Abstract
Objectives
Resveratrol (RV), a natural polyphenol derived from red wine, recently showed the potential of anticancer and radiosensitizing effects. A recent study has suggested that the cancer stem cells (CSCs) may reflect the clinical refractory malignancy of brain tumors, including medulloblastoma (MB). The aim of the present study is to investigate the possible role of RV in radiosensitivity of MB cells and MB-associated CSCs.
Materials and methods
MB-associated CSCs were isolated and cultured by serum-free medium with basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF). The parental MB cells and MB-CSCs were treated with RV in different concentrations and assessed for cell viability. The treatment includes RV alone, radiation alone, or radiation combined with RV.
Results
MB-CSCs selected by serum-free medium with bFGF and EGF can form 3D spheroid formation and display enhanced self-renewal and highly co-expressed “stem cell” genes (Oct-4, Nanog, Nestin, and Musashi-1) as well as antiapoptotic genes (Bcl-2 and Bcl-xL). These MB-CSCs showed significant resistance to radiotherapy as compared to the parental MB cells. Importantly, 100 μM RV could effectively inhibit the proliferation of MB-CSCs and significantly enhance the radiosensitivity in RV-treated MB-CSCs.
Conclusions
Our data suggest that RV can effectively inhibit the proliferation and tumorigenicity of MB-CSCs and significantly synergistically enhance radiosensitivity in RV-treated MB-CSCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Medulloblastoma (MB) is a malignant cerebellum tumor predominantly found in children, accounting for 13–20% of all pediatric central nervous system tumors [7, 19, 33]. As for remedy of MB, surgical excision alone is not curative[21, 33]. Virtually all tumors recur and patients die within 3 years without adjuvant treatment [21]. Local or disseminated whole neuroaxis relapse can be seen even after standard radiation treatment, including craniospinal axis irradiation and focal boost [7, 19, 21, 33]. Besides, radiotherapy has its own side effects and may cause pituitary hormone dysfunction and behavior problems [16, 21]. Thus, to improve the therapeutic outcome and promote the life quality of the survivors, novel therapeutic agents and radiosensitizers are urgently needed.
Resveratrol (RV; 3,4′,5-tri-hydroxy-trans-stilbene), a natural polyphenol, is mostly found in grapes, red wine, and peanuts [14]. RV possesses many pharmacological effects that are closely related to health therapies including antioxidant stress, anti-inflammatory, antiviral, cardiac protection, neuroprotection, antiaging activities, and life span extension [6, 14, 15]. Importantly, recent researches demonstrated that RV has an anticancer effect and inhibits tumorigenesis by inducing apoptosis via Fas-, P53-, and P21WAF/CIP1-mediated pathways [1, 26, 30]. Furthermore, some reports indicated that RV can also increase radiosensitivity in several cancer cell lines including melanoma, cervix carcinoma, chronic myeloid leukemia (K-562), and multiple myeloma (IM-9) [2, 23]. However, the RV-mediated radiosensitizing effects in the treatment of MB and MB-associated cancer stem cells were still undetermined.
In the previous study, we used serum-free medium that contained basic fibroblast growth factor (bFGF) and epidermal growth factor (EGF) growth factors to enrich the subpopulation of normal neural stem cell from hippocampal region of rodent brain [9] and cancer stem-like cells (CSCs) derived from brain tumors [10]. In this present study, the specific aim is to investigate the possible role of RV in antiproliferative and radiosensitizing effects in MB cells and MB-CSCs and further clarify the mechanism.
Materials and methods
Isolation of the subsets of cancer stem-like cells from medulloblastoma tissues
This research followed the tenets of the Declaration of Helsinki and all samples were obtained after patients had given informed consent. Dissociated cells from the samples of medulloblastoma patients were cultured in serum-free Dulbecco’s modified Eagle’s medium (DMEM)/F12 (GIBCO) medium supplemented with N2 supplement (R&D), 10 ng/ml human recombinant bFGF (R&D), and 10 ng/ml EGF [10]. Gamma radiation was delivered by a Theratronic cobalt unit T-1000 (Theratronic International, Inc., Ottawa, Canada) at a dose rate of 1.1 Gy/min (SSD = 57.5 cm). For evaluation of cell proliferation, cells were seeded on 24-well plates at a density of 2 × 104 cells per well in medium, followed by the methyl thiazole tetrazolium assay (MTT assay; Sigma-Aldrich Co.) [10].
Immunophenotypic analysis
For cell surface antigen phenotyping, the different passage cells were detached and stained with anti-CD133 with secondary phycoerythrin-coupled antibodies (Miltenyi Biotec). Bone-marrow-derived stromal cells were fixed with 2% paraformaldehyde until they were ready for analysis using FACSCalibur apparatus (Becton-Dickinson).
Real-time RT-PCR
Real-time reverse-transcription polymerase chain reaction (RT-PCR) was performed as previously described [25]. Briefly, total RNA (1 μg) of each sample was reverse-transcribed in a 20 μl volume using 0.5 μg oligo dT and 200 U Superscript II RT (Invitrogen, Carlsbad, CA, USA). The primer sequences used for real-time RT-PCR are shown in Table 1. The amplification was carried out in a total volume of 20 μl containing 0.5 μM in each primer, 4 mM MgCl2, 2 μl LightCycler™-FastStart DNA Master SYBR green I (Roche Molecular Systems, Alameda, CA, USA), and 2 μl of 1:10 diluted complementary DNA. PCR reactions were prepared in duplicate and heated to 95°C for 10 min followed by 40 cycles of denaturation at 95°C for 10 s, annealing at 55°C for 5 s, and extension at 72°C for 20 s. Standard curves (cycle threshold values versus template concentration) were prepared for each target gene and for the endogenous reference (GAPDH) in each sample. Quantification of unknown samples was performed using LightCycler Relative Quantification Software version 3.3 (Roche Molecular Systems, Alameda, CA, USA).
In vitro cell invasion analysis and soft agar assay
The 24-well plate Transwell® system with a polycarbonate filter membrane (8-μm pore size; Corning, UK) was used. Cell suspensions were seeded in the upper compartment of the Transwell chamber at a density of 1 × 105 cells in 100-μl serum-free medium. The opposite surface of the filter membrane, which faced the lower chamber, was stained with Hoechst 33342 for 3 min and migrating cells were visualized under an inverted microscope. The soft agar assay was performed as follows. The bottom of each well (35 mm) of a six-well culture dish was coated with 2-ml agar mixture (DMEM, 10% (v/v) fetal calf serum (FCS), 0.6% (w/v) agar). After the bottom layer solidified, 2-ml top agar–medium mixture (DMEM, 10% (v/v) FCS, 0.3% (w/v) agar) containing 2 × 104 cells was added and incubated at 37°C for 4 weeks. The plates were stained with 0.5 ml 0.005% Crystal Violet and then the number of colonies was counted using a dissecting microscope.
Statistical analysis
The results are reported as mean ± SD. Statistical analysis was performed using Student’s t test or a one-way or two-way analysis of variance test followed by Turkey’s test, as appropriate. p < 0.05 was considered to be statistically significant.
Results
Isolation and characterization of cancer stem-like cells from medulloblastoma
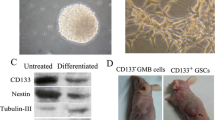
It has been reported that cancer stem cells and glioma stem cells can be cultured and enriched in suspension to generate floating spheroid-like bodies (SB) and maintain the self-renewal capabilities in serum-free media with bFGF and EGF [20, 29, 31]. For 1 month under DF-12 serum-free medium with bFGF and EGF [9, 10], the suspended SB CSCs were successfully isolated from medulloblastoma samples (Fig. 1a, b). These CSCs derived from MB could stably proliferate to form SB in serum-free medium with bFGF and EGF. The result of a FACSscan showed that MB-CSCs derived from SB under serum-free/bFGF/EGF medium could be stained positively for the marker of brain tumor stem cell—CD133. In contrast, the parental tumor cells derived from MB were primarily cultured and attached on the dish under serum-contained medium (traditional formula; Fig. 1c). It only presented the very low level of CD133 antigen in these parental MB cells (Fig. 1d). Importantly, the percentage of CD133-positive cells in MB-CSC was gradually and significantly increased under serum-free media with bFGF and EGF for a 3-month culture (Fig. 2a; p < 0.01) but not detected in primary parental cancer cells even after a 3-month serum-condition culture (Fig. 2a). Furthermore, quantitative real-time RT-PCR showed that the messenger RNA expression levels of stem-cell-related genes (Oct-4, Nanog, Nestin, and Musashi-1) and antiapoptotic (Bcl-2 and Bcl-xL) were upregulated in MB-CSC as compared to the parental MB cells (Fig. 2b; p < 0.01). In sum, our data indicated that the spheroid-like MB cells (MB-CSCs) selected by serum-free media with bFGF and EGF present the characteristics of cancer stem-like cells.
Isolation cancer stem-like cells from medulloblastoma. a Under DF-12 serum-free medium with bFGF and EGF, MB-derived cancer stem-like cells were isolated in suspension to generate floating spheroid-like bodies and maintain the self-renewal capabilities in serum-free media with bFGF and EGF (bar 100 mm). b The result of flow cytometry showed that the high percentage of CD133-positive cells was detected in MB-CSCs. c The parental tumor cells derived from MB were primarily cultured and attached on the dish under serum-contained medium (traditional formula: DMEM containing 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 units per milliliter penicillin and 100 μg/ml streptomycin; bar 60 μm). d In contrast to MB-CSCs, only the very low level of CD133 antigen was detected in these parental MB cells
Detection of the expression levels of stem cell markers in MB-CSCs and parental MB cell. a The result of a FACSscan showed that the percentage of CD133-positive cells in MB-CSC was gradually and significantly increased under serum-free conditional media for a 3-month culture. It only presented the very low level of CD133 antigen in these parental MB cells even after a 3-month serum-condition culture. b Real-time RT-PCR analyses revealed the amounts of Oct-4, Nanog, Nestin, Musashi, Bcl-2, Bcl-xL, and Bax transcripts in MB-CSCs and parental MB cells. The internal control is housekeeping gene-GAPDH and every stemness gene expression of MB-CSCs was normalized with parental MB cells. Data shown are the mean ± SD of three experiments
Evaluation of cytotoxic effects of resveratrol in MB-CSC and parental tumors
RV has been recently suggested to inhibit tumor growth [2, 23, 26, 30]. However, it remains undetermined whether RV can inhibit the CSC properties of MB-CSC. To answer this question, MB-CSCs were treated with different doses of RV and cell viability was analyzed using the MTT assay. As shown in Fig. 3, MB-CSCs (Fig. 3a) were treated with RV at different concentrations (0, 10, 50, 100, and 150 μM) for 48 h. Cell viability of MB-CSC was not significantly affected if the concentration of RV was lower than 50 μM (p > 0.05; Fig. 3c). After 48-h treatment with 100 μM RV, the spheroid-like MB-CSCs detached and became a single suspension (Fig. 3b). The total cell number and growth rates of MB-CSCs after being treated with 150 μM RV for 48 h were significantly decreased (p < 0.01; Fig. 3c). In contrast, the cell viability of parental MB cells (serum-condition culture) could be moderately affected by 50 μM RV and significantly decreased by the treatment of 50 μM RV (p < 0.01; Fig. 3c)
Evaluation of cytotoxic effects of resveratrol in MB-CSCs and parental MB cells. a The morphology of MB-CSCs without any treatment (control). b The morphology of MB-CSCs treated with 100 mM RV for 48 h. c The cell viability of MB-CSCs and parental MB cells treated with 0, 10, 50, 100, and 150 μM RV for 48 h was analyzed by MTT assay. Data shown are the mean ± SD of three experiments
Enhanced radiosensitivity of MB-CSCs after treatment with resveratrol
Recent studies supported that RV plays a key role in the synergetic treatment for anticancer [2, 23]. In Fig. 3, the viability of MB-CSCs was reduced by 40–45% when the concentration of RV was 100 μM, and these data suggested that 100 μM RV leads to a significant cytotoxic effect in treated MB-CSCs. To further investigate the role of RV in synergetic treatment for clinical use of MB and MB-CSCs, the optimal concentration of RV as a radiosensitizer for radiotherapy against MB-CSCs was further tested. By applying ionizing radiation (IR) doses from 0 to 10 Gy to the two groups of cells, the results further confirmed that MB-CSCs showed greater radioresistance than the parental MB cells (p < 0.01; Fig. 4). Furthermore, the treatment effect of IR-2 Gy on MB-CSCs was also significantly improved with the addition of 100 μM RV (p < 0.01; Fig. 4). We further evaluated the in vitro tumorigenic ability of MB-CSCs before and after the RV treatment. Compared with the IR (2 Gy) treatment alone, migration/invasion (Fig. 5a) and tumor colony formation (Fig. 5b) were significantly inhibited in MB-CSCs treated with 100 μM RV alone or 100 μM RV combined with 2-Gy IR. These data provide evidence that the effectiveness and radiosensitivity of radiation treatment for MB-CSCs can be improved with RV treatment.
Determination of radiotherapy effect in MB-CSCs and parental MB cells with or without RV. a To determine the effect of radiation on tumor growth rate, an ionizing radiation dose from 0 to 10 Gy was used to treat MB-CSCs and parental MB cells. *p < 0.01: MB-CSCs vs. parental MB cells. Survival fraction of MB-CSCs treated with RV (100 μM) and exposed to 0–10-Gy radiation. Data were compared with IR-treated parental MB cells. Data shown are the mean ± SD of three experiments
Discussion
The clinicopathological observations have found that perhaps only a few subsets of cancer cells were able to proliferate extensively, whereas most cancer cells had only a limited ability to proliferate [13, 32]. Recent studies demonstrated that tumors contain a small subpopulation of cells, i.e., cancer stem cells or cancer-initiating cells, which exhibit a self-renewing capacity and are responsible for tumor heterogeneity, maintenance, and metastasis [32]. CD133 (prominin-1: PROM1), a five-transmembrane glycoprotein, was identified as an important marker for a subset of CSCs in leukemia, retinoblastoma, colon cancer, prostate carcinoma, brain tumor, and hepatoma [20, 28, 29, 31]. Recent reports suggested that expression of CD133 antigen in gliomas, medulloblastoma, and other brain tumors could serve as a prognostic indicator for tumor regrowth, malignant progression, and patient survival [4, 20, 31, 35].
In this study, we reported that the MB-CSCs can form the 3D spheroid bodies and exhibit the high percentage of CD133 surface antigen under the serum-free conditional media culture (Figs. 1 and 2). These cells showed strong capabilities of migration and tumor formation in vitro and displayed significant resistance to radiotherapy (Figs. 3 and 5). The expression of embryonic stem cell genes such as Oct-4 and Nanog has been correlated with tumorigenesis and self-renewing activity and can affect some aspects of tumor behavior such as recurrence and resistance to therapy [5, 24, 28]. Recently, the expression of Oct-4 and Nanog was shown in CSC derived from human oral, breast, and brain tumors, suggesting that their expression may be implicated in self-renewal and tumorigenesis via activating downstream target genes [5, 11, 17]. Furthermore, Nestin and Musashi-1 constitutively express in the neural crest, embryonic brain tissues, and hippocampal progenitors [18]. Indeed, Nestin, Musashi-1, and CD133 have been suggested to be the markers for neural stem cells or neural progenitors [18, 32]. Using real-time quantitative RT-PCR method, we showed that enriched MB-CSCs highly expressed embryonic stem cell markers (Oct-4 and Nanog) and neural stem cell markers (Nestin, Musashi-1, and CD133) as well as antiapoptotic genes (Bcl-2 and Bcl-xL; Fig. 2). This finding suggested that the spheroid-like MB cells selected by serum-free conditional media present CSC properties, self-renewal capability, and the high expression of embryonic stem cell genes as well as neural stem cell genes.
The property of resistance to radiation therapy or chemotherapy is the major clinical criteria to characterize “CSCs” [24]. The existence of CSCs may explain why the conventional anticancer therapies only can suppress a tumor but often cannot completely eradicate it resulting in tumor relapse [12, 13, 24]. Thus, the characteristics of CSCs are their resistance to therapy and are suggested to be responsible for disease recurrence and possibly even metastasis [32]. Consistent with this CSC hypothesis, our data showed that spheroid-body-like MB-CSCs were significantly resistant to radiotherapy as compared to the parental MB cells. Recently, Bao et al. [3] demonstrated that the fraction of tumor cells expressing CD133, a marker for both neural stem cells and brain cancer stem cells, was enriched after radiation in gliomas. These CD133-positive cells (CD133+) play a critical role not only in the restoration of tumor cells and CSCs but also in their resistance to radiotherapy [3]. In order to improve the therapeutic outcome of MB by targeting MB-CSCs or CD133+, novel effective agents and radiosensitizers are needed. Previous studies suggested that RV could increase radiosensitivity via several mechanisms, including inactivation of nuclear factor κB and increased S-phase cell cycle arrest [27]. Yu et al. [34] has suggested that RV could inhibit the activation of STAT3 signaling of MB cells and may further commit MB cells to growth arrest and apoptosis. Furthermore, the recent studies showed that RV-induced apoptosis not only inhibits tumor growth but also acts as a radiochemosensitizer for anticancer therapy [2, 8, 22, 23]. To further explore and establish RV as a possible future treatment for brain-tumor-associated CSCs, we demonstrated that RV can inhibit the growth of MB-CSCs and that pretreatment with 150 μM RV markedly decreased the viability of MB-CSCs (Fig. 2). Importantly, the treatment of 100 μM RV effectively enhanced radiosensitivity in RV-treated MB-CSCs (Fig. 4) and further significantly inhibited the in vitro tumorigenic capabilities in RV-treated MB-CSCs. These findings support the antiproliferative, radiosensitizing, and anticancer stem cell effects of RV on MB-CSCs and RV’s potential use to improve the clinical treatment of malignant MB as well as cancer stem cells.
Conclusion
In summary, we demonstrated that the spheroid-like MB cells selected by serum-free condition medium exhibit CSC properties and are refractory to IR treatment. These MB-CSCs also present the higher self-renewal ability that might represent a reservoir with unlimited proliferative potential for generating brain tumors. This CSC property in MB and other tumors should not be neglected in future translational oncology studies. Indeed, our results indicate that RV treatment plays crucial roles in both anticancer and radiosensitizing effects on treated MB-CSCs. RV may therefore improve the clinical radiation therapy for patients with pediatric brain tumors.
References
Atten MJ, Godoy-Romero E, Attar BM, Milson T, Zopel M, Holian O (2005) Resveratrol regulates cellular PKC alpha and delta to inhibit growth and induce apoptosis in gastric cancer cells. Invest New Drugs 23:111–119
Baatout S, Derradji H, Jacquet P, Ooms D, Michaux A, Mergeay M (2004) Enhanced radiation-induced apoptosis of cancer cell lines after treatment with resveratrol. Int J Mol Med 13:895–902
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444:756–760
Beier D, Wischhusen J, Dietmaier W, Hau P, Proescholdt M, Brawanski A, Bogdahn U, Beier CP (2008) CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol 18:370–377
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40:499–507
Bradamante S, Barenghi L, Villa A (2004) Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev 22:169–188
Cervoni L, Cantore G (1995) Medulloblastoma in pediatric age: a single-institution review of prognostic factors. Childs Nerv Syst 11:80–84, discussion 85
Chakraborty PK, Mustafi SB, Ganguly S, Chatterjee M, Raha S (2008) Resveratrol induces apoptosis in K562 (chronic myelogenous leukemia) cells by targeting a key survival protein, heat shock protein 70. Cancer Sci 99:1109–1116
Chiou SH, Ku HH, Tsai TH, Lin HL, Chen LH, Chien CS, Ho LL, Lee CH, Chang YL (2006) Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotonergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol 148:587–598
Chiou SH, Kao CL, Chen YW, Chien CS, Hung SC, Lo JF, Chen YJ, Ku HH, Hsu MT, Wong TT (2008) Identification of CD133-positive radioresistant cells in atypical teratoid/rhabdoid tumor. PLoS ONE 3:e2090
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF (2008) Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res 14:4085–4095
Costea DE, Tsinkalovsky O, Vintermyr OK, Johannessen AC, Mackenzie IC (2006) Cancer stem cells—new and potentially important targets for the therapy of oral squamous cell carcinoma. Oral Dis 12:443–454
Dalerba P, Cho RW, Clarke MF (2007) Cancer stem cells: models and concepts. Annu Rev Med 58:267–284
Das S, Das DK (2007) Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6:168–173
de la Lastra CA, Villegas I (2005) Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res 49:405–430
Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML (1996) Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol 29:91–101
Ezeh UI, Turek PJ, Reijo RA, Clark AT (2005) Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 104:2255–2265
Fong SP, Tsang KS, Chan AB, Lu G, Poon WS, Li K, Baum LW, Ng HK (2007) Tropism of neural progenitor cells to embryonic stem cells: neural induction and transplantation in a mouse ischemic stroke model. J Neurosci Res 85:1851–1862
Habrand JL, De Crevoisier R (2001) Radiation therapy in the management of childhood brain tumors. Childs Nerv Syst 17:121–133
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI (2003) Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci USA 100:15178–15183
Hoppe-Hirsch E, Brunet L, Laroussinie F, Cinalli G, Pierre-Kahn A, Renier D, Sainte-Rose C, Hirsch JF (1995) Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst 11:340–345, discussion 345–346
Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK (2008) Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res 314:1163–1176
Johnson GE, Ivanov VN, Hei TK (2008) Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 13:790–802
Jordan CT, Guzman ML, Noble M (2006) Cancer stem cells. N Engl J Med 355:1253–1261
Kao CL, Chiou SH, Chen YJ, Singh S, Lin HT, Liu RS, Lo CW, Yang CC, Chi CW, Lee CH, Wong TT (2005) Increased expression of osteopontin gene in atypical teratoid/rhabdoid tumor of the central nervous system. Mod Pathol 18:769–778
Kuo PL, Chiang LC, Lin CC (2002) Resveratrol-induced apoptosis is mediated by p53-dependent pathway in Hep G2 cells. Life Sci 72:23–34
Liao HF, Kuo CD, Yang YC, Lin CP, Tai HC, Chen YY, Chen YJ (2005) Resveratrol enhances radiosensitivity of human non-small cell lung cancer NCI-H838 cells accompanied by inhibition of nuclear factor-kappa B activation. J Radiat Res (Tokyo) 46:387–393
Neuzil J, Stantic M, Zobalova R, Chladova J, Wang X, Prochazka L, Dong L, Andera L, Ralph SJ (2007) Tumour-initiating cells vs. cancer ‘stem’ cells and CD133: what’s in the name? Biochem Biophys Res Commun 355:855–859
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R (2007) Identification and expansion of human colon-cancer-initiating cells. Nature 445:111–115
Roccaro AM, Leleu X, Sacco A, Moreau AS, Hatjiharissi E, Jia X, Xu L, Ciccarelli B, Patterson CJ, Ngo HT, Russo D, Vacca A, Dammacco F, Anderson KC, Ghobrial IM, Treon SP (2008) Resveratrol exerts antiproliferative activity and induces apoptosis in Waldenstrom’s macroglobulinemia. Clin Cancer Res 14:1849–1858
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432:396–401
Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8:755–768
Whelan HT, Krouwer HG, Schmidt MH, Reichert KW, Kovnar EH (1998) Current therapy and new perspectives in the treatment of medulloblastoma. Pediatr Neurol 18:103–115
Yu LJ, Wu ML, Li H, Chen XY, Wang Q, Sun Y, Kong QY, Liu J (2008) Inhibition of STAT3 expression and signaling in resveratrol-differentiated medulloblastoma cells. Neoplasia 10:736–744
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC (2008) Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res 14:123–129
Acknowledgments
This study was supported by research grants from NSC-(97-3111-B-075-001-MY3), Taipei Veterans General Hospital (V97E1-008, ER2-018, ER3-005, F-001), Taipei City Hospital (96002-62-092 and 97001-62-015), and National Yang-Ming University (Ministry of Education, Aim for the Top University Plan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Kai-Hsi Lu, Yi-Wei Chen, Ping-Hsing Tsai, and Ming-Long Tsai contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lu, KH., Chen, YW., Tsai, PH. et al. Evaluation of radiotherapy effect in resveratrol-treated medulloblastoma cancer stem-like cells. Childs Nerv Syst 25, 543–550 (2009). https://doi.org/10.1007/s00381-009-0826-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-009-0826-6