Abstract
Objective
Antibiotic-impregnated shunt (AIS) systems have been designed to prevent the colonization of shunt components by skin flora that occurs at surgery. Although such systems may decrease the incidence of early shunt infections (those occurring within 6 months of shunt placement), it is unclear if such exposure to prolonged antibiotics leads to an increased incidence or virulence of late shunt infections (those occurring later than 6 months after shunt placement). In this study, the authors evaluate the incidence of late shunt infection after the introduction of an AIS system in a pediatric hydrocephalus population.
Materials and methods
We prospectively reviewed all pediatric patients undergoing antibiotic-impregnated CSF shunt insertion or shunt revision operations at our institution for the 33 month period between October 1, 2002 and June 31, 2005. All shunt-related complications, including shunt infection, were evaluated in those patients with later than 6 months of follow-up.
Results
A total of 153 pediatric patients (between 1 and 21 years of age) underwent 262 shunting procedures involving the use of antibiotic-impregnated catheters. All patients were followed-up for later than 6 months with a mean follow-up of 21.7 months (range 13–46 months). Ten patients (3.82%) experienced an early shunt infection within the 6-month follow-up period. No patients experienced a late shunt infection.
Conclusion
Although concern exists that AIS systems may delay shunt infections or even increase the rate or virulence of such infections, introduction of such catheters into a pediatric hydrocephalus cohort does not significantly increase incidence of late CSF shunt infection compared to historic controls.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite advances in surgical technique and technology, shunt infections remain a persistent obstacle for those treating pediatric hydrocephalus. The majority of shunt infections occur within 4 months after implantation [8, 11, 16, 21], and it is believed that perioperative colonization of shunt components by skin flora is the etiology [8, 9]. The incidence of late shunt infections (LSI) is not established (although reported to be 12.7%) [25], and the sources of contamination are poorly understood. Peritonitis (generally appendicitis), hematogenous contamination, bowel perforation, direct inoculation (abdominal surgery or traumatic exposure of the shunt), and persistence of bacterial colonization from the original shunt implantation surgery have all been considered potential sources.
Antibiotic-impregnated shunt (AIS) components are designed to release antibiotics over the course of several weeks. In this way, colonization is prevented, and early shunt infections are decreased. Recent studies have suggested that the introduction of antibiotic-impregnated shunt (AIS) systems into hydrocephalic patient populations may lead to decreased incidences of early shunt infections (occurring less than 6 months postoperatively) [12, 23]. However, reluctance to use such systems still exists secondary to increased cost compared to standard shunt components and concern that prolonged exposure to antibiotics in such systems may increase the incidence or virulence of late shunt infections. In this clinical study, the primary objective was to determine if the incidence of late shunt infection (later than 6 months postoperatively) was increased compared to previously published historic controls in a pediatric patient population after the introduction of an AIS system used to treat hydrocephalus.
Materials and methods
All pediatric patients (1–21 years of age) undergoing AIS CSF shunt insertion or shunt revision involving ventriculoperitoneal (VP), ventriculoatrial (VA), ventriculopleural (VPl), and cystoperitoneal (CP) shunts systems at the Johns Hopkins Hospital were prospectively reviewed from October 1, 2002 to July 31, 2005. Patient demographics, CSF shunting history, clinical presentation, radiological studies, operative variables, and shunt types and configurations were reviewed in all cases. Since the introduction of the Bactiseal AIS system (Codman, Johnson & Johnson, Boston, MA, USA) in October 2002, greater than 95% of CSF shunts have included either an antibiotic-impregnated medium pressure unishunt system, antibiotic-impregnated ventricular and distal catheters attached to a non-impregnated valve of the surgeon’s choice or placement of an antibiotic-impregnated ventricular or distal catheter to an existing shunt system in cases where only a proximal or distal shunt revision was indicated, respectively. Shunt valve designs have included Medtronic PS Medical® Delta® valve and Medtronic Strata® valve (Medtronic Neurosurgery, Goleta, CA, USA), Codman® Hakim™ Programmable valve (Codman, Johnson & Johnson, Raynham, MA, USA), and a small minority of other valve designs.
All patients were followed-up for later than 6 months after shunt surgery. Shunt-related complications, and date and etiology of shunt failure were recorded. Shunt malfunction was defined as any event leading to shunt removal, replacement or revision, and etiologies for malfunction included shunt infection, proximal, distal or valve obstruction, distal catheter migration, overshunting, shunt disconnection, wound breakdown involving shunt or any combination of these etiologies. Shunt infection was further defined as those patients with clinical suspicion of shunt infection (fever, increased WBC/ESR/CRP, low CSF glucose, low CSF:plasma glucose ratio, and/or wound breakdown involving the shunt) with positive cultures of CSF and/or hardware. Patients experiencing shunt malfunction in the absence of both clinical suspicion of shunt infection and positive cultures were not considered “infected”. Incidence of late shunt infection (occurring later than 6 months after surgery) was noted in this population and compared to previously published historic controls for early [8, 11, 16, 21] and late [1, 25] shunt infections.
Results
Patient population
A total of 153 pediatric patients with hydrocephalus underwent 262 CSF shunt operations involving the use of antibiotic-impregnated catheters at Johns Hopkins Hospital from October 1, 2002 to July 31, 2005. Etiology of hydrocephalus leading to initial shunt placement was related to a congenital abnormality in 59 (39%) patients, intracranial hemorrhage in 34 (22.2%), myelodysplasia in 19 (12.4%), tumor in 17 (11%), Dandy–Walker malformation in 4 (2.6%), posterior fossa cyst in 4 (2.6%), meningitis in 3 (2%), aqueductal stenosis in 2 (1.3%), and other etiology in 11 (7%). Communicating hydrocephalus was diagnosed in 82 (53.5%) patients, whereas non-communicating hydrocephalus was present in 62 (40.5%) (Table 1). Eighty-one (53%) patients were male and 72 (47%) patients were female, all ranging in age from 1 to 21 years. Of the patients, 52 (34%) had a history of premature birth (less than 36 weeks gestation) (Table 2).
Shunt systems implanted or revised included 239 (91%) ventriculoperitoneal (VP) shunts, 10 (3.8%) ventriculoatrial (VA) shunts, 7 (2.8%) ventriculopleural (VPl) shunts, and 5 (2%) cystoperitoneal (CP) shunts. Of the operations, 143 (54.5%) included a programmable valve, 98 (37.4%) included a set pressure valve, 72 (27.5%) involved initial placement of the shunt system, while 190 (72.5%) were for shunt revision. Of these revision surgeries, 124 (62.3%) operations were proximal revisions, 43 (22.6%) operations were distal revisions, and 23 (12%) operations were entire shunt replacements. All revision surgeries were accounted for by 55 (36%) of the 153 patients. Of these patients, 43 had a revision surgery within 6 months of a previous operation and 20 had multiple revision surgeries within 6 months of a previous operation (78% and 36%, respectively, of all patients undergoing revision surgery) (Table 2). All revision surgeries were done for shunt malfunction; none were done for presumed infection.
Of these 153 patients, 129 (84.3%) were followed-up closely for at least 1 year (range 13–46 months). Twenty-one patients were lost to follow-up, usually after the first postoperative visit within 2 months. Three patients died at 2 months, 2 months, and 4 months, respectively. None of these three patients died of shunt malfunction or shunt infection.
Shunt infection
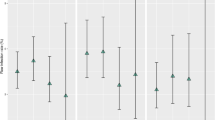
Of the 262 procedures performed, there were 10 cases of shunt infection occurring within the first 6 months postoperatively. The majority of these infections were caused by gram-positive organisms (7/10, 70%), namely, Staphylococcus species Table 3. All Staphylococcus aureus infections were oxacillin/methacillin-sensitive. The average length of time after surgery at which the shunt infection was detected was 4.5 weeks (range 2–8 weeks). No repeat infections occurred in the same patient after shunt replacement. In addition, there were no cases of shunt infection occurring after 6 months postoperatively, even after including those patients previously infected in the acute setting who underwent successive shunt placement with AIS catheters (Fig. 1).
Incidence of shunt infection as a function of time after shunt insertion in patients receiving antibiotic-impregnated catheters for the treatment of pediatric hydrocephalus. Forty months after shunt placement, 10 (3.82%) of the antibiotic-impregnated shunt catheters were infected. No infections occurred after 2 months postoperatively
Discussion
In North America, roughly 1 in 10 implanted CSF shunts become infected [10], leading to significant neurologic morbidity in the pediatric population. Reduced IQ and school performance, increased risk of seizures, and psychomotor retardation have all been associated with such infections [4, 6, 7, 9, 12, 20, 26, 27]. In addition, shunt infection is a common cause of shunt failure with its additional risk of morbidity and mortality [24]. The exact medical cost to society of such infections is not clear. Given that CSF shunt placement, the majority of which are revision surgeries, have been estimated to account for greater than 100 million dollars (US) of national health care expenditures annually [5], it is not unreasonable to conclude that shunt infections contribute significantly to these health care costs.
The majority of CSF shunt infections appear within the first 6 months postoperatively [8, 11, 16, 21]. It is generally accepted that these “early” infections most commonly result from the direct inoculation of shunt components by nonpathogenic skin flora at the time of surgery [10]. Although meticulous surgical technique and use of prophylactic perioperative antibiotics has been shown to significantly reduce the risk of CSF shunt infection [13, 19, 25], once colonization of shunt is established and infection ensures, eradication of the colonizing organism with intravenous antibiotics alone is often unsuccessful [17], necessitating shunt externalization, removal, and replacement. Antibiotic-impregnated shunt (AIS) systems have been developed to decrease the progression of colonization to infection, mainly by preventing staphylococci colonization of the catheter surface [17, 18].
The AIS system used in this study contains 0.054% rifampicin and 0.15% clindamycin, antibiotics, which have been shown to be bacteriocidal against multiple species of staphylococci [3, 14, 17, 18, 22]. In addition, a small number of studies have suggested that the introduction of AIS systems into hydrocephalic patient populations may be associated with a decrease in the incidence of early shunt infections [12, 23]. However, even though the bacteriocidal action of such AIS catheters has been shown to last for 60 days or more [2, 3, 15, 18, 22], such components have not been shown to be protective against reinoculation after 6 months. Thus, the efficacy of antibiotic-impregnated catheters in preventing late shunt infections is unclear. Moreover, there is concern that providing prolonged exposure to antibiotics in the early preoperative period potentially increases the incidence and/or virulence of late shunt infections.
Late shunt infections (LSI) have been defined as those occurring more than 9–12 months postoperatively [1, 25]. Although bacterial colonization from the original surgery has been considered a possibility when no other obvious source is found, most LSIs have been linked to peritonitis, hematogenous seeding from an established localized or systemic infection, bowel perforation by the distal shunt catheter or direct inoculation via surgical or traumatic exposure of the shunt.
In this study, 153 pediatric patients with hydrocephalus treated with AIS components in 262 procedures were prospectively followed-up paying particular attention to shunt malfunction and shunt infection. Of these patients, 129 (84.3%) were followed-up for at least 1 year (range 13–46 months). Ten shunt infections were detected within 6 months postoperatively with an average of 4.5 weeks after surgery, suggesting roughly a 3.82% incidence of early shunt infection. No infections occurred beyond 6 months postoperatively.
Although there is neither an established definition nor an established incidence of LSI, most define it as an infection later than 9–12 months after shunt insertion, and Vinchon et al. found an incidence of 12.7% in their large series of 1,700 patients [25], serving as the largest available published historic control. Limitations of this study include restricted long-term follow-up for the majority of the patients (mean 21.7 months), no internal control from our institution involving the incidence of LSI before introduction of the AIS system, and a small number of total patients that may underpower our conclusions. Despite such shortcoming however, our data suggest no increase in the incidence of LSI in patients treated with AIS systems compared to previously published historical controls. Furthermore, it appears that such “long-term” exposure to antibiotics with impregnated catheters does not necessarily impart an increased risk of shunt colonization and infection by more virulent organisms, such as methicillin-resistant S. aureus (MRSA), gram-negative organisms or fungus.
Conclusion
In this study, no increase in the incidence of late CSF shunt infections was noted after the introduction of an antibiotic-impregnated shunt (AIS) system into a pediatric hydrocephalus population. Although continued long-term follow-up is necessary to get an estimate of the true incidence of LSI, it appears that the use of such systems may contribute to a decreased incidence of early shunt infections without placing patients at risk for delayed complications.
References
Baird C, O’Connor D, Pittman T (1999) Late shunt infections. Pediatr Neurosurg 31:269–273
Bayston R, Grove N, Siegel J, Lawellin D, Barsham S (1989) Prevention of hydrocephalus shunt catheter colonisation in vitro by impregnation with antimicrobials. J Neurol Neurosurg Psychiatry 52:605–609
Bayston R, Lambert E (1997) Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J Neurosurg 87:247–251
Blount J, Campbell J, Haines S (1993) Complications in ventricular cerebrospinal fluid shunting. Neurosurg Clin N Am 4:633–656
Bondurant CP, Jimenez DF (1995) Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 23:254–258 (discussion 259)
Chadduck W, Adametz J (1988) Incidence of seizures in patients with myelomeningocele: a multifactorial analysis. Surg Neurol 30:281–285
Chapman PH, Borges LF (1984) Shunt infections: prevention and treatment. Clin Neurosurg 32:652–664
Enger P, Svendsen F, Wester K (2003) CSF shunt infections in children: experiences from a population-based study. Acta Neurochir (Wien) 145:243–248 (discussion 248)
Fan-Havard P, Nahata MC (1987) Treatment and prevention of infections of cerebrospinal fluid shunts. Clin Pharm 6:866–880
Gardner P, Leipzig T, Phillips P (1985) Infections of central nervous system shunts. Med Clin North Am 69:297–314
George R, Leibrock L, Epstein M (1979) Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg 51:804–811
Govender S, Nathoo N, van Dellen J (2003) Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg 99:831–839
Haines SJ, Walters BC (1994) Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery 34:87–92
Hampl J, Schierholz J, Jansen B, Aschoff A (1995) In vitro and in vivo efficacy of a rifampin-loaded silicone catheter for the prevention of CSF shunt infections. Acta Neurochir (Wien) 133:147–152
Hampl J, Weitzel A, Bonk C, Kohnen W, Roesner D, Jansen B (2003) Rifampin-impregnated silicone catheters: a potential tool for prevention and treatment of CSF shunt infections. Infection 31:109–111
Kanev P, Sheehan J (2003) Reflections on shunt infection. Pediatr Neurosurg 39:285–290
Kockro R, Hampl J, Jansen B, Peters G, Scheihing M, Giacomelli R, Kunze S, Aschoff A (2000) Use of scanning electron microscopy to investigate the prophylactic efficacy of rifampin-impregnated CSF shunt catheters. J Med Microbiol 49:441–450
Kohnen W, Schaper J, Klein O, Tieke B, Jansen B (1998) A silicone ventricular catheter coated with a combination of rifampin and trimethoprim for the prevention of catheter-related infections. Zentralbl Bakteriol 287:147–156
Langley J, LeBlanc J, Drake J, Milner R (1993) Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta-analysis. Clin Infect Dis 17:98–103
McGirt MJ, Leveque JC, Wellons JC 3rd, Villavicencio AT, Hopkins JS, Fuchs HE, George TM (2002) Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg 36:248–255
Ronan A, Hogg G, Klug G (1995) Cerebrospinal fluid shunt infections in children. Pediatr Infect Dis J 14:782–786
Schierholz J, Pulverer G (1997) Development of a new CSF-shunt with sustained release of an antimicrobial broad-spectrum combination. Zentralbl Bakteriol 286:107–123
Sciubba DM, Stuart RM, McGirt MJ, Woodworth GF, Samdani AF, Carson BS, Jallo GI (2005) Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg 103:131–136
Smith E, Butler W, Barker FG 2nd (2004) In-hospital mortality rates after ventriculoperitoneal shunt procedures in the United States, 1998 to 2000: relation to hospital and surgeon volume of care. J Neurosurg 100:90–97
Vinchon M, Lemaitre M, Vallee L, Dhellemmes P (2002) Late shunt infection: incidence, pathogenesis, and therapeutic implications. Neuropediatrics 33:169–173
Walters BC, Goumnerova L, Hoffman HJ, Hendrick EB, Humphreys RP, Levinton C (1992) A randomized controlled trial of perioperative rifampin/trimethoprim in cerebrospinal fluid shunt surgery. Childs Nerv Syst 8:253–257
Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP (1984) Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 60:1014–1021
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sciubba, D.M., McGirt, M.J., Woodworth, G.F. et al. Prolonged exposure to antibiotic-impregnated shunt catheters does not increase incidence of late shunt infections. Childs Nerv Syst 23, 867–871 (2007). https://doi.org/10.1007/s00381-007-0334-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-007-0334-5