Abstract
Purpose
The incidence of ventriculoperitoneal (VP) shunt infection accounts for about 5–15 %, but it can rise up to 70 % in specific high-risk subgroups. Antibiotic-impregnated catheters (AICs) have been designed to reduce shunt infections, but reports on their efficacy are discordant, especially in young children. The aim of this study is to assess, for the first time, the efficacy of AICs in newborns and infants at very high risk for shunt infection.
Methods
We reviewed the medical records of newborns and infants treated with a VP shunt for newly diagnosed hydrocephalus. Patients were divided in two groups: Group A was composed by children who received AICs, whereas Group B included children implanted with standard silicone catheters (non-AICs). We compared the shunt infection rate in both groups, and analyzed differences in specific high-risk subgroups (preterm newborns, children with posthemorrhagic or postinfective hydrocephalus, and children with a previous external ventricular drainage).
Results
Forty eight children younger than 1 year old were included in our study. Twenty two patients were implanted with an AIC, whereas 26 patients received a standard silicone catheter. The follow-up was at least 1 year (mean 8 ± 3 years). The overall infection rate decreased from 34 % in non-AIC group to 9 % in the AIC group. Moreover, AICs showed to have a protective effect against shunt infections in all the specific high-risk subgroups analyzed.
Conclusions
This study demonstrates for the first time that AICs are effective in reducing VP shunt infection in high-risk pediatric patients younger than 1 year old.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is the most common neurosurgical disease in children, with an incidence of three to five cases per 1,000 live births [1]. For more than 50 years, the standard of care consists of the implant of a shunt. Although this strategy has resulted in a significant prognostic improvement, the procedure is still burdened with serious complications still, to date, not completely resolved. One of the most common and critical problems is represented by shunt infections, being frequently associated with long-term neurological complications, such as reduced intelligence quotient, increased risk of seizures, psychomotor retardation, and shunt failure [2, 3]. In addition, about one third of the shunt-related deaths are subsequent to shunt infections and their complications [4].
Despite the infections rate in the overall shunted population reaches about 15 % [5–12], it is significantly higher in some specific patient subgroups, such as young children [2, 6, 13–15], postmeningitis or posthemorrhagic hydrocephalus [2, 6, 8, 10, 11, 16, 17], and patients initially submitted to external ventricular drainage (EVD) [11, 18], rising up to 70 % in premature newborns [2, 4, 5, 10, 11, 13, 19–28].
The leading cause of shunt infection is due to colonization from Staphylococcus epidermidis, being Staphilococcus aureus, other bacteria, and fungi, including yeasts, implicated as well [15, 29, 30]. These pathogens produce a glycoprotein biofilm that allows their adhesion to the catheter, avoiding antibiotics penetration and reducing the effect of immunological response [17, 31, 32]. Therefore, once established, shunt colonization is difficult to eradicate and requires shunt conversion to EVD.
In vitro and in vivo studies demonstrated that antibiotics incorporated into silicone rubber catheter provide local antimicrobial activity [33, 34]. Based on this research, antibiotic-impregnated catheters (AICs) have been introduced in the market with the aim to reduce the incidence of cerebrospinal fluid (CSF) shunt infection. Several studies evaluated the efficacy and safety of AICs, providing conflicting results [4, 8, 18, 19, 35–42].
In the literature, to date, only five studies have compared AIC vs. non-AIC efficacy in reducing shunt infection rates in pediatric patients [4, 18, 19, 38, 43]. However, in all these studies, patients had a mean age greater than 1 year, and most importantly, the authors did not analyze differences of AICs efficacy in specific high-risk subgroups or differences according to the patients’ age.
In the present study, we assessed, for the first time, the efficacy of AICs as compared to non-AICs in reducing shunt infection rates exclusively in newborns and infants younger than 1 year old. We also analyzed the AICs protective effect in specific high-risk subgroups, consisting of premature newborns, children with posthemorrhagic or postinfective hydrocephalus, and children converted from a previous EVD to a definitive ventriculoperitoneal (VP) shunt.
Materials and methods
We examined the medical records of children younger than 1 year (neonates: 1–30 days; infants: 1–12 months) admitted to the Neonatal and Pediatric ICU with the initial diagnosis of hydrocephalus and submitted to their first VP shunt at the Neurosurgical Clinic of the University of Messina, Italy, between 2002 and 2012.
Relatives signed a written informed consent for the purpose of publication of clinical data, according to the internal regulation approved by the Ethical Committee at our institution.
We collected data about age at VP shunt insertion, sex, gestational age, etiology of the hydrocephalus, eventual previous EVD, type of shunt catheters (AICs or non-AICs), eventual shunt-related infections, and time to shunt infection.
Patients were divided in two groups according to the type of catheters used for the VP shunt: Group A consisted of newborns and infants who were implanted with ventricular and peritoneal AICs (Bactiseal®, Codman Johnson & Johnson, Raynham, MA, USA), connected to a programmable Hakim valve (Hakim Medos, Codman Johnson & Johnson). Group B included all patients treated with non-AICs, consisting of a standard silicone ventricular and peritoneal catheter connected to the same shunt hardware of Group A. The choice of a programmable valve was due to the possibility to easily and noninvasively adjust the opening pressure to adapt to changes in patient conditions. All patients who received non-AICs were treated before 2005, because of the lack of AICs at our institution before that period.
All cases have been submitted to antibiotic prophylaxis consisting of intravenous (i.v.) cefazolin (first-generation cephalosporin) 1 h prior to begin surgery, according to our internal protocol. During surgery, we used all the known measures for reducing as much as possible the incidence of shunt infection. We used a meticulous sterile technique, paying particular attention in draping patients, using washing solutions with the addition of antibiotics (vancomycin 500 mg in 500 ml of 0.9 % NaCl solution, applied to the shunt catheters and valve prior to the insertion and used for washing during surgery, if needed), and reducing the number of operators and visitors in the operating theater.
We assessed the VP shunt infection rate in our series, comparing Group A vs. Group B. Then we weighted the shunt infection rate in both groups according to the patients’ age (newborns or infants) and to specific risk factors: high-risk categories were defined a priori and included premature infants (shunt placement occurred during the birth admission in neonates less than 37 weeks gestational age), children with postinfective (who received a VP shunt for newly diagnosed hydrocephalus in the acute setting following a treated meningitis or meningoencephalitis) or posthemorrhagic hydrocephalus (who were implanted with a VP shunt after a cerebral intraventricular hemorrhage), and children converted from a previous EVD to a definitive VP shunt (who underwent the insertion of a new VP shunt following a previous EVD or the removal of a previous externalized shunt system). In Group A, the EVD consisted of a ventricular antibiotic-impregnated catheter, whereas in Group B, it consisted of a standard silicone ventricular catheter.
Our analysis included only those patients receiving follow-up care over time at our institution, consisting of clinical examination at 1 week, 1 month, 3 months, 12 months, and yearly thereafter following the procedure. The minimum follow-up was 1 year for each patient (mean 8 ± 3 years).
All patients who presented skin lacerations and/or ulcerations near the shunt subcutaneous route or postoperative CSF leakage, or patients who were submitted to other neurosurgical procedures that could be responsible of secondary CSF infection, were excluded from the present study to avoid sampling bias. Therefore, in this study, each case of infection, regardless of the time of presentation, was considered as presumably acquired at the time of the shunt insertion.
The presence of a shunt infection was defined exclusively in case of a CSF culture positive to a microorganism. Other criteria considered suspicious of infection were CSF pleocytosis (leukocyte count, >50 leukocytes/mm3), an increase of C-reactive protein serum concentration, fever, shunt malfunction, or neurological symptoms [11, 18]. The contemporary presence of increased C-reactive protein, fever, and clinical signs of shunt malfunction, including neurologic deterioration, were considered highly suspicious for shunt infection, leading to shunt conversion to EVD and CSF microbiological analysis. However, shunt infection was confirmed exclusively in case of a microbiologically positive CSF culture.
If shunt infection occurred or was highly suspected, an empirical antibiotic treatment was immediately started. When three CSF successive samples became sterile and pleocytosis had been resolved, a new VP shunt was reinserted. The time of onset of infection from shunt insertion was also recorded and was defined as time to shunt infection.
Statistical analysis
The homogeneity of the study population among the two groups (Group A vs. Group B) was assessed using the unpaired Student’s t test for continuous data (age) and the Chi-square test with Yates’ correction for nominal data (sex, prematurity, etiology of hydrocephalus, and previous EVD). Comparison of VP shunt infection rate in AIC vs. non-AIC group and in specific high-risk subpopulations was performed by using the Fisher’s exact test. Protective efficacy of AICs was expressed as odds ratio (OR) with 95 % confidence interval (CI). Time to shunt infection analysis was performed using the Kaplan–Meier plots, the log–rank (Mantel–Cox) test, the hazard ratio (HR), and the reciprocal HR. Statistical significance was defined as a p value < 0.05. Data analysis was performed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla, CA, USA (www.graphpad.com).
Results
Patients’ characteristics are presented in Table 1. Forty eight children younger than 1 year old were treated at our Institution with the insertion of a shunt for a newly diagnosed hydrocephalus in the period examined. Twenty two patients were implanted with AICs (Group A), whereas 26 patients received standard ventricular silicone catheters (Group B). The mean age at VP shunt insertion was 66 days (range 1 day–1 year old). Statistical analysis demonstrated that the two groups were homogeneous for age at insertion, sex, etiology of hydrocephalus, prematurity, and for previous EVD (Table 1).
In Group A, 2 of 22 patients (9 %) were affected by shunt infection. These two patients were infants and have been treated for posthemorrhagic and malformative hydrocephalus, respectively. Infections were sustained by multidrug resistant S. epidermidis (MDRSE) in both cases. Antibiograms showed resistance to different antibiotics, including clindamycin and rifampicin. In Group B, we recorded a shunt infection in 9 of 26 patients (34 %). Of these, five were premature newborns, and four were infants. The cause of hydrocephalus was secondary to malformations in three patients, simultaneously posthemorrhagic and postinfective in two, exclusively posthemorrhagic in three, and secondary to spinal disraphism in one. Infections were sustained by S. epidermidis in five cases, S. aureus in two cases, and Pseudomonas aeruginosa in the remaining two cases.
When we compared the overall shunt infection rates in Group A vs. Group B, the use of AICs was associated with a significant protective effect against shunt contaminations (p = 0.045; OR 0.188, 95 % CI 0.035–0.996; Fig. 1).
As the risk of infection is higher in newborns than older children, especially if born prematurely, we compared the infection rate according to the age at insertion in the two groups. Among newborns, we did not observe infections in AICs implanted patients, Group A (0 of 12), whereas we recorded five shunt infections in non-AIC cases, Group B (5 of 15–33 %). Therefore, the use of AICs was able to significantly reduce the infection rate in this high-risk population (p = 0.047; OR 0.076, 95 % CI 0.003–1.549; Fig. 2). Conversely, among infants, the infection rate was 20 % (2 of 10) in Group A and 36.3 % (4 of 11) in Group B. Despite a trend toward a reduction in the shunt infection rate in the AIC group, the difference was not statistically significant (p = 0.635; Fig. 3).
Among premature neonates, no patients (0 of 10) in Group A and 5 of 10 in Group B (50 %) were affected by shunt infection. Statistical analysis showed a significant difference between the two groups (p = 0.032; OR 0.047, 95 % CI 0.002–1.030; Fig. 4a).
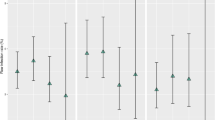
Shunt infection rates in high-risk subpopulations. a Among preterm newborns, the infection rate decreased from 50 % (5 of 10 patients) in non-AIC group to zero in AIC group (p = 0.032). b Among children with posthemorrhagic hydrocephalus, the infection rate was reduced from 71.4 % (5 of 7 patients) in non-AIC group to 12.5 % (1 of 8) in AIC group (p = 0.040). c Among patients with postinfective hydrocephalus, the infection rate decreased from 66.6 % (2 of 3 patients) in non-AIC group to zero in AIC group (p = 0.142). d Among patients with previous EVD, the infection rate was reduced from 83.3 % (5 of 6 patients) in non-AIC group to 14.2 % (5 of 6) in AIC group (p = 0.029)
Considering patients with posthemorrhagic hydrocephalus, 1 of 8 in Group A (12.5 %) and 5 of 7 in Group B (71.4 %) had a shunt infection. The observed trend toward a reduction in the AICs patients was statistically significant (p = 0.040; OR 0.057, 95 % CI 0.003–0.818; Fig. 4b).
Among patients with postinfective hydrocephalus, no patients in Group A (0 of 4) and 2 of 3 in Group B (66.6 %) developed a CSF infection. However, the difference was not statistically significant (p = 0.142; Fig. 4c).
When assessing the efficacy of AICs in hydrocephalic cases who were converted from a previous EVD to a definitive shunt, 1 of 7 in Group A (14.2 %) and 5 of 6 in Group B (83.3 %) were affected by a shunt-related infection. Statistical analysis showed a significant protective effect for AICs vs. non-AICs. (p = 0.029; OR 0.033, 95 % CI 0.001–0.680; Fig. 4d).
Analyzing the odds ratio values for each subgroups, we observed a protective effect of AICs in all our pediatric population, being the OR always less than 1 (Fig. 5). The highest protective effect was observed in children converted from a previous EVD to a definitive VP shunt and in newborns. A less powerful, but significant protective effect was recorded in premature patients and in children with posthemorrhagic hydrocephalus. Conversely, despite a trend toward reduction of the infection rate in the AIC group, the protective effect was not significant in children with postinfective hydrocephalus and in infants.
Forest plot depicting the protective effect of AICs against shunt infections in each high risk pediatric subgroups analyzed, expressed as odds ratio with 95 % confidence interval. AICs showed to have a protective activity in all subgroups, especially in patients with previous EVD. However, in infants and in children with postinfective hydrocephalus, this protection was not statistically significant
Lastly, we analyzed the overall shunt infection rate according to the time of infection onset during the first year of follow-up. The median time to shunt infection was 25 days for AICs and 160 for non-AICs patients. The log–rank analysis confirmed that shunt infections occurred significantly later in Group B as compared with patients of Group A (p = 0.049; HR 0.245, 95 % CI 0.092–0.985; reciprocal HR 4.069, 95 % CI 1.015–10.82; Fig. 6).
Kaplan–Meier plot of time to shunt infection analysis. The median time to shunt infection from the initial surgery was 25 days for AIC group and 160 for non-AIC group. The log–rank analysis showed that shunt infection rate was reduced in patients treated with AICs (Group A) as compared with patients implanted non-AICs (Group B; p = 0.049). The hazard ratio was 0.245 and the reciprocal hazard ratio was 4.069 suggesting that patients treated with non-AICs have a fourfold increased risk for shunt infection during the first year from surgery as compared with patients who received AICs
Discussion
Despite the advances in the treatment of hydrocephalus observed in the last decades, shunt infections still remain responsible for high morbidity and mortality rate, especially in the pediatric population. Antibiotic-impregnated shunt catheters (AICs) are relatively new devices consisting of silicon tubes whose matrix is impregnated with dual drug antibiotics, usually rifampicin (0.54 %) and clindamycin (0.15 %), which are simultaneously released after insertion. They have been designed to provide a time-limited protection from infections, especially from that sustained by Gram-positive bacteria, such as Staphylococcus spp.
In the literature, different studies have been focused on the analysis of the AICs efficacy in reducing shunt infections, providing nonuniform results [4, 8, 18, 19, 35–39, 41, 42]. A limitation of most of them is the concomitant evaluation of the AICs efficacy in adults and in children. To our knowledge, only five studies have, to date, specifically addressed the protective effect of AICs exclusively in children [4, 18, 19, 38, 43]. However, in all these series, the mean age of patients was greater than 1 year, and the reported findings were discordant. Aryan et al. [19] in 2005 reported their initial experience with AICs in 31 children (age range 6 months to 17 years, mean 4.5 years), suggesting a lower incidence of infections by using AICs as compared with standard implanted shunts. In 2008, Eymann et al. [4] evaluated the safety and efficacy of AICs in two separated cohorts composed by 208 adults and 41 children, respectively, and reported that from both clinical and economical perspectives, AICs represented a valuable addition in hydrocephalus therapy, significantly reducing the shunt infection rate. However, apart from the mixed characteristics of the population, the age range of the children cohort was 2 months–12 years, and the authors did not provide any consideration about infection rate differences according to the age at insertion. In 2009, Parker et al. [43] analyzed the efficacy of AICs in a large pediatric cohort demonstrating a protective effect against shunt infection in all the analyzed population including high-risk patients such us premature neonates and children with postinfective hydrocephalus. Again, in this study, the population age range was quite large, being the mean age at insertion 6.5 years (range 1 day–20 years). Except for premature neonates, the efficacy of AICs in reducing the infection rate in the other high-risk groups was not referred to the age at insertion (i.e., distinguishing between neonates, infants, children, and adults). Moreover, the authors did not include posthemorrhagic hydrocephalus as a high-risk category. Conversely, in the present study, young age at insertion and posthemorrhagic hydrocephalus were considered as important risk factors for patients’ stratification. In contrast with the aforementioned studies, in 2008, Hayhurst and colleagues [18] reported a similar incidence of shunt infections in pediatric patients treated with AICs (9.8 %) as compared to cases implanted with non-AICs (10.4 %), suggesting that AICs are efficient only in specific pediatric subgroups. In particular, the authors reported only an encouraging trend toward a reduction of the shunt infection rate in neonates implanted with a de novo shunt in the AIC group (from 27 to 10.7 %). Nevertheless, the difference was not statistically significant. Therefore, the young age at insertion was considered as a main risk factor to stratify the study population but the final findings were hampered by the fact that the neonate group was almost exclusively composed by patients treated with a de novo shunt (28 of 33 cases). Therefore, no data were reported about the efficacy of AICs in neonates belonging to other specific risk categories (i.e., previous EVD and posthemorrhagic or postinfective hydrocephalus). Conversely, in the present paper, the young age at insertion was the main risk factor used to select the study population. Then patients were further stratified according to other specific risk factors, in particular, prematurity, postinfective and posthemorrhagic hydrocephalus, and a previous EVD. Lastly, Kan and Kestle [38] reported the lack of a protective effect of AICs in children both in terms of infection rate and average time to infection (mean age at insertion: AIC group 7.9 years–non-AIC group 6.8 years).
Several studies confirmed that age at shunt insertion represents one of the most important risk factors for shunt infections. In particular, infection rate is reported to be as higher as earlier the shunt is implanted [2, 6, 13–15]. Pople et al. [14] reported a shunt infection rate almost three times higher in patients younger than 6 months old, as compared to older children. Similarly, Renier et al. [15] reported that shunt infection was 2.6 times more frequent before 6 months than after 1 year of age. These findings have been explained through a relative deficiency of the immune response in younger children against bacteria [14].
Starting from these evidences, in the present study, we analyzed the efficacy of AICs in a cohort composed only by children younger than 1 year old. We observed an overall reduction of shunt infections from 34 % in children treated with non-AICs to 9 % in those implanted with AICs. When assessing the infection rate according to age at insertion, we observed a trend in the decrease of infection rate in infants, but it was not statistically significant. Conversely, there was a significant difference in the newborn subgroup, where the infection rate dropped from 33.3 % in non-AICs to as low as 0 in AICs, respectively.
Prematurity, defined as a gestational age <37 weeks at birth, has been reported as being another of the most significant risk factors for shunt infection [2, 10, 13]. The association between premature birth and shunt infection can be explained with the weakness of the immune response of neonates. In addition, premature babies are characterized by an immaturity of the skin, responsible for a different bacterial skin colonization with predominance of coagulase-negative Staphylococci, especially S. epidermidis, which is known as the most frequent microorganism responsible of shunt infections [44]. In our series of patients, the use of AICs significantly reduced the shunt infection rate in premature children from 50 % in patients who received non-AICs to 0 in the ones who were treated with AICs.
Intraventricular hemorrhages and CNS infections as causes of hydrocephalus are associated with a high risk of shunt infection [2, 6, 8, 10, 11, 16, 45, 46]. In particular, posthemorrhagic hydrocephalus has been reported as a risk factor for shunt infection, with a relative risk (RR) of 2.07, twofolds higher than other etiologies [16]. We observed a statistically significant reduction of shunt infections from 71.4 % in patients with posthemorrhagic hydrocephalus implanted with non-AICs to 12.5 % in those treated with AICs. Similar results were obtained for patients with postinfective hydrocephalus, with a reduction from 66.6 % (non-AICs) to 0 cases (AICs). However, for the latter category, the reduction was not statistically significant, presumably due to the small size of available cases included in the present series.
The shunt insertion in hydrocephalic cases initially treated with the implant of an EVD is frequently correlated with a significant risk of infection [11]. This specific subgroup of pediatric patients has been reported to be particularly vulnerable to shunt bacterial colonization, associated to an increasing possibility of intrinsic bacterial resistance [18]. In our cases, we recorded a protective effect of AICs demonstrated by a statistically significant decrease of shunt infections from 83.3 % in the non-AIC group to 14.2 % in the AIC group. These findings are in contrast to that of Hayhurst et al. [18], who reported an unacceptable increase of the shunt infection rate up to 20.3 % in patients treated after the insertion of previous AIC used as EVD. They speculated that such an increase could be due to the selection of rifampicin- and/or clindamycin-resistant Gram-positive bacteria or Gram-negative microorganisms. However, the authors underlined that no CSF microbiological samples were tested for resistance against clindamycin or rifampicin, and, moreover, the Gram-negative infection rate was comparable with data reported in literature (15–20 %). Therefore, the hypothesis that AICs used for EVD could select resistant microorganisms was not demonstrated. In the present study, we did not record Gram-negative infections in the AICs post-EVD group. Moreover, our findings are concordant with that of Parker et al. [43], who reported a similar dramatic reduction of the infection rate in the AICs post-EVD group (from 13.3 % to zero). Maybe the different findings of Hayhurst et al. [18] could be due to a limited number of post-EVD patients in the control group (11 cases) that could have affected the comparison with the AIC group (54 cases).
To better assess the protective effect of AICs, we evaluated the odds ratio for each high-risk subgroup. AICs were able to significantly protect children younger than 1 year from shunt infection. The protection was higher in newborns than in infants and among the other high-risk categories. The strongest protection of AICs was observed in children converted from a previous EVD to a definitive VP shunt. A less powerful, but significant protective effect was recorded in premature patients and in children with posthemorrhagic hydrocephalus. Lastly, AIC’s protective effect in children with postinfective hydrocephalus was slightly strong, although this finding was not statistically significant due to the limited number of patients available for this specific category.
Another important aspect that needs to be clarified to better assess the efficacy of AICs in preventing infections is the source of the shunt colonization. It has been demonstrated that the most part of shunt infections are acquired during surgery due to the contamination of the surgical field with microorganisms from surgeon’s gloves or from patients’ skin [29, 47, 48]. These contaminations have been defined as “early acquired infections” and have to be distinguished from the less frequent “late acquired infections.” The latter are frequently determined by a secondary contamination of the shunt system from different causes such as the presence of skin lacerations or ulceration near the catheters subcutaneous route or valve or the successive neurosurgical procedures that can cause a CSF leak [49]. However, infections can become clinically evident after a variable time interval from contamination. About two thirds of cases are diagnosed within 30 days from shunt surgery and more than 90 % within 1 year [50]. Only 10 % of infections become clinically evident after 1 year, and they usually are late acquired infections [51]. AICs have been designed to protect patients only from early acquired infections, being active up to 56 days in vitro [33]. Therefore, they cannot give any protection against late acquired shunt infections. This could be the explanation for some literature evidences, reporting similar shunt infection rates using AICs and non-AICs [41]. Starting from these evidences, we compared shunt infection rate in AIC vs. non-AIC groups according to the period of presentation during the first year of follow-up. In order to avoid a bias in collecting patients, we excluded from our series all patients who presented risk factors for late acquired infection such as skin ulcerations near the shunt course or as neurosurgical procedures that could determine CSF leakage or infections. Therefore, all the infection cases reported in the present study have to be considered as early infections acquired during the shunt insertion. The median time to shunt infection was 25 days for the AIC group and 160 days for the non-AIC group. The log–rank analysis showed a statistically significant difference between the two curves, showing that patients treated with non-AICs had a fourfold increased risk of shunt infection during the first year after surgery as compared to patients treated with AICs. The shorter time to shunt infection observed in the AIC group could be the effect of a higher protection against infection. In fact, in case of AIC failure, the infection becomes clinically evident more rapidly than using the non-AICs. Conversely, the absence of symptoms and signs of infections after the first month from shunt insertion could be considered as a reasonable sign of the efficacy of the infection prevention strategy in the AICs patients, but not in the non-AICs ones. These findings seem to confirm the protective effect of AICs.
Nevertheless, this study has some limitations that can reduce the strength of our findings. First of all, the present study is a retrospective case–control study (AIC vs. non-AIC group). It reports a single-center experience in a relatively small number of patients. This limitation was more evident especially when we compared the infection rate among the different high-risk subgroups, due to the small number of patients included in each category. This reduced the strength of the statistical analysis. Although the odds ratio analysis showed a significant protective effect of AICs for almost all the high-risk subgroups, the 95 % confidence intervals appeared to be quite large. Moreover, in children with postinfective hydrocephalus and in infants, the protective effect of AICs did not reach a statistical significance. Lastly, the monoinstitutional design of the present study did not allow us to reduce some important bias such as the difference in shunt infection rates due to different procedures for infection prevention that could be used in different institutions (type of antibiotic prophylaxis, change of gloves during surgery, use of antibiotics in washing solutions, number of surgeon in the operating theater, etc.). Taking into account all these limitations, an objective analysis of our findings suggests that the present study provides only preliminary data about the efficacy of AICs in the most vulnerable subgroups of pediatric patients. In fact, the aim of the authors was to shed light on lack of literature and to underline the need of a multicenter randomized controlled trial for assessing the real efficacy of AICs in high-risk infants and newborns.
Conclusions
This study demonstrates, for the first time, that AICs are effective in reducing the shunt infection rate in very high-risk pediatric patients younger than 1 year old (newborn and infants). We observed a significant reduction of the shunt infection rates in all the analyzed subgroups. In particular, the AIC protective activity was more significant in newborns and in specific high-risk subgroups such as children with previous EVD, posthemorrhagic and postinfective hydrocephalus, and prematures. However, the retrospective observational case–control nature of the present study and the relatively small number of patients, especially considering the specific subgroups, cannot allow us to draw definitive conclusions. A larger prospective, blinded, randomized controlled trial with adequate power is still needed to confirm these results and to provide any recommendation for or against the routine use of AICs in young children, who still need a real and effective protection against shunt infections.
References
Chi JH, Fullerton HJ, Gupta N (2005) Time trends and demographics of deaths from congenital hydrocephalus in children in the United States: National Center for Health Statistics data, 1979 to 1998. J Neurosurg 103(2 Suppl):113–118. doi:10.3171/ped.2005.103.2.0113
Vinchon M, Dhellemmes P (2006) Cerebrospinal fluid shunt infection: risk factors and long-term follow-up. Childs Nerv Syst 22(7):692–697. doi:10.1007/s00381-005-0037-8
Walters BC, Hoffman HJ, Hendrick EB, Humphreys RP (1984) Cerebrospinal fluid shunt infection. Influences on initial management and subsequent outcome. J Neurosurg 60(5):1014–1021. doi:10.3171/jns.1984.60.5.1014
Eymann R, Chehab S, Strowitzki M, Steudel WI, Kiefer M (2008) Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr 1(6):444–450. doi:10.3171/PED/2008/1/6/444
Choux M, Genitori L, Lang D, Lena G (1992) Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 77(6):875–880. doi:10.3171/jns.1992.77.6.0875
Dallacasa P, Dappozzo A, Galassi E, Sandri F, Cocchi G, Masi M (1995) Cerebrospinal fluid shunt infections in infants. Childs Nerv Syst 11(11):643–648, discussion 649
Gardner P, Leipzig T, Phillips P (1985) Infections of central nervous system shunts. Med Clin North Am 69(2):297–314
Klimo P Jr, Thompson CJ, Ragel BT, Boop FA (2011) Antibiotic-impregnated shunt systems versus standard shunt systems: a meta- and cost-savings analysis. J Neurosurg Pediatr 8(6):600–612. doi:10.3171/2011.8.PEDS11346
Kontny U, Hofling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ (1993) CSF shunt infections in children. Infection 21(2):89–92
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis: Off Publ Infect Dis Soc Am 36(7):858–862. doi:10.1086/368191
Quigley MR, Reigel DH, Kortyna R (1989) Cerebrospinal fluid shunt infections. Report of 41 cases and a critical review of the literature. Pediatr Neurosci 15(3):111–120
Younger JJ, Simmons JC, Barrett FF (1987) Operative related infection rates for ventriculoperitoneal shunt procedures in a children’s hospital. Infect Control: IC 8(2):67–70
Kulkarni AV, Drake JM, Lamberti-Pasculli M (2001) Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94(2):195–201. doi:10.3171/jns.2001.94.2.0195
Pople IK, Bayston R, Hayward RD (1992) Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg 77(1):29–36. doi:10.3171/jns.1992.77.1.0029
Renier D, Lacombe J, Pierre-Kahn A, Sainte-Rose C, Hirsch JF (1984) Factors causing acute shunt infection. Computer analysis of 1174 operations. J Neurosurg 61(6):1072–1078. doi:10.3171/jns.1984.61.6.1072
Lee JK, Seok JY, Lee JH, Choi EH, Phi JH, Kim SK, Wang KC, Lee HJ (2012) Incidence and risk factors of ventriculoperitoneal shunt infections in children: a study of 333 consecutive shunts in 6 years. J Korean Med Sci 27(12):1563–1568. doi:10.3346/jkms.2012.27.12.1563
Parker SL, Anderson WN, Lilienfeld S, Megerian JT, McGirt MJ (2011) Cerebrospinal shunt infection in patients receiving antibiotic-impregnated versus standard shunts. J Neurosurg Pediatr 8(3):259–265. doi:10.3171/2011.6.PEDS11257
Hayhurst C, Cooke R, Williams D, Kandasamy J, O’Brien DF, Mallucci CL (2008) The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst 24(5):557–562. doi:10.1007/s00381-007-0521-4
Aryan HE, Meltzer HS, Park MS, Bennett RL, Jandial R, Levy ML (2005) Initial experience with antibiotic-impregnated silicone catheters for shunting of cerebrospinal fluid in children. Childs Nerv Syst 21(1):56–61. doi:10.1007/s00381-004-1052-x
Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE (1995) Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir 136(1–2):1–7
Boynton BR, Boynton CA, Merritt TA, Vaucher YE, James HE, Bejar RF (1986) Ventriculoperitoneal shunts in low birth weight infants with intracranial hemorrhage: neurodevelopmental outcome. Neurosurgery 18(2):141–145
Chapman PH, Borges LF (1985) Shunt infections: prevention and treatment. Clin Neurosurg 32:652–664
Cochrane DD, Kestle JR (2003) The influence of surgical operative experience on the duration of first ventriculoperitoneal shunt function and infection. Pediatr Neurosurg 38(6):295–301. doi:10.1159/000070413
Ersahin Y, Mutluer S, Guzelbag E (1994) Cerebrospinal fluid shunt infections. J Neurosurg Sci 38(3):161–165
Faillace WJ (1995) A no-touch technique protocol to diminish cerebrospinal fluid shunt infection. Surg Neurol 43(4):344–350
Kanev PM, Sheehan JM (2003) Reflections on shunt infection. Pediatr Neurosurg 39(6):285–290. doi:10.1159/000075255
Mancao M, Miller C, Cochrane B, Hoff C, Sauter K, Weber E (1998) Cerebrospinal fluid shunt infections in infants and children in Mobile, Alabama. Acta Paediatr 87(6):667–670
O’Kane MC, Richards H, Winfield P, Pickard JD (1997) The United Kingdom Shunt Registry. Eur J Pediatr Surg: Off J Austrian Assoc Pediatr Surg [et al] = Zeitschrift fur Kinderchirurgie 7(Suppl 1):56
Rehman AU, Rehman TU, Bashir HH, Gupta V (2010) A simple method to reduce infection of ventriculoperitoneal shunts. J Neurosurg Pediatr 5(6):569–572. doi:10.3171/2010.2.PEDS09151
Schoenbaum SC, Gardner P, Shillito J (1975) Infections of cerebrospinal fluid shunts: epidemiology, clinical manifestations, and therapy. J Infect Dis 131(5):543–552
Gray ED, Peters G, Verstegen M, Regelmann WE (1984) Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet 1(8373):365–367
Peters G, Locci R, Pulverer G (1982) Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis 146(4):479–482
Bayston R, Lambert E (1997) Duration of protective activity of cerebrospinal fluid shunt catheters impregnated with antimicrobial agents to prevent bacterial catheter-related infection. J Neurosurg 87(2):247–251. doi:10.3171/jns.1997.87.2.0247
Pattavilakom A, Kotasnas D, Korman TM, Xenos C, Danks A (2006) Duration of in vivo antimicrobial activity of antibiotic-impregnated cerebrospinal fluid catheters. Neurosurgery 58(5):930–935. doi:10.1227/01.NEU.0000210155.65102.E7, discussion 930–935
Demetriades AK, Bassi S (2011) Antibiotic resistant infections with antibiotic-impregnated Bactiseal catheters for ventriculoperitoneal shunts. Br J Neurosurg 25(6):671–673. doi:10.3109/02688697.2011.575478
Govender ST, Nathoo N, van Dellen JR (2003) Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg 99(5):831–839. doi:10.3171/jns.2003.99.5.0831
Gutierrez-Gonzalez R, Boto GR, Fernandez-Perez C, del Prado N (2010) Protective effect of rifampicin and clindamycin impregnated devices against Staphylococcus spp. infection after cerebrospinal fluid diversion procedures. BMC Neurol 10:93. doi:10.1186/1471-2377-10-93
Kan P, Kestle J (2007) Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Child’s Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg 23(7):773–777. doi:10.1007/s00381-007-0296-7
Pattavilakom A, Xenos C, Bradfield O, Danks RA (2007) Reduction in shunt infection using antibiotic impregnated CSF shunt catheters: an Australian prospective study. J Clin Neurosci: Off J Neurosurg Soc Australasia 14(6):526–531. doi:10.1016/j.jocn.2006.11.003
Richards HK, Seeley HM, Pickard JD (2009) Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatr 4(4):389–393. doi:10.3171/2009.4.PEDS09210
Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE (2007) Do antibiotic-impregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis 7:38. doi:10.1186/1471-2334-7-38
Steinbok P, Milner R, Agrawal D, Farace E, Leung GK, Ng I, Tomita T, Wang E, Wang N, Wong GK, Zhou LF (2010) A multicenter multinational registry for assessing ventriculoperitoneal shunt infections for hydrocephalus. Neurosurgery 67(5):1303–1310. doi:10.1227/NEU.0b013e3181f07e76
Parker SL, Attenello FJ, Sciubba DM, Garces-Ambrossi GL, Ahn E, Weingart J, Carson B, Jallo GI (2009) Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Child’s Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg 25(1):77–83. doi:10.1007/s00381-008-0743-0, discussion 85
D’Angio CT, McGowan KL, Baumgart S, St Geme J, Harris MC (1989) Surface colonization with coagulase-negative staphylococci in premature neonates. J Pediatr 114(6):1029–1034
Lamprecht D, Schoeman J, Donald P, Hartzenberg H (2001) Ventriculoperitoneal shunting in childhood tuberculous meningitis. Br J Neurosurg 15(2):119–125
Sil K, Chatterjee S (2008) Shunting in tuberculous meningitis: a neurosurgeon’s nightmare. Child’s Nerv Syst: ChNS: Off J Int Soc Pediatr Neurosurg 24(9):1029–1032. doi:10.1007/s00381-008-0620-x
Bayston R, Ashraf W, Bhundia C (2004) Mode of action of an antimicrobial biomaterial for use in hydrocephalus shunts. J Antimicrob Chemother 53(5):778–782. doi:10.1093/jac/dkh183
Bayston R, Lari J (1974) A study of the sources of infection in colonised shunts. Dev Med Child Neurol 16(6 Suppl 32):16–22
Baird C, O’Connor D, Pittman T (1999) Late shunt infections. Pediatr Neurosurg 31(5):269–273. doi:10.1159/000028874
George R, Leibrock L, Epstein M (1979) Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg 51(6):804–811. doi:10.3171/jns.1979.51.6.0804
Owen R, Pittman T (2004) Delayed external ventriculoperitoneal shunt infection. J Kentucky Med Assoc 102(8):349–352
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raffa, G., Marseglia, L., Gitto, E. et al. Antibiotic-impregnated catheters reduce ventriculoperitoneal shunt infection rate in high-risk newborns and infants. Childs Nerv Syst 31, 1129–1138 (2015). https://doi.org/10.1007/s00381-015-2685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2685-7