Abstract
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has potent antitumor effects in glioma cell lines but has shown little clinical benefit for patients. We investigated whether the widely used chemotherapeutic agent temozolomide (TMZ) can sensitize glioma stem-like cells (GSCs) from human glioblastoma multiforme (GBM) to TRAIL-induced apoptosis. GSCs were isolated from GBM, and stem cell properties were confirmed by immunocytochemistry and in vivo tumorigenicity. Primary GSCs (PGCs) were produced by serum treatment of GBM-derived cells. Changes in expression levels of various TRAIL-related signaling factors before and after TRAIL or TRAIL + TMZ treatment were measured by Western blotting. Overexpression vectors and siRNAs were used to investigate mechanism of TRAIL sensitivity. GSCs showed greater resistance to TRAIL-induced apoptosis than PGCs and had lower basal caspase activity. Caspase knockdown in PGCs reduced TRAIL sensitivity. Expression levels of c-Fas-associated death domain-like interleukin 1-converting enzyme-like inhibitory protein long and short isoforms (c-FLIPL and c-FLIPS) were significantly higher in GSCs than PGCs, and siRNA-mediated c-FLIP knockdown in GSCs enhanced TRAIL-induced apoptosis. TMZ enhanced TRAIL-induced apoptosis in GSCs and downregulated c-FLIP expression. Add of TMZ also upregulated the expression of the E3 ubiquitin ligase casitas B-lineage lymphoma (c-Cbl). Moreover, overexpression of c-Cbl alone reduced c-FLIP expression, and c-Cbl knockdown both enhanced c-FLIP expression and reduced the potentiating effect of TMZ on TRAIL-induced apoptosis. The result indicated that TMZ may overcome TRAIL resistance in GSCs by suppressing c-FLIP expression through c-Cbl-mediated ubiquitination and degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas are the most common tumors of the central nervous system and have poor prognosis. Despite advances in treatment, the recurrence rate for gliomas is still high, with an average survival time of only 14.6 months [1]. Thus, new therapeutic approaches are urgently needed. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) has great promise as an anticancer drug because of its potent pro-apoptotic effects on cancer cell and negligible toxicity on healthy cells, including human astrocytes [2]. Glioma stem cells, a self-renewing and tumorigenic subpopulation of glioma cells, have been implicated in the treatment resistance of glioblastoma (GBM) and could therefore be responsible for resistance to TRAIL-induced cell death observed in gliomas [3]. For in vitro studies, cultured glioma spheres are widely used as a source of glioma stem-like cells [4].
TRAIL resistance is associated with expression of the endogenous cell death signaling pathway inhibitor c-Fas-associated death domain-like interleukin 1-converting enzyme-like inhibitory protein (c-FLIP) in tumor necrosis factor (TNF)-treated B cell chronic lymphocytic leukemia cells [5]. Recent studies have suggested that ubiquitin ligase is involved in ubiquitination and degradation of c-FLIPS, the short isoform of c-FLIP [6]. Ubiquitin ligase acts as a negative regulator of multiple signal transduction pathways by means of its E3 ubiquitin ligase activity, thereby modulating the balance between proliferation and apoptosis through the regulation of protein degradation [7]. However, whether the insensitivity of glioma stem-like cells (GSCs) to TRAIL is attributable to lack of c-FLIP degradation by ubiquitin ligase is not yet clear.
In some cancers, resistance to TRAIL treatment can be overcome if it is used in combination with other types of therapeutics, including radiation and chemotherapeutic drugs [8, 9]. The present study examined whether a similar approach could be applied to enhance the TRAIL sensitivity of GSCs. Temozolomide upregulated the E3 ligase casitas B-lineage lymphoma (c-Cbl) and downregulated both c-FLIPS and the long isoform c-FLIPL, thereby disinhibiting caspase-8 and restoring the TRAIL apoptotic pathway in GSCs.
Materials and methods
Statement of ethics
We obtained written informed consent from all participating patients who were informed of, and understood, the purpose and risks in providing specimens. This study was approved by the Medical Ethics Committee of the First Hospital of China Medical University, Liaoning, China. The animal study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the Chinese Institute of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of China Medical University. All surgeries were performed under sodium pentobarbital anesthesia, and every effort was made to minimize animal suffering.
Glioma sphere culture
The study protocol was approved by the institutional review board of the First Hospital of China Medical University, and written informed consent was obtained from each tissue donor. Glioblastoma multiforme (GBM) specimens were obtained from glioma patients treated at the Department of Neurosurgery of the First Affiliated Hospital of China Medical University. Glioma stem-like cells were isolated from two GBM specimens (referred to as GSC-no. 1 and GSC-no. 2 cells) and cultured in serum-free medium modified from a previously published protocol [10]. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 containing B27 and supplemented with basic fibroblast growth factor (bFGF, 20 ng/ml) and epidermal growth factor (EGF, 20 ng/ml) (all from Invitrogen, Carlsbad, CA, USA).
To confirm the stem-like characteristics of glioma sphere cells, tumor marker expression and in vivo tumorigenicity were examined. Tumor spheres from the third or fourth generation were cultured for 4 h until they became adherent, washed, fixed in [fixative], and incubated overnight at 4 °C with mouse monoclonal antibodies against the stem cell markers cluster of differentiation (CD)133, nestin, and SOX-2 (all at 1:200; Abcam, Cambridge, UK), or rabbit glial fibrillary acidic protein (GFAP) antibody (1:200; Abcam). Immunolabeled cells were then incubated with a Cy3-labeled goat anti-mouse secondary antibody (1:50; Sigma, St. Louis, MO, USA) for 30 min at 37 °C and counterstained for 5 min with 4′,6-diamidino-2-phenylindole (DAPI). Cultures were then examined under a BX61 fluorescence microscope (Olympus, Tokyo, Japan). For tumorigenicity analysis, 100 dissociated glioma spheres were injected into the brains of immunodeficient NOD-SCID mice. After xenografts were detected by magnetic resonance imaging (data not shown), mice were sacrificed for histological examination.
Cell death assay
Cells isolated from glioma spheres grown in the presence or absence of serum (GSCs and PGCs) were seeded in 96-well plates at 5 × 103 cells/well. Cells were left untreated or treated with recombinant human TRAIL (Cytolab/Peprotech Asia, Rehovot, Israel), TMZ (Tasly Pharmaceutical Co., Ltd., Tianjin, China), or both for 24 h. Cell viability was analyzed using the CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The rate of cell death was calculated by the following formula: 1 − (luminescent density of treated cells/luminescent density of untreated cells) × 100 %.
Determination of caspase activity by absorption spectroscopy
Caspase activity was measured using a colorimetric assay kit (Keygen Biotech. Co. Ltd., Nanjing, China). Cells were incubated with various concentrations of TRAIL, TMZ, or both for varying lengths of time and then suspended in 50 ml chilled cell lysis buffer containing 50 mM HEPES (pH 7.4), 5 mM CHAPS, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), and 5 mM dithiothreitol (DTT). After incubation on ice for 60 min and centrifugation for 1 min at 10,000 g, supernatants (50 ml) were added to reaction buffer (40 mM HEPES, pH 7.4; 3 mM CHAPS; 10 mM DTT, and 4 mM EDTA) with 5 ml caspase substrate (4 mM) and incubated at 37 °C. Optical density was measured at 405 nm using a microplate reader (Model 550; Bio-Rad Laboratories, Hercules, CA, USA).
Cell transfection
Knockdown
A caspase-8-targeting siRNA and scrambled control siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). An siRNA against c-Cbl (CUG CCG AUG UGA AAU UAA ATT) and a sequence targeting nucleotides 535–555 of the c-FLIP transcript (Gene Bank accession number U97074) were synthesized by Genechem Co. (Shanghai, China). For siRNA knockdown, GSCs were transfected with 300 nM siRNA using the Amaxa Cell Line Nucleofector Kit V and Nucleofector II electroporator (Lonza Cologne GmbH, Cologne, Germany) according to the supplier’s instructions. After transfection, cells were cultured in serum-free medium for 18 h before additional experiments were performed.
Overexpression
Human c-Cbl and c-FLIPL cDNAs were purchased from GenePharma (Shanghai, China) and cloned into the pcDNA3.1 plasmid (Invitrogen). GSC-no. 1 and -no. 2 and primary glioma cell lines PGC-no. 1 and PGC-no. 2 were seeded in six-well plates and grown to 90–95 % confluence, washed twice with phosphate-buffered saline, and incubated in 2 ml DMEM/F12 medium without antibiotics. GSC-no. 1 and -no. 2 cells were transfected with c-Cbl plasmid (2 μg) and PGC-no. 1 and -no. 2 with c-FLIPL plasmid (2 μg) using Lipofectamine 2000 reagent (Invitrogen). Cells transfected with the empty pcDNA3.1 vector served as the negative control.
Western blotting
GSCs treated with TRAIL, TMZ, or both were lysed in cell lysis buffer (50 mM Tris, 150 mM NaCl, 2 mM EDTA, 10 % glycerol, 1 % Triton X-100, 1 % protease inhibitor cocktail, and 1 mM PMSF). Total protein from each lysate (50 μg per sample) was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After blocking with 5 % skim milk in Tris-buffered saline with 1 % Triton X-100, membranes were incubated with appropriate primary and secondary antibodies. Immunolabeled bands were detected using the SuperSignal Western Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA).
c-FLIP expression in human glioma tissue specimens
The mRNA expression profiles of c-FLIP in human glioma tissues, including whole genome mRNA expression microarray data and corresponding clinical information, were obtained from the Chinese Glioma Genome Atlas (CGGA) database. A total of 295 samples were examined in this study to determine the relationship between c-FLIP mRNA expression and glioma grade (Supplementary Fig. 3).
Statistical analysis
SigmaPlot v. 13.0 software (Systat Software Inc., Chicago, IL, USA) was used for statistical analyses. Differences in c-FLIP expression across tumor grades were compared with the Student’s t test. For in vitro studies, statistical analyses were performed on data from triplicate experiments. A P value < 0.05 was considered statistically significant.
Results
Glioma sphere culture and characterization
In our previous report, primary glioma cell (PGC)-derived glioma spheres were shown to retain the properties of cancer stem cells and the genomic signature of parental tumors [10]. In this study, two GSC cultures derived from separate human GBM tumors (GSC-no. 1 and -no. 2) and matched serum-grown primary cultures (PGC-no. 1 and -no. 2) were generated from surgically resected GBM tissues. Approximately 15 % of cells in GSC-no. 1 and 10 % in GSC-no. 2 were capable of forming glioma spheres; in contrast, few spheres were generated from PGC-no. 1 and PGC-no. 2 under the same culture conditions (Fig. 1a). These results indicate that cells from tumor spheres but not serum-grown PGC cultures retain the self-renewal capacity of cancer stem cells. Cells from glioma spheres expressed the stem cell markers CD133, nestin, and SOX-2, but not the differentiated glial marker GFAP (Fig. 1b), confirming their stem cell identity. These markers were not expressed by PGC-no. 1 or -no. 2 (data not shown).
Generation of human glioma spheres from GBM tumors and demonstration of stem cell properties and in vivo tumorigenicity. a Glioma spheres and matched serum-grown cultures from primary glioma cell (PGC) lines no. 1 and no. 2 were cultured after 7 days under neurosphere culture conditions. Data are expressed as mean ± SEM (n = 6). b Expression of the stem cell markers CD133, nestin, and SOX-2 and differentiated glial marker GFAP in glioma spheres as detected by immunocytochemistry. c Xenograft formation by injection of GSC-no. 1 in the brains of NOD-SCID mice. GSC-no. 1-derived xenografts were observed in tissue sections stained with hematoxylin and eosin (×10). Three mitotic bodies can be seen in the xenograft (×400)
To investigate the tumor-forming capacity of GSCs, 100 cells were injected into the brains of immunodeficient NOD-SCID mice. Tumors were visible on the injected side of the brain (Fig. 1c). Microscopic examination of brain sections revealed that the tumors possessed the histological features of GBM, such as diffuse infiltration throughout the cerebral tissue. These results confirm that GBM-derived GSCs have the characteristics of stem-like cells with glioma properties.
Stem-like cells from glioma spheres are resistant to TRAIL-induced apoptosis
Apoptosis of GSCs and PGCs in response to TRAIL treatment was then assessed. Dissociated GSC-no. 1, GSC-no. 2, PGC-no. 1, and PSC-no. 2 cells were cultured in the presence of 100 ng/ml TRAIL for 24 h (Fig. 2). TRAIL treatment induced substantial apoptosis in PGC cultures, but not in GSC-no. 1 and GSC-no. 2 cultures (Fig. 2a). Moreover, caspase activity, which is necessary for TRAIL-induced apoptosis [11], was detected in PGCs but not in GSCs (Fig. 2b) by colorimetric assay. Knockdown of caspase-8 expression by a targeted siRNA inhibited TRAIL-induced apoptosis in PGCs (Fig. 2c), indicating that TRAIL-induced cell death in PGCs occurs via activation of caspase-8.
Resistance of glioma stem-like cells (GSC) to TRAIL-induced apoptosis and TRAIL sensitive of primary glioma cells (PGC) associated with high caspase-8 expression. a Substantial apoptosis was induced by TRAIL (100 ng/ml, 24 h) in PGCs but not in GSCs. b Basal caspase activity was higher in PGCs than GSCs. c Knockdown of caspase-8 in PGCs by 72 h of transfection with caspase-8 siRNA (confirmed by Western blotting) significantly reduced apoptosis induced by TRAIL (100 ng/ml for 24 h). Data are expressed as mean ± SEM (n = 4). *P < 0.05
TRAIL-induced apoptosis in GSCs is inhibited by c-FLIP
TRAIL-resistant tumor lines such as U87MG express high levels of c-FLIPL and c-FLIPS relative to TRAIL-sensitive lines such as LN18 [12], and c-FLIP overexpression inhibits caspase cleavage and suppresses TRAIL-induced apoptosis in resistant cells [13]. Both c-FLIPL and c-FLIPS were expressed at higher levels in GSCs (no. 1 and no. 2) than in PGC-no. 1 or PGC-no. 2 (Fig. 3a). To test if this higher c-FLIP expression contributes to TRAIL resistance, expression was knocked down prior to TRAIL exposure and apoptosis assays. GS-no. 1 cells were transfected with siRNA against nucleotides 535–555 common to both isoforms of the c-FLIP transcript, and transfected cells were grown under glioma sphere culture conditions. Knockdown of both c-FLIP proteins was confirmed 72 h after siRNA transfection by Western blotting (Fig. 3b). Cultures of GSC-no. 1 transfected with the siRNA exhibited a markedly higher percentage of apoptotic cells after 24 h in 100 ng/ml TRAIL compared to GC-no. 1 cells transfected with a scrambled control siRNA. Thus, under reduced c-FLIP expression, GSC phenotype was transformed from TRAIL-resistant to TRAIL-sensitive (Fig. 3b), strongly suggesting that endogenous c-FLIP inhibits TRAIL-induced apoptosis in GSCs. Furthermore, c-FLIPL overexpression induced TRAIL resistance in PGCs (S1 Fig).
Reciprocal relationship between expression levels of c-Fas-associated death domain-like interleukin 1-converting enzyme-like inhibitory protein (c-FLIP) and sensitivity to TRAIL-induced apoptosis. a Expression levels of both the long (c-FLIP L ) and short (c-FLIP S ) isoforms of the protein were higher in GSC-no. 1 and -no. 2 compared to TRAIL-sensitive PGC-no. 1 and -no. 2 as determined by Western blotting. b Knockdown of c-FLIPL and c-FLIPS protein expression in GSC-no. 1 by siRNA transfection for 72 h (as detected by Western blotting) significantly increased TRAIL-induced apoptosis in GSCs (100 ng/ml TRAIL for 24 h). Data are expressed as mean ± SEM (n = 4). *P < 0.05
TMZ stimulates c-Cbl and suppresses c-FLIP expression in GSCs
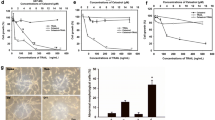
To determine whether TRAIL resistance in GSCs can be overcome by treatment with a chemotherapeutic agent, GSC-no. 1 and -no. 2 cultures were incubated with serial dilutions of TMZ with or without TRAIL. While GSCCs were resistant to the pro-apoptotic effects of TMZ alone, the combination of TMZ and TRAIL induced substantial apoptosis in both GSC-no. 1 and -no. 2 (Fig. 4a). Moreover, the combination of 100 ng/ml TRAIL and 20 μmol/l TMZ increased caspase activity in GSCs (Fig. 4b). These findings indicate that TMZ and TRAIL synergistically promote apoptosis of stem-like cells from glioma spheres. We then tested if the toxicity of TMZ plus TRAIL was mediated by changes in c-FLIP expression. Increased c-Cbl expression and decrease in c-FLIPL and c-FLIPS expression levels were detected in GSC-no. 1 and -no. 2 following TMZ drug treatment (Fig. 4c). Ubiquitination is a post-translational modification that targets cellular proteins for degradation; c-Cbl is a member of the Cbl E3 ubiquitin ligase family. Recent studies have implicated c-Cbl in the ubiquitination and degradation of c-FLIPS in TNF-treated mouse macrophage cells [6]. Overexpression of the E3 ubiquitin ligase c-Cbl downregulated both c-FLIPL and c-FLIPS expression in GSCs (S2 Fig.). To determine whether c-Cbl-mediated ubiquitination and degradation of c-FLIPS mediate the effects of TMZ on TRAIL sensitivity, GSC-no. 1 and-no. 2 were also pretreated for 30 min with the proteasome inhibitor PS341 prior to combined application of 100 ng/ml TRAIL and 20 μmol/l TMZ for 24 h. Upregulation of c-FLIPL and c-FLIPS expression relative to control cells was observed under these conditions (Fig. 4d). In addition, siRNA knockdown of c-Cbl in GSC cells resulted in upregulation of c-FLIPL and c-FLIPS protein levels (Fig. 4e) and abolished the toxic effect of TMZ and TRAIL (Fig. 4f, g).
The chemotherapeutic agent temozolomide (TMZ) sensitizes GSCs to TRAIL-induced apoptosis through upregulation of the E3 ubiquitin ligase casitas B-lineage lymphoma (c-Cbl) and concomitant c-FLIPS downregulation. a Analysis of cell death in GSC-no. 1 and -no. 2 after 24 h of treatment with the indicated doses of TMZ alone or TMZ plus 100 ng/ml TRAIL. b Caspase activity as evaluated by absorption spectroscopy was markedly enhanced by addition of TMZ. c Protein expression levels of c-Cbl were enhanced, while c-FLIPL and c-FLIPS levels were reduced by TMZ treatment (24 h) as measured by Western blotting. d Expression levels of c-Cbl, c-FLIPL, and c-FLIPS were measured in the presence or absence of the protease inhibitor PS341 (10 nM) after combined treatment with 100 ng/ml TRAIL and 20 μmol/l TMZ for 24 h. e Knockdown of c-Cbl in GS-No. 1 was detected by Western blotting after transfection with c-Cbl siRNA for 72 h. f Cell death was examined by the apoptosis assay in c-Cbl-siRNA-transfected GSC-No. 1 cells after 24 h of treatment with the indicated doses of TMZ alone or in combination with 100 ng/ml TRAIL. g GSC-No. 1-transfected glioma spheres were treated with 100 ng/ml TRAIL for 24 h, and cell death was assessed by the apoptosis assay. Data are expressed as mean ± SEM (n = 4). *P < 0.05
c-FLIP expression is correlated with glioma grade
Expression data in the CGGA database showed that c-FLIP expression was significantly higher in grade IV tumors (GBM) than in grades II and III (P < 0.0001 versus both), indicating a strong correlation between high c-FLIP expression and glioma malignancy (S3 Fig.).
Discussion
Gliomas are the most common intracranial malignancies in adults, and the prognosis remains poor despite advances in chemotherapeutic, surgery, and radiation treatments. Multiple studies have demonstrated the existence of cancer stem cells in tumors, including brain [14, 15], colon [16, 17], and breast [18, 19] cancers, that likely have important roles in tumor proliferation, invasion, recurrence, and resistance to conventional treatments. In the last decade, extensive research has been conducted on GBM-derived cell lines in order to establish novel therapeutic approaches to GBM treatment, including targeting of the TRAIL apoptotic pathway [20]. Indeed TRAIL has proven cytotoxicity in GBM-derived cell lines and their derivative xenografts [21, 22]. However, there are many disadvantages of cell lines that have been cultured for many generations for treatment evaluation, so many recent studies have used tumor stem cells as a model to test the efficacy of new drugs. The present study demonstrates for the first time that TMZ enhances TRAIL-induced apoptosis of human GSCs by promoting c-Cbl-mediated proteasomal degradation of the anti-apoptotic c-FLIP proteins.
Glioma spheres were used to evaluate the efficacy of TRAIL treatment. Glioma spheres propagated from human malignant glioma tissue consist mainly of brain tumor stem cells [23] as confirmed by genetic analysis [16, 24, 25] and CD133-positive status [15, 23]. Based on these reports, CD133 as well as nestin and SOX-2 were used to identify GSCs in this study; indeed all three markers, but not the differentiated glial marker GFAP, were expressed by glioma spheres. Significantly, only a small number of these GSCs were required to form brain tumors in NOD-SCID mice, confirming that they possess stem cell properties.
Chemotherapy is the predominant maintenance treatment for delaying the recurrence of glioma. Extensive research has been conducted on TRAIL therapy in GBM cell lines [20], with potent TRAIL-induced apoptosis through death receptors 4 and 5 widely reported [26, 27]. In a previous study, TRAIL-sensitive and -resistant glioma cell lines expressed c-FLIP at similar levels [12]. However, in this study, the expression levels of c-FLIPL and c-FLIPS were significantly higher in GSCs than (TRAIL-sensitive) PGCs, and GSCs acquired sensitivity to TRAIL-induced apoptosis after siRNA-mediated knockdown of c-FLIP proteins. These findings suggest that high levels of c-FLIP can suppress TRAIL-induced apoptosis, thereby conferring TRAIL tolerance in GSC-derived cells. We further showed that the apoptosis induced by TRAIL in glioma cells was dependent on the expression and enzymatic activity of caspase, consistent with the results of other studies.
TRAIL resistance in other types of cancer can be overcome by combined radio- and chemotherapy [28–30]. TMZ is widely used to treat glioma patients, and we therefore attempted to determine whether the concurrent application of TMZ could overcome TRAIL resistance in GSCs. Upregulation of caspase 8 expression has been linked to TRAIL plus TMZ therapeutic efficiency in glioblastoma [31, 32]. The present study found that TMZ enhanced TRAIL-induced apoptosis in GSCs, consistent with the results of other studies [33, 34]. Following TMZ application, the expression of the E3 ligase c-Cbl was upregulated, while c-FLIPL and c-FLIPS were downregulated, suggesting that potentiation of TRAIL toxicity by TMZ is due to c-Cbl-mediated degradation of c-FLIP proteins, which normally inhibit receptor-activated cell death pathways. The role of c-Cbl protein as a mediator of TMZ-induced apoptosis in TRAIL-treated GSCs was confirmed by application of the protease inhibitor PS341 and siRNA knockdown of c-Cbl, both of which restored TRAIL insensitivity of GSCs. Taken together, these results strongly suggest that TMZ enables GSCs to overcome TRAIL resistance by suppressing c-FLIP protein expression, possibly by promoting c-Cbl-mediated ubiquitination and protein degradation (Fig. 5). Most interestingly, when treated with TMZ alone, knocking down of CbI protein would enhance the cell death rate of glioma stem cells. However, as showed in Fig. 4f, when treated with TMZ combined with TRAIL, knocking down of CbI protein decreased the cell death rate, which is consistent with the result of Fig. 4g. This is an interesting phenomena, which indicated that CbI my play different role when treated with TMZ alone or treated with TMZ combined with TRAIL, further experiments are needed to clarify this phenomena.
Proposed model of increased glioma stem-like cell (GSC) sensitivity to TRAIL-induced apoptosis in the presence of TMZ. TMZ promotes c-Cbl-mediated ubiquitination and degradation of cell death pathway inhibitor c-FLIP in GSCs, thereby allowing TRAIL to activate death receptors (DR4 and DR5) and downstream apoptotic pathways
Recently, It is demonstrated that Bak and Mcl-1 are essential for Temozolomide induced cell death in human glioma [35] and TMZ treatment could downreguate Bcl2 protein in glima [36, 37]. We have tested the expression of Bcl2 family proteins in glioma stem-like cells after treatment with TMZ, but the expression level of Bcl-2 family proteins did not show any difference compared with the control group (data not shown), which is different with the result in non-stem-like glioma cells [35–37]. This result may indicate the difference between glioma stem-like cells and non-stem-like cells. Recently, targeting and/or co-targeting Bcl-2 and Bax were proposed as promising strategies for cancer therapy [38–40]. Combination therapy with TMZ and TRAIL and Bcl-2 inhibitor and/or Bax activator for the treatment of the GSCs may be worthy of further investigation.
Experiments using cell lines established from various cancers have tended to overestimate the clinical efficacy of TRAIL for several reasons. First, the blood–brain barrier may limit the anti-glioma activity of TRAIL as well as antibodies in patients. Second, certain molecules that are highly expressed in GSCs may disrupt the TRAIL-mediated apoptotic pathway. Using cells that more accurately reflect the in vivo behavior of glioma cells should provide a better indication of drug efficacy. Microarray data from the GCCG database indicate that c-FLIP expression is higher in grade IV than in grades II or III gliomas, suggesting that the grade IV type (GBM) is resistant to TRAIL treatment, a resistance that may potentially be overcome by concurrent treatment with TMZ. This study provides experimental basis for the combined treatment of glioma stem cells with TRAIL and TMZ. The effectiveness of this treatment strategy needs to be evaluated in animal models or clinically. We did not perform in vivo study in this experiment since this is not the focus of this study. A future in vivo study is thus needed.
Conclusion
The results of this study demonstrate that TMZ treatment sensitizes GSCs to TRAIL-induced apoptosis by negatively regulating c-FLIPL and c-FLIPS expression via the ubiquitin ligase activity of c-Cbl. These findings indicate that combining TMZ with TRAIL may be an effective treatment for glioma, warranting future studies in a glioma animal model.
References
Clarke J, Butowski N, Chang S. Recent advances in therapy for glioblastoma. Arch Neurol. 2010;67(3):279–83. doi:10.1001/archneurol.2010.5.
Lo Cicero A, Schiera G, Proia P, Saladino P, Savettieri G, Di Liegro CM, et al. Oligodendroglioma cells shed microvesicles which contain TRAIL as well as molecular chaperones and induce cell death in astrocytes. Int J Oncol. 2011;39(6):1353–7. doi:10.3892/ijo.2011.1160.
Syed V, Mukherjee K, Godoy-Tundidor S, Ho SM. Progesterone induces apoptosis in TRAIL-resistant ovarian cancer cells by circumventing c-FLIPL overexpression. J Cell Biochem. 2007;102(2):442–52.
Sciuscio D, Diserens AC, van Dommelen K, Martinet D, Jones G, Janzer RC, et al. Extent and patterns of MGMT promoter methylation in glioblastoma- and respective glioblastoma-derived spheres. Clin Cancer Res. 2011;17(2):255–66. doi:10.1158/1078-0432.CCR-10-1931.
MacFarlane M, Harper N, Snowden RT, Dyer MJ, Barnett GA, Pringle JH, et al. Mechanisms of resistance to TRAIL-induced apoptosis in primary B cell chronic lymphocytic leukaemia. Oncogene. 2002;21(44):6809–18.
Kundu M, Pathak SK, Kumawat K, Basu S, Chatterjee G, Pathak S, et al. A TNF- and c-Cbl-dependent FLIP(S)-degradation pathway and its function in Mycobacterium tuberculosis-induced macrophage apoptosis. Nat Immunol. 2009;10(8):918–26. doi:10.1038/ni.1754.
Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–72.
Ivanov VN, Hei TK. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis. 2014;19(3):399–413. doi:10.1007/s10495-013-0925-4.
Morizot A, Mérino D, Lalaoui N, Jacquemin G, Granci V, Iessi E, et al. Chemotherapy overcomes TRAIL-R4-mediated TRAIL resistance at the DISC level. Cell Death Differ. 2011;18(4):700–11. doi:10.1038/cdd.2010.144.
Qiu B, Zhang D, Wang C, Tao J, Tie X, Qiao Y, et al. IL-10 and TGF-b2 are overexpressed in tumor spheres cultured from human gliomas. Mol Biol Rep. 2011;38(5):3585–91. doi:10.1007/s11033-010-0469-4.
Crowder RN, El-Deiry WS. Caspase-8 regulation of TRAIL-mediated cell death. Exp Oncol. 2012;34(3):160–4.
Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir EG, Yong VW, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res. 2001;61(3):1162–70.
Bellail AC, Tse MC, Song JH, Phuphanich S, Olson JJ, Sun SY, et al. DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J Cell Mol Med. 2010;14(6A):1303–17. doi:10.1111/j.1582-4934.2009.00777.x.
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401.
Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23(58):9392–400.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–5.
O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–10.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63.
Liao MJ, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, et al. Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 2007;67(17):8131–8.
Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4(1):34–41.
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104(2):155–62.
Roth W, Isenmann S, Naumann U, Kügler S, Bähr M, Dichgans J, et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265(2):479–83.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8.
Huang S, Yang ZJ, Yu C, Sinicrope FA. Inhibition of mTOR kinase by AZD8055 can antagonize chemotherapy-induced cell death through autophagy induction and down-regulation of p62/sequestosome 1. J Biol Chem. 2011;286(46):40002–12. doi:10.1074/jbc.M111.297432.
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403.
Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8(10):782–98. doi:10.1038/nrc2465.
Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002;277(28):25020–5.
Niemoeller OM, Belka C. Radiotherapy and TRAIL for cancer therapy. Cancer Lett. 2013;332(2):184–93. doi:10.1016/j.canlet.2011.07.003.
Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6(2):335–46.
Fiveash JB, Gillespie GY, Oliver PG, Zhou T, Belenky ML. Enhancement of glioma radiotherapy and chemotherapy response with targeted antibody therapy against death receptor 5. Int J Radiat Oncol Biol Phys. 2008;71(2):507–16. doi:10.1016/j.ijrobp.2008.02.005.
Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64(19):6858–62.
Hingtgen S, Ren X, Terwilliger E, Classon M, Weissleder R, Shah K. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and temozolomide. Mol Cancer Ther. 2008;7(11):3575–85. doi:10.1158/1535-7163.
Uzzaman M, Keller G, Germano IM. Enhanced proapoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand on temozolomide-resistant glioma cells. J Neurosurg. 2007;106(4):646–51.
Kim SM, Woo JS, Jeong CH, Ryu CH, Jang JD, Jeun SS. Potential application of temozolomide in mesenchymal stem cell-based TRAIL gene therapy against malignant glioma. Stem Cells Transl Med. 2014;3(2):172–82. doi:10.5966/sctm.2013-0132.
Gratas C, Séry Q, Rabé M, Oliver L, Vallette FM. Bak and Mcl-1 are essential for temozolomide induced cell death in human glioma. Oncotarget. 2014;5(9):2428–35.
Dai C, Zhang B, Liu X, Ma S, Yang Y, Yao Y, et al. Inhibition of PI3K/AKT/mTOR pathway enhances temozolomide-induced cytotoxicity in pituitary adenoma cell lines in vitro and xenografted pituitary adenoma in female nude mice. Endocrinology. 2013;154(3):1247–59. doi:10.1210/en.2012-1908.
Qi XC, Xie DJ, Yan QF, Wang YR, Zhu YX, Qian C, et al. RIG1 dictates the chemo-sensitivity of temozolomide (TMZ) in U251 glioblastoma cells via down-regulation of EGFR/topoisomerase-2/Bcl-2. Biochem Biophys Res Commun. 2013;437(4):565–72. doi:10.1016/j.bbrc.2013.06.116.
Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24(1):120–9. doi:10.1016/j.ccr.2013.06.002.
Xin M, Li R, Xie M, Park D, Owonikoko TK, Sica GL, et al. Small-molecule Bax agonists for cancer therapy. Nat Commun. 2014;5:4935. doi:10.1038/ncomms5935.
You S, Li R, Park D, Xie M, Sica GL, Cao Y, et al. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol Cancer Ther. 2014;13(3):606–16. doi:10.1158/1535-7163.
Acknowledgments
This work was supported by the Chinese National Natural Science Foundation (nos. 81101917 and 81172409) (http://www.nsfc.gov.cn/) SL and by the Liaoning Province Natural Science Foundation (no. 2013021045) (http://www.lninfo.gov.cn/).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1 Fig
Role of c-Fas-associated death domain-like interleukin 1-converting enzyme-like inhibitory protein long isoform (c-FLIPL) in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in primary glioma cells (PGC-No.1 and PGC-No.2). (PDF 94 kb)
S2 Fig
Immunoblot analysis c-FLIPL and c-FLIPS expression in GSC-No.1 and GSC-No.2 transfected with control or c-Cbl cDNA. (PDF 26 kb)
S3 Fig
A total of 295 samples from the Chinese Glioma Genome Atlas (CGGA) database were analyzed; data included whole genome mRNA expression profiles from microarray analyses and corresponding clinical information. The relationship between c-FLIP mRNA expression and glioma grade (II–IV) is shown. (PDF 30 kb)
Rights and permissions
About this article
Cite this article
Zhitao, J., Long, L., Jia, L. et al. Temozolomide sensitizes stem-like cells of glioma spheres to TRAIL-induced apoptosis via upregulation of casitas B-lineage lymphoma (c-Cbl) protein. Tumor Biol. 36, 9621–9630 (2015). https://doi.org/10.1007/s13277-015-3720-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3720-8