular Systems, Alameda, CA).
Statistic in vitro cell invasion analysis and soft agar assay
The 24-well plate Transwell® system with a polycarbonate filter membrane (8-µm pore size; Corning, UK) was used. Cell suspensions were seeded in the upper compartment of the Transwell chamber at a density of 1 × 105 cells in 100 µl serum-free medium. The opposite surface of the filter membrane, which faced the lower chamber, was stained with Hoechst 33342 for 3 min, and migrating cells were visualized under an inverted microscope. The soft agar assay was performed as follows. The bottom of each well (35 mm) of a six-well culture dish was coated with 2 ml agar mixture (DMEM, 10% (v/v) FCS, 0.6% (w/v) agar). After the bottom layer solidified, 2 ml top agar-medium mixture (DMEM, 10% (v/v) FCS, 0.3% (w/v) agar) containing 2 × 104 cells was added and incubated at 37°C for 4 weeks. The plates were stained with 0.5 ml 0.005% Crystal Violet, and then the number of colonies was counted using a dissecting microscope.
Radiation treatment for cell survival analysis
The Gamma Radiation (ionizing irradiation (IR)) was delivered by Theratronic cobalt unit T-1000 (Theratronic Internation, Inc., Ottawa, Canada) at a dose rate of 1.1 Gy/min (SSD = 57.5 cm).Briefly, cells in the control and irradiation groups were exposed to different radiation dosages (0, 2, 4, 6, 8, and 10 Gy). After incubation for 10 days, colonies (>50 cells per colony) were fixed and stained for 20 min with a solution containing crystal violet and methanol. Cell survival was determined by means of colony formation assay. The plating efficiency (PE) and survival fraction (92) were calculated as follows: \( {\hbox{PE = }}\left( {{{\hbox{colony number}} \mathord{\left/{\vphantom {{\hbox{colony number}} {\hbox{inoculating cell number}}}} \right.} {\hbox{inoculating cell number}}}} \right) \times 100\% \). \( {\hbox{SF = }}{{\hbox{colonies counted}} \mathord{\left/{\vphantom {{\hbox{colonies counted}} {\left( {{\hbox{cells seeded }} \times \left( {{{\hbox{PE}} \mathord{\left/{\vphantom {{\hbox{PE}} {100}}} \right.} {100}}} \right)} \right)}}} \right.} {\left( {{\hbox{cells seeded }} \times \left( {{{\hbox{PE}} \mathord{\left/{\vphantom {{\hbox{PE}} {100}}} \right.} {100}}} \right)} \right)}} \).
Statistical analysis
All data were presented as mean ± standard deviation (SD). One- or two-way ANOVA was employed to determine whether the results had statistical significance. P value less than 0.05 was considered a significant difference. The statistics software used in this study was Sigma Stat 3.0.1 (SPSS Inc., Chicago, IL).
Results
Isolation and functional characterization of medulloblastoma-derived tumor stem-like cells
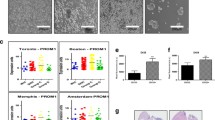
Sphere body formation of TSC is enriched under the cultivation of defined serum-free medium with growth factors from individual solid tumors, including colon cancer, lung cancer, and breast tumors where the serum-free culture condition helps maintain the TSC undifferentiated [21, 30, 32]. It has been reported that glioma and medulloblastoma stem cells can be cultured and enriched in suspension to generate floating spheroid-like bodies (SB) and maintain the self-renewal capabilities in serum-free media with bFGF and EGF [6, 23]. To investigate the existence of TSC in MB cancers cells, Doay, a MB cancer cell line, was cultured in defined serum-free medium with bFGF and EGF. After being cultured for 2 weeks, cancer cells gradually detached from culture dishes, aggregated, and formed sphere-like bodies. Significant sphere-like bodies were observed after 3 weeks of serum-free medium culture (Fig. 1a). These TSCs derived from MB could stably proliferate to form SB in serum-free medium with bFGF and EGF (Fig. 1a). To further characterize the progenitor/stem cells properties of our enriched MB-TSC, we used flow cytometry to detect the expression profile of progenitor/stem cell surface markers. As shown in Fig. 1c, we detected that the majority of isolated MB-TSC were positively stained with CD133, being the specific cell surface markers of brain tumor stem cells. In contrast, it only presented the very low level of CD133 antigen in these parental MB cells (Fig. 1b). Furthermore, quantitative real-time RT-PCR showed that the mRNA expression levels of stemness-related genes (CD133, Sox-2, Bmi1, Nestin; Fig. 2a; p < 0.01). Overall, our data indicated that the sphere-like MB-TSCs under serum-free media with bFGF and EGF selection display the characteristics of TSCs.
Isolation tumor stem-like cells from medulloblastoma. a MB cancer cell line Doay was used and cultured in DMEM/F-12 serum-free medium with bFGF and EGF. After being in culture for 3 weeks, cancer cells gradually detached from culture dishes, aggregated, and formed SB. (bar 100 μm). b In contrast to MB-TSC, only the very low level of CD133 antigen was detected in these parental MB cells. c The results of flow cytometry showed that the high percentage of CD133-positive cells was detected in MB-TSC cells
Detection of the expression levels of stemness markers and antiapoptotic genes in MB-TSC and parental MB cell. a Real-time RT-PCR analyses revealed that the transcript amounts of Sox2, Bmi1, and Nestin in MB-TSC and parental MB cells. The internal control is housekeeping gene GAPDH, and every stemness gene expression of MB-TSC was normalized with parental MB cells. Data shown are the mean ± SD of three experiments. b Real-time RT-PCR analyses revealed that the transcript amounts of c-FLIP, Bcl-2, Bax, and Caspase 8 in MB-TSC and parental MB cells. The internal control is housekeeping gene GAPDH, and every stemness gene expression of MB-TSC was normalized with parental MB cells. Data shown are the mean ± SD of three experiments. c Total proteins were prepared from parental Doay or MB-TSC cells and analyzed by Western blotting with anti-c-FLIP and anti-Bcl-2. The amount of β-actin protein of different cell extracts was referred as loading control
Upregulated expression of antiapoptotic genes and protein in MB-derived TSC
Expression of antiapoptotic genes in parental MB cells and MB-TSC were examined transcriptionally and translationally. The results showed that antiapoptotic (c-FLIP, Caspase 8, Bcl-2 and Bax) genes were upregulated in MB-TSC as compared to those of parental MB cells (Fig. 2b). In accordance with the real-time RT-PCR results, the western blotting data showed that the protein levels of c-FLIP and Bcl-2 in MB-TSCs were also significantly upregulated compared to those of parental MB cells (Fig. 2c).
Evaluation of cytotoxic effects of TRAIL in MB-TSC and parental MB cells
TRAIL has been recently suggested to inhibit tumor growth [12]. However, it remains undetermined whether TRAIL can inhibit the TSC properties of MB. To answer this question, MB-TSC were treated with different doses of TRAIL, and cell viability was analyzed using the MTT assay. As shown in Fig. 3, MB cells were treated with TRAIL at different concentrations (0, 100, 200, and 500 ng/mL) for 24 h detached and rounded up (Fig. 3a). However, MB-TSC also displayed spheroid-like morphology (Fig. 3b).Subsequently, cell viability of MB-TSC was not significantly affected (Fig. 3c). In contrast, the total cell number and growth rates of parental MB cells after being treated with were significantly decreased (p < 0.05; Fig. 3c). Lastly, the apoptotic cells also increased in parental MB cells treated with TRAIL than MB cells. However, apoptotic ability of MB-TSC treated with TRAIL was not significantly affected (Fig. 3d).
Evaluation of cytotoxic effects of TRAIL in MB-TSC and parental MB cells. a The morphology of parental MB cells treated with 100 ng/mL TRAIL for 24 h. b The morphology of MB-TSC treated with 100 ng/mL TRAIL for 24 h. c The cell viability of MB-TSC and parental MB cells treated with 0, 10, 200, 500 ng/mL TRAIL for 24 h were analyzed by MTT assay. Data shown are the mean ± SD of three experiments. d Daoy cells were treated with or without 100 ng/mL TRAIL for 6, 12, 24 h and stained with apoptotic assay. The percentage of apoptotic cells (%) was shown. *p < 0.05 compared with control group. Data shown here are the mean ± SD of three experiments
Tumorigenic properties in MB-TSC and parental MB cells with or without TRAIL
To determine the TRAIL effect on tumorigenicity of parental MB cells and MB-TSC, parental MB cells and MB-TSC treated with TRAIL was analyzed for invasion and soft agar colony formation abilities. Invasion ability and colony formation of MB-TSC treated with TRAIL were not significantly affected. In contrast, colony formation (Fig. 4a) and migration/invasion (Fig. 4b) were significantly inhibited in MBs treated with 100 ng/mL TRAIL.
Determination of tumorigenic properties in MB-TSC and parental MB cells with or without TRAIL. a Soft agar tumor colony formation assay were performed in parental MB cells and MB-TSC with or without TRAIL. *p < 0.05. Data shown here are the mean ± SD of three experiments. b Migration/invasion as determined by in vitro Matrigel Transwell invasion assay
MB-TSC-enhanced resistance to TRAIL-induced radiosensitivity
Recent studies supported that TRAIL plays a key role in the synergetic treatment for anticancer [1]. The property of resistance to radiation therapy is the one of major clinical criteria to characterize “tumor stem-like cells.” The existence of TSC cells may explain why the conventional anticancer therapies only can suppress or shrink a tumor but often cannot completely eradicate it resulting in eventual recurrence. To determine the effect of radiation on tumor growth rate, an IR dose from 0 to 10 Gy was used to treat the four groups of cells. As shown in Fig. 5, the survival rate and number of MB-TSCs after IR treatment were significantly higher than those of parental MB cells (p < 0.05; Fig. 5). Furthermore, the data revealed that the cell survival rate in IR-treated MB-TSC was not significantly decreased by the IR treatment combined with TRAIL treatment. On the contrary, cell survival significantly declined after chemotherapy with TRAIL treatment in parental MB cells (Fig. 5).
MB-TSC displayed resistance to TRAIL-induced radiosensitivity. a To determine the effect of radiation on tumor growth rate, an IR dose from 0 to 10 Gy was used to treat MB-TSC and parental MB cells. *p < 0.05 MB-TSC vs. parental MB cells. Survival fraction of MB-TSC treated with TRAIL (100 ng/mL) and exposed to 0–10 Gy radiation. Data were compared with IR-treated parental MB cells. Data shown are the mean ± SD of three experiments
Discussion
While recent decades have witnessed a revolution in therapeutic strategies yielding significant clinical responses measured in terms of tumor regression and disease-free survival, overall survival of MB has failed to substantially improve [6]. The recent identification of TSC subpopulations with the unique ability to fuel tumor growth may shed light on the disconnecting between response rates and overall survival [3, 13, 26, 29]. These cells possess unique survival mechanisms and distinctive stem cell properties, including the ability to self-renew and differentiate, and also exhibit a marked ability to proliferate following a prolonged period of quiescence [9, 10, 20]. That is, therapies that fail to adequately target TSC populations, which represent a minority of most epithelial tumors, will fail to eliminate those cells capable of regenerating the tumor after therapy has ceased [3]. Furthermore, familiarity with both the means of resistance to a particular chemotherapeutic agent and the phenotypic identity of those cells that harbor resistance mechanisms should help facilitate the discovery of therapies better able to clear minimum residual disease and prolong overall survival [17]. In this study, we reported that the MB-TSC can form the spheroid bodies and exhibit the high percentage of CD133 surface antigen under the serum-free conditional media culture. CD133 (prominin-1: PROM1), a 5-transmembrane glycoprotein, was identified as an important marker for a subset of tumor stem cells in leukemia, retinoblastoma, colon cancer, prostate carcinoma, brain tumor, hepatoma, and oral cancer [6, 16, 24, 30, 33]. Recent reports suggested that expression of CD133 antigen in gliomas, medulloblastoma, and other brain tumors could serve as a prognostic indicator for tumor regrowth, malignant progression, and patient survival [4, 37].
By analogy to normal stem cells, TSC will be inherently resistant to chemotherapy through mechanisms that serve to protect stem cells from DNA and cellular damage. First, most current agents destroy rapidly proliferating cells [2, 8]. Because TSC, like all stem cells, are relatively quiescent, the current chemotherapeutic approaches maybe ineffective [11]. Second, stem cells tend to be more resistant to chemotherapeutic agents and radiation than are more mature cell types from the same tissue [11]. This is believed to be due to the presence of multidrug resistance, antiapoptotic proteins, and enhanced DNA repair mechanisms [2, 3]. Using real-time quantitative RT-PCR method, we showed that enriched MB-TSCs highly expressed antiapoptotic genes (Bcl-2, Bax, c-FLIP, Caspase 8; Fig. 2). This higher expression of Bcl-2 and c-FLIP suggests that MB-TSC cells may be resistant to TRAIL. Consistent with our finding, breast and liver TSC are resistant to TRAIL-induced apoptosis [38]. Since this appears to hold true for TSC, we would predict that they would also display resistance to therapeutics. The majority of knowledge regarding cancer gene expression has been obtained using material obtained from whole-tumor preparations. Establishing mechanisms of resistance to chemotherapeutic drugs can be difficult, especially with heterogeneous tumors. Because TSC often make up only a tiny proportion of all the cancer cells in a tumor, their genotype will be obscured by all of the other cancer cells when this methodology is utilized. It is necessary to isolate the TSC subpopulation from tumors and evaluate their gene expression to identify molecular pathways that are critical to the TSC population.
Conclusion
In conclusion, we demonstrated that MB-TSC display a higher antiapoptotic genes (c-FLIP, Caspase 8, Bcl-2, and Bax) expression with the ability to represent an enhanced TRAIL resistance. The resistance of in vitro chemotherapy is partially due to preferential activation of c-FLIP and Bcl-2 genes expression. The more understanding of the characterization of MB-TSC population will shed new light on the underlying biological mechanism of this specific population and, thus, will ultimately lead to the development of more specific therapeutic agents for the treatment of this deadly cancer.
References
Ashkenazi A (2008) Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov 7:1001–1012
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Baumann M, Krause M, Hill R (2008) Exploring the role of cancer stem cells in radioresistance. Nat Rev Cancer 8:545–554
Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, Beier CP (2008) CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol 18:370–377
Cervoni L, Cantore G (1995) Medulloblastoma in pediatric age: a single-institution review of prognostic factors. Childs Nerv Syst 11:80–84, discussion 85
Chiou SH, Kao CL, Chen YW, Chien CS, Hung SC, Lo JF, Chen YJ, Ku HH, Hsu MT, Wong TT (2008) Identification of CD133-positive radioresistant cells in atypical teratoid/rhabdoid tumor. PLoS One 3:e2090
Chiou SH, Ku HH, Tsai TH, Lin HL, Chen LH, Chien CS, Ho LL, Lee CH, Chang YL (2006) Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol 148:587–598
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF (2008) Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res 14:4085–4095
Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58:267–284
Dalerba P, Clarke MF (2007) Cancer stem cells and tumor metastasis: first steps into uncharted territory. Cell Stem Cell 1:241–242
Dean M, Fojo T, Bates S (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5:275–284
Griffith TS, Stokes B, Kucaba TA, Earel JK Jr, VanOosten RL, Brincks EL, Norian LA (2009) TRAIL gene therapy: from preclinical development to clinical application. Curr Gene Ther 9:9–19
Gupta PB, Chaffer CL, Weinberg RA (2009) Cancer stem cells: mirage or reality? Nat Med 15:1010–1012
Habrand JL, De Crevoisier R (2001) Radiation therapy in the management of childhood brain tumors. Childs Nerv Syst 17:121–133
Hao C, Song JH, Hsi B, Lewis J, Song DK, Petruk KC, Tyrrell DL, Kneteman NM (2004) TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res 64:8502–8506
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A 100:15178–15183
Hill RP, Perris R (2007) “Destemming” cancer stem cells. J Natl Cancer Inst 99:1435–1440
Ho MM, Ng AV, Lam S, Hung JY (2007) Side population in human lung cancer cell lines and tumors is enriched with stem-like cancer cells. Cancer Res 67:4827–4833
Hoppe-Hirsch E, Brunet L, Laroussinie F, Cinalli G, Pierre-Kahn A, Renier D, Sainte-Rose C, Hirsch JF (1995) Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst 11:340–345, discussion 345–346
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355:1253–1261
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA (2006) Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 9:391–403
Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC (2008) Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst 100:672–679
Lu KH, Chen YW, Tsai PH, Tsai ML, Lee YY, Chiang CY, Kao CL, Chiou SH, Ku HH, Lin CH, Chen YJ (2009) Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv Syst 25:543–550
Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, Prochazka L, Dong L, Andera L, Ralph SJ (2007) Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem Biophys Res Commun 355:855–859
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997) The receptor for the cytotoxic ligand TRAIL. Science 276:111–113
Park CY, Tseng D, Weissman IL (2009) Cancer stem cell-directed therapies: recent data from the laboratory and clinic. Mol Ther 17:219–230
Phillips TM, McBride WH, Pajonk F (2006) The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 98:1777–1785
Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A (1996) Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem 271:12687–12690
Polyak K, Weinberg RA (2009) Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 9:265–273
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115
Rosen JM, Jordan CT (2009) The increasing complexity of the cancer stem cell paradigm. Science 324:1670–1673
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB (2003) Identification of a cancer stem cell in human brain tumors. Cancer Res 63:5821–5828
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G (2007) Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1:389–402
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8:755–768
Whelan HT, Krouwer HG, Schmidt MH, Reichert KW, Kovnar EH (1998) Current therapy and new perspectives in the treatment of medulloblastoma. Pediatr Neurol 18:103–115
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC (2008) Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 14:123–129
Zobalova R, Stantic M, Prokopova K, Dong LF, Neuzil J (2009) Cancer cells with high expression of CD133 exert FLIP upregulation and resistance to TRAIL-induced apoptosis. Biofactors 34:231–235
Acknowledgement
This study was supported by research grants from the National Science Council (NSC-97-3111-B-075-001-MY3, 97-2314-B-075-056-MY3), Taipei Veterans General Hospital (V97E1-008, V97F-001), Yen-Tjing-Ling Medical Foundation, Taipei City Hospital (96001-62-014, 96001-62-018, 96002-62-092), and National Yang-Ming University (Ministry of Education, Aim for the Top University Plan), Taiwan.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yu, Chiou, and Lee contributed equally.
Rights and permissions
About this article
Cite this article
Yu, CC., Chiou, GY., Lee, YY. et al. Medulloblastoma-derived tumor stem-like cells acquired resistance to TRAIL-induced apoptosis and radiosensitivity. Childs Nerv Syst 26, 897–904 (2010). https://doi.org/10.1007/s00381-010-1087-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-010-1087-0