Abstract
Purpose
Cerebrospinal fluid shunt infection is associated with patient morbidity and high cost. We conducted a systematic review of the current evidence of comprehensive surgical protocols or individual interventions designed to reduce shunt infection incidence.
Methods
A systematic review using PubMed and SCOPUS identified studies evaluating the effect of a particular intervention on shunt infection risk. Systemic prophylactic antibiotic or antibiotic-impregnated shunt efficacy studies were excluded. A total of 7429 articles were screened and 23 articles were included.
Results
Eight studies evaluated the effect of comprehensive surgical protocols. Shunt infection was reduced in all studies (absolute risk reduction 2.2–12.3 %). Level of evidence was low (level 4 in seven studies) due to the use of historical controls. Compliance ranged from 24.6 to 74.5 %. Surgical scrub with antiseptic foam and omission of a 5 % chlorhexidine gluconate preoperative hair wash were both associated with increased shunt infection. Twelve studies evaluated the effect of a single intervention. Only antibiotic-impregnated suture, a no-shave policy, and double gloving with glove change prior to shunt handling, were associated with a significant reduction in shunt infection. In a hospital with high methicillin-resistant staphylococcus aureus (MRSA) prevalence, a randomized controlled trial found that perioperative vancomycin rather than cefazolin significantly reduced shunt infection rates.
Conclusion
Despite wide variation in compliance rates, the implementation of comprehensive surgical protocols reduced shunt infection in all published studies. Antibiotic-impregnated suture, a no-shave policy, double gloving with glove change prior to device manipulation, and 5 % chlorhexidine hair wash were associated with significant reductions in shunt infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus is one of the most frequently encountered conditions in neurosurgery, with 70,000 hospital admissions annually in the USA [1]. Using the Nationwide Inpatient Sample database, Patwardhan et al. estimated approximately 30,000 primary shunt-related procedures in 2000 for the management of hydrocephalus, contributing $1.1 billion toward health care spending for that year [2]. Children comprise the majority of cerebrospinal fluid (CSF) shunt patients, and in 2003, the pediatric population alone accounted for $1.2 billion and 250,000 in-hospital days [3].
Even though CSF shunts have been refined over decades of experience, the failure rate can be as high as 40 % by 1 year after placement [4]. Infection is the most significant complication, affecting 8–12 % of patients within 2 years of initial shunt placement [4–6]. Multiple risk factors for infection exist, including female gender, young age, etiology of hydrocephalus [7], presence of a perioperative CSF leak [8], premature birth, previous shunt infection [9], hospital volume, and surgeon case volume [6]. Long-term consequences of infection include an increased risk of seizure disorder, cognitive disorders, and other neurologic disabilities, and an increased mortality rate [8, 9]. Furthermore, there is a greater increase in length of stay and hospital costs compared to other shunt complications because management of infection requires at least two surgical interventions (removal of the existing shunt system and insertion of a new one after achieving negative CSF cultures) and intensive antibiotic therapy [10]. Attenello et al. assessed a cohort of patients in the USA who developed shunt infections within 18 months of surgery; the mean hospital cost per shunt infection was close to $50,000 [11].
Given the enormous risk to patients and the health care burdens associated with CSF shunt infections, additional interventions for risk reduction are necessary. Prior studies have investigated the role of perioperative antibiotics and, more recently, antibiotic-impregnated shunts (AIS). A Cochrane meta-analysis of 15 trials found that perioperative administration of systemic, prophylactic antibiotics in intracranial ventricular shunt procedures reduced infection (odds ratio (OR) 0.52, 95 % confidence interval (CI) 0.36–0.74) compared to placebo or no antibiotics [12]. A Cochrane meta-analysis of two trials found that AIS reduced infection (OR 0.21, 95 % CI 0.08–0.55) compared to standard shunt catheters [12]. A meta-analysis by Parker et al. comparing AIS versus non-AIS systems also identified a significant improvement in infection rate (3.3 vs 7.2 %, p < 0.0001) [13].
Beyond the use of systemic antibiotics and AISs—which are widely accepted—many institutions have initiated perioperative protocols designed to minimize infection; these take into account factors such as double gloving, antimicrobial drapes and sutures, solutions for prepping the surgical site, and the structure or function of operating room (OR) processes or personnel. No analysis has consolidated the findings from these institution-level studies. This would be of value to any institution seeking to construct and implement a perioperative protocol. To address this, we conducted a systematic review of the current literature on interventional measures, beyond systemic antibiotics and AISs, which have been designed to reduce shunt infection rates.
Methods
Inclusion criteria
Only studies evaluating the effect of a particular intervention on the incidence of shunt infection were included. Studies primarily evaluating external ventricular drain (EVD) infection were excluded. Non-English articles, animal and in vitro studies were excluded. There were no restrictions on publication year or status. For clinical studies using duplicate data, only the study with the most recent results were included. Because the efficacy of perioperative prophylactic intravenous antibiotics is no longer controversial, trials utilizing a control group that did not receive perioperative intravenous antibiotics were excluded [12]. The efficacy of antibiotic-impregnated shunts (AIS) has been evaluated extensively in randomized controlled trials, several meta-analyses, and a Cochrane review [12–17]. Therefore, we excluded studies evaluating the efficacy of AIS compared to non-AIS.
Data collection
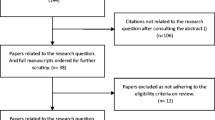
We searched PubMed and SCOPUS using the terms “shunt” and “infection,” which returned 10,602 results (Fig. 1—PRISMA flow diagram [18]). Abstracts were screened for relevance, which narrowed the group to 86. Full text was assessed in the resulting 86 articles for eligibility criteria, resulting in 23 articles that were included in the final systematic review. References of full-text articles were searched for any additional references not identified in the original search. The search period ended November 25, 2014. Two reviewers conducted data extraction from the 23 articles independently, and results were concordant in all cases. Strength of evidence of the included articles was assessed and assigned a score using the Oxford Centre for Evidence Based Medicine (OCEBM) Level of Evidence 2 classification system (Table 1) [19].
PRISMA flow diagram [18]
Results
Comprehensive surgical protocols: We identified eight studies evaluating the effect of comprehensive surgical protocols on the incidence of shunt infection (Table 2). Seven studies used historical control groups, and one study had no control group. Due to the use of historical controls in most studies, the level of evidence was low: level 3 in one large, multicenter study and level 4 in the remaining studies. In the seven studies with a control group, historical infection rate was high (6.4–13.2 %), and an institutional protocol was developed to decrease shunt infection. Infection rates were reduced after protocol initiation in all studies, ranging from 0.17 to 5.7 % (absolute risk reduction 2.2–12.3 %, relative risk reduction 33.8–97.4 %, Table 2). Only three studies provided statistical comparisons between cohorts, and significant reduction in shunt infection was found in two of these studies (Table 2) [20, 21, 25].
The earliest reported implementation of a surgical protocol was by Welch et al. (level 4 evidence) in 1979 [27]. Their protocol required glove change prior to handling of the shunt and multiple levels of antibiotics (intravenous, intrathecal, intrashunt, and antibiotic irrigation). In 1992, Choux et al. (level 4) evaluated 1197 surgeries under a different protocol involving preoperative povidone-iodine hair wash with no shaving, repeat hair wash on postoperative day 1, and irrigation of the shunt with gentamicin intraoperatively [26]. The protocol also standardized the time of day of shunt cases, duration, and composition of OR personnel. The resulting infection rate of 0.17 % was also the lowest infection rate observed among all of the protocol studies. Subsequently, other groups have modified the Choux protocol and observed reductions in shunt infection, albeit not to rates as low as those reported by Choux et al. Modifications of the Choux protocol have included the following: opening the implant just prior to use [21], postoperative IV vancomycin [24], and excluding the use of Holter valves [23].
The remaining three studies assessing comprehensive surgical protocols were prospective in design. These protocols adopted several key elements of the Choux protocol, with individual study modifications, such as morning scheduling and limiting OR traffic, 5 % chlorhexidine gluconate preoperative hair wash followed by 5 % chlorhexidine alcohol skin prep, use of Ioban™ (3 M, St. Paul, MN) drapes, double gloving (followed by removal of the outer glove for catheter opening and manipulation), opening of the abdomen first, “no-touch” policy in which contact of implants with gloves and skin edges was avoided (otherwise implants were replaced), injection of vancomycin/gentamicin into the shunt reservoir, and closing of the cranium first. Of note, the studies by Kestle et al. and Pirotte et al. did not allow AIS catheters during the study period [22, 25], whereas in the trial by Hommelstad et al., AIS catheters were used in patients considered “high risk” [20]. Furthermore, of the eight protocol-based studies, only the three prospective studies reported the proportion of surgeries demonstrating adherence to all components of the protocol. Hommelstad et al. (level 4) reported the lowest compliance, 24.6 %, and demonstrated, on multivariate analysis, that only noncompliance with preoperative hair wash with 5 % chlorhexidine gluconate had a trend toward an association with shunt infection (p = 0.051) [20]. Kestle et al. (level 3) had the highest compliance at 74.5 %; on multivariate analysis, they identified that surgical scrub with antiseptic foam was associated with shunt infection [25].
Single variable intervention studies
We identified 12 studies—none of which were multicenter—that evaluated the effect of a single intervention on the incidence of shunt infection (Table 3). Multiple studies addressed preparation of the surgical field. Hirsch et al. (level 3) demonstrated a three-fold reduction in infections with the use of a plastic cell isolation system [39]. Both Horgan et al. (level 4) and Ratanalert et al. (level 3) evaluated the effect of eliminating the practice of shaving, which led to a reduction in infection rates in both studies, with the latter demonstrating significant between group differences [36, 37]. A prospective, nonrandomized study by Haliosis et al. (level 3) compared Ioban™ with plain surgical drapes. The only two cases of infection occurred with plain drapes, but the sample size was too small to demonstrate significance differences between groups [28].
Other studies assessed intraoperative factors. In Tulipan et al. (level 4) and Rehman et al. (level 4), implementation of double gloving—and removing the outer glove prior to implant handling in the latter study—resulted in significant reductions in infection rates compared with historical controls [31, 35]. Faillace et al. (level 4) implemented a “no touch” policy (described previously) and additionally separated skin and shunt instruments to reduce contamination, resulting in a three-fold reduction in infection (p = 0.058), but may have been inadequately powered [38]. Two studies examined wound irrigation. Theophilus et al. (level 2) noted that a methicillin-containing solution yielded an 11.1 % (nonsignificant) absolute reduction in shunt infection compared to saline without antibiotics [29]. Hayashi et al. (level 3) found that irrigation with saline or an amikacin-containing solution yielded significant and similar reductions in infection when compared with no irrigation at all [30].
With regards to wound closure, Rozzelle et al. (level 1), in a randomized, controlled, double-blinded trial, observed that triclosan-impregnated antimicrobial sutures compared to polyglactin sutures (Vicryl™, Ethicon, Somerville, NJ) for closure of the galea resulted in a significant reduction in infection rate (4.3 vs 21.1 %, p = 0.038) [33]. Eyman et al. (level 3) compared skin closure with Dermabond® (Ethicon, Somerville, NJ) alone to standard nonabsorbable suture and found an absolute reduction in shunt infection of 17 % with Dermabond® but did not provide any statistical analysis [32].
Nonstandard antibiotic prophylaxis
Three studies were identified that investigated nonstandard options for antibiotic prophylaxis (Table 4). Tacconelli et al. (level 1) found that perioperative prophylaxis with intravenous vancomycin, compared to cefazolin, significantly reduced shunt infection rate in a hospital known to have a high methicillin-resistant Staphylococcus aureus (MRSA) prevalence [40]. Ragel et al. (level 3), in a single-center, retrospective analysis, found that a surgeon who used IT gentamicin and vancomycin had a reduced infection rate (0.4 %) compared to surgeons that used either IT gentamicin alone (5.4 %) or no IT antibiotics (6.6 %) [41]. No statistics were provided for these analyses. In Nejat et al. (level 1), use of ceftriaxone or sulfamethoxazole-trimethoprim was associated with similar rates of postoperative infection [34].
Discussion
Shunt infection represents a major complication with significant patient morbidity and an associated large, and potentially largely preventable, cost. To our knowledge, this is the first systematic review that examines the effect of interventions designed to reduce shunt infection, outside of studies describing AISs and systemic antibiotic prophylaxis. Overall level of evidence was low in studies utilizing comprehensive protocols. While every study observed a decrease in infection rate, few studies demonstrated significance. Only noncompliance with preoperative 5 % chlorhexidine gluconate hair wash (p = 0.051) and surgical scrub with antiseptic foam (p = 0.01) were associated with shunt infection in the studies reviewed. In the single-variable intervention studies, only double gloving, with a glove change prior to implant handling, antimicrobial suture, or a no-shave policy were associated with a statistically significant reduction in shunt infection. Although systemic prophylactic vancomycin decreased shunt infection compared with cefazolin, the study was performed in a hospital with a high prevalence of MRSA, and it is unclear if the effects would be similar in a hospital with average or lower MRSA prevalence.
This study has limitations. First, the study design of the comprehensive protocol studies limits the scientific validity of their results. All studies with control groups used historical controls. In these centers, infection rate was noted to be high, leading to the construction and implementation of surgical protocols to reduce infection. As such, the participating surgeons and OR personnel are subject to the Hawthorne effect; study participants are more likely to alter their behavior knowing that prior infection rates were high and compliance and outcome were being closely monitored [42]. In addition, given that most protocols implemented more than one variable, it is difficult to determine which variables actually may have led to the reduction in shunt infection. Prospective, randomized, controlled studies assessing each intervention separately—or a few interventions grouped together—are needed to further our understanding of the optimal protocols to prevent shunt infections. Because of the heterogeneity of study methods and intervention variables, no summary statistics could be reasonably derived from the results of this systematic review. Last, six of seven comprehensive protocol studies specifying population age were limited to the pediatric population. Therefore, the results of these studies might not be generalizable to the adult hydrocephalus population. Conversely, many of the single variable intervention studies included both pediatric and adult patients.
While we have focused on the literature studying infection in shunt surgery, data from studies evaluating infection in craniotomy or general surgical literature may be applicable to shunt surgery. One area of contention in the neurosurgical community is the practice of hair removal; while some argue that this improves visualization and optimizes skin closure, there is no evidence to suggest that hair sparing increases infection rates [43, 44]. However, if hair removal is performed, clipping is associated with a lower infection rate than shaving with razors [45]. Two studies in our review demonstrated higher infection rates with shaving compared to no shaving, but hair clipping was not specifically evaluated.
The choice of surgical preparation solutions has been investigated by multiple groups. Darouiche et al. conducted a randomized trial across 6 hospitals in 849 patients comparing chlorhexidine-alcohol with povidone-iodine for clean-contaminated non-neurosurgical surgery [46]. They observed a statistically significant reduction in superficial and deep wound infections in the chlorhexidine-alcohol group. A meta-analysis by Noorani et al. reported similar benefits of chlorhexidine-alcohol for non-neurosurgical preoperative antisepsis in clean-contaminated wounds [47]. A recent Cochrane meta-analysis suggested that alcohol-based solutions may be the most effective at preventing surgical site infection in clean surgery [48]. Interestingly, in the present systematic review, we noted that chlorhexidine-based prep was integrated into several comprehensive and single-variable protocols. One study further identified that noncompliance with 5 % chlorhexidine-gluconate hair wash was associated with increased infection [20]. Due to the underpowered nature of some studies, the effect of skin surgical prep as an independent variable could not be fully ascertained.
Given the heterogeneity of studies captured through our systematic review, as well as individual habits and practices, it is not surprising that current practices to control infection rates following shunt surgery remain variable. A 2009 Web-based survey given to pediatric neurosurgeons who were members of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons revealed wide variation in both knowledge and use of certain interventions [49]. For example, intraventricular antibiotics were used by 27 % of respondents while antibiotic-impregnated sutures were used by 14 out of the 59 respondents who were familiar with them. In reviewing other surgical factors, 62 % of those surveyed used double gloving, 90 % limited shunt contact with skin, and 45 % handled shunt components only with instruments. The only universally applied intervention was perioperative systemic antibiotic prophylaxis, either with vancomycin or a cephalosporin. With the emergence of higher quality studies in recent years and through systematic consolidation of the literature, as in the current review, we hope that practice patterns in reducing shunt infection will become more standardized and evidence-based.
Conclusion
To our knowledge, this is the first systematic review of studies evaluating the effect of interventions to reduce shunt infection, beyond the accepted use of intravenous, perioperative antibiotics and AISs. The use of surgical scrub with antiseptic foam and the omission of a 5 % chlorhexidine gluconate preoperative hair wash were both associated with an increase in shunt infection. Only antibiotic-impregnated suture, a no-shave policy, and double gloving with glove change prior to shunt handling, significantly reduces the incidence of shunt infection. For hospitals with high MRSA prevalence, use of perioperative intravenous vancomycin rather than cefazolin significantly reduces shunt infection. This analysis will aid institutions that seek to design and implement a surgical protocol or adopt surgical practices aimed at reducing the incidence of shunt infection.
References
Bondurant CP, Jimenez DF (1995) Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 23:254–258
Patwardhan RV, Nanda A (2005) Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 56:139–145
Simon TD, Riva-Cambrin J, Srivastava R, Bratton SL, Dean JM, Kestle JRW, Hydrocephalus Clinical Research Network (2008) Hospital care for children with hydrocephalus in the United States: utilization, charges, comorbidities, and deaths. J Neurosurg Pediatr 1:131–137
Browd SR, Ragel BT, Gottfried ON, Kestle JRW (2006) Failure of cerebrospinal fluid shunts: part i: obstruction and mechanical failure. Pediatr Neurol 34:83–92
Duhaime A-C (2006) Evaluation and management of shunt infections in children with hydrocephalus. Clin Pediatr (Phila) 45:705–713
Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, LaFleur B, Dean JM, Kestle JR, Hydrocephalus Clinical Research Network (2009) Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. J Neurosurg Pediatr 4:156–165
Reddy GK, Bollam P, Caldito G (2012) Ventriculoperitoneal shunt surgery and the risk of shunt infection in patients with hydrocephalus: long-term single institution experience. World Neurosurg 78:155–163
Jeelani NU O, Kulkarni AV, DeSilva P, Thompson DNP, Hayward RD (2009) Postoperative cerebrospinal fluid wound leakage as a predictor of shunt infection: a prospective analysis of 205 cases. J Neurosurg Pediatr 4:166–169
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 36:858–862
Shannon CN, Simon TD, Reed GT, Franklin FA, Kirby RS, Kilgore ML, Wellons JC 3rd (2011) The economic impact of ventriculoperitoneal shunt failure. J Neurosurg Pediatr 8:593–599
Attenello FJ, Garces-Ambrossi GL, Zaidi HA, Sciubba DM, Jallo GI (2010) Hospital costs associated with shunt infections in patients receiving antibiotic-impregnated shunt catheters versus standard shunt catheters. Neurosurgery 66:284–289
Ratilal B, Costa J, Sampaio C (2006) Antibiotic prophylaxis for surgical introduction of intracranial ventricular shunts. Cochrane Database Syst Rev CD005365
Parker SL, Anderson WN, Lilienfeld S, Megerian JT, McGirt MJ (2011) Cerebrospinal shunt infection in patients receiving antibiotic-impregnated versus standard shunts. J Neurosurg Pediatr 8:259–265
Eymann R, Chehab S, Strowitzki M, Steudel W-I, Kiefer M (2008) Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr 1:444–450
Klimo P Jr, Thompson CJ, Ragel BT, Boop FA (2011) Antibiotic-impregnated shunt systems versus standard shunt systems: a meta- and cost-savings analysis. J Neurosurg Pediatr 8:600–612
Richards HK, Seeley HM, Pickard JD (2009) Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatr 4:389–393
Thomas R, Lee S, Patole S, Rao S (2012) Antibiotic-impregnated catheters for the prevention of CSF shunt infections: a systematic review and meta-analysis. Br J Neurosurg 26:175–184
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 6:e1000097
Howick J, Chalmers I, Glasziou P, Greenhalgh T, Heneghan C, Liberati A, Moschetti I, Phillips B, Thornton H (2011) Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxf Cent Evid-Based Med. http://www.cebm.net/index.aspx?o=5653
Hommelstad J, Madsø A, Eide PK (2013) Significant reduction of shunt infection rate in children below 1 year of age after implementation of a perioperative protocol. Acta Neurochir (Wien) 155:523–531
Kestle JR, Hoffman HJ, Soloniuk D, Humphreys RP, Drake JM, Hendrick EB (1993) A concerted effort to prevent shunt infection. Childs Nerv Syst 9:163–165
Pirotte BJM, Lubansu A, Bruneau M, Loqa C, Van Cutsem N, Brotchi J (2007) Sterile surgical technique for shunt placement reduces the shunt infection rate in children: preliminary analysis of a prospective protocol in 115 consecutive procedures. Childs Nerv Syst 23:1251–1261
Mottolese C, Grando J, Convert J, Abdoulrahman M, Lelievre H, Vandenesch F, Bret P, Lapras C (2000) Zero rate of shunt infection in the first postoperative year in children–dream or reality? Childs Nerv Syst 16:210–212
Rotim K, Miklic P, Paladino J, Melada A, Marcikic M, Scap M (1997) Reducing the incidence of infection in pediatric cerebrospinal fluid shunt operations. Childs Nerv Syst 13:584–587
Kestle JRW, Riva-Cambrin J, Wellons JC 3rd, Kulkarni AV, Whitehead WE, Walker ML, Oakes WJ, Drake JM, Luerssen TG, Simon TD, Holubkov R, Hydrocephalus Clinical Research Network (2011) A standardized protocol to reduce cerebrospinal fluid shunt infection: the Hydrocephalus Clinical Research Network Quality Improvement Initiative. J Neurosurg Pediatr 8:22–29
Choux M, Genitori L, Lang D, Lena G (1992) Shunt implantation: reducing the incidence of shunt infection. J Neurosurg 77:875–880
Welch K (1979) Residual shunt infection in a program aimed at its prevention. Z Kinderchir Grenzgeb 28:374–377
Haliasos N, Bhatia R, Hartley J, Thompson D (2012) Ioban drapes against shunt infections? Childs Nerv Syst 28:509–510
Theophilus SC, Adnan JS (2011) A randomised control trial on the use of topical methicillin in reducing post-operative ventriculoperitoneal shunt infection. Malays J Med Sci 18:30–37
Hayashi T, Shirane R, Yokosawa M, Kimiwada T, Tominaga T (2010) Efficacy of intraoperative irrigation with saline for preventing shunt infection. J Neurosurg Pediatr 6:273–276
Rehman A-U, Rehman T-U, Bashir HH, Gupta V (2010) A simple method to reduce infection of ventriculoperitoneal shunts. J Neurosurg Pediatr 5:569–572
Eymann R, Kiefer M (2010) Glue instead of stitches: a minor change of the operative technique with a serious impact on the shunt infection rate. Acta Neurochir Suppl 106:87–89
Rozzelle CJ, Leonardo J, Li V (2008) Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J Neurosurg Pediatr 2:111–117
Nejat F, Tajik P, El Khashab M, Kazmi SS, Khotaei GT, Salahesh S (2008) A randomized trial of ceftriaxone versus trimethoprim-sulfamethoxazole to prevent ventriculoperitoneal shunt infection. J Microbiol Immunol Infect 41:112–117
Tulipan N, Cleves MA (2006) Effect of an intraoperative double-gloving strategy on the incidence of cerebrospinal fluid shunt infection. J Neurosurg 104:5–8
Ratanalert S, Musikawat P, Oearsakul T, Saeheng S, Chowchuvech V (2005) Non-shaved ventriculoperitoneal shunt in Thailand. J Clin Neurosci 12:147–149
Horgan MA, Piatt JH Jr (1997) Shaving of the scalp may increase the rate of infection in CSF shunt surgery. Pediatr Neurosurg 26:180–184
Faillace WJ (1995) A no-touch technique protocol to diminish cerebrospinal fluid shunt infection. Surg Neurol 43:344–350
Hirsch JF, Renier D, Pierre-Kahn A (1978) Influence of the use of a surgical isolator on the rate of infection in the treatment of hydrocephalus. Childs Brain 4:137–150
Tacconelli E, Cataldo MA, Albanese A, Tumbarello M, Arduini E, Spanu T, Fadda G, Anile C, Maira G, Federico G, Cauda R (2008) Vancomycin versus cefazolin prophylaxis for cerebrospinal shunt placement in a hospital with a high prevalence of meticillin-resistant Staphylococcus aureus. J Hosp Infect 69:337–344
Ragel BT, Browd SR, Schmidt RH (2006) Surgical shunt infection: significant reduction when using intraventricular and systemic antibiotic agents. J Neurosurg 105:242–247
Parsons HM (1974) What Happened at Hawthorne? New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science 183:922–932
Broekman MLD, van Beijnum J, Peul WC, Regli L (2011) Neurosurgery and shaving: what’s the evidence? J Neurosurg 115:670–678
Sebastian S (2012) Does preoperative scalp shaving result in fewer postoperative wound infections when compared with no scalp shaving? A systematic review. J Neurosci Nurs 44:149–156
Tanner J, Norrie P, Melen K (2011) Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev CD004122
Darouiche RO, Wall MJ, Itani KMF, Otterson MF, Webb AL, Carrick MM, Miller HJ, Awad SS, Crosby CT, Mosier MC, Alsharif A, Berger DH (2010) Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 362:18–26
Noorani A, Rabey N, Walsh SR, Davies RJ (2010) Systematic review and meta-analysis of preoperative antisepsis with chlorhexidine versus povidone-iodine in clean-contaminated surgery. Br J Surg 97:1614–1620
Dumville JC, McFarlane E, Edwards P, Lipp A, Holmes A (2013) Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev 3, CD003949
Gruber TJ, Riemer S, Rozzelle CJ (2009) Pediatric neurosurgical practice patterns designed to prevent cerebrospinal fluid shunt infection. Pediatr Neurosurg 45:456–460
Conflict of interest
AGM is a consultant for Spinal Modulation and Functional Neuromodulation. AGM has distribution rights related to intellectual property with ATI, Cardionomics and Enspire. RJW was supported by the Melvin Burkhardt chair in neurosurgical oncology and the Karen Colina Wilson research endowment within the Brain Tumor and Neuro-oncology Center at the Cleveland Clinic. None of the funders played a role in data collection, analysis, interpretation, or the writing or editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarmey, N., Kshettry, V.R., Shriver, M.F. et al. Evidence-based interventions to reduce shunt infections: a systematic review. Childs Nerv Syst 31, 541–549 (2015). https://doi.org/10.1007/s00381-015-2637-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2637-2