Abstract
The management of idiopathic dilated cardiomyopathy (DCM) is well established. However, a subset of patients do not have recovery from or have recurrences of left ventricular (LV) dysfunction despite receiving optimal medical therapy. There are limited long-term follow-up data about LV function and the predictive value of iodine-123-metaiodobenzylguanidine (123I-MIBG) scintigraphy, especially among the Japanese population. We retrospectively investigated 81 consecutive patients with DCM (mean LV ejection fraction (EF) 28 ± 7.5%) who had undergone 123I-MIBG scintigraphy before starting β-blockers. According to chronological changes in LVEF, study patients were classified into three subgroups: sustained recovery group, recurrence group, and non-recovery group. The outcome measure was cardiac death. Mean age was 59 ± 11 years and median follow-up was 11.5 (5.8–15.0) years. Thirty-six patients had recovery, 11 had recurrences, and 34 did not have recovery. The sustained recovery group had the best cardiac death-free survival, followed by the recurrence and non-recovery groups. Prolonged time to initial recovery was associated with recurrence of LV dysfunction. Large LV end-diastolic diameter and reduced heart to mediastinum ratio were associated with poor prognosis. In conclusion, with β-blocker therapy, 14% of patients showed recurrences of LV dysfunction. Thus, careful follow-up is needed, keeping in mind the possibility of recurrence, even if LVEF once improved, especially in patients whose time to initial recovery was long. 123I-MIBG scintigraphy provides clinicians with additional prognostic information.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Non-ischemic dilated cardiomyopathy (DCM), a relatively common disorder, is characterized by left ventricular (LV) dilation and impaired systolic function leading to heart failure [1, 2]. Its management is well established, β-blockers being considered the first-line drug [3, 4]. The sympathetic nervous system is activated in patients with DCM and β-blockers are thought to reduce the detrimental effects of catecholamine stimulation [5, 6]. However, despite optimal medical therapy, a subset of patients do not show improvement or have recurrence of LV dysfunction [7,8,9].
Iodine-123-metaiodobenzylguanidine (123I-MIBG), an analogue of the adrenergic neuron blocking agent guanethidine, is thought to use the same myocardial uptake and release mechanism as norepinephrine [10]. 123I-MIBG scintigraphic findings reflect the status of myocardial sympathetic innervation and may be related to severity of myocardial damage [11]. Previous studies have reported that such findings can predict the prognosis of patients with DCM [12, 13]. However, the duration of follow-up in these previous studies was relatively short and long-term follow-up data are limited, especially among Japanese population. The purpose of this study was to investigate long-term chronological changes in LV function after β-blocker therapy and long-term prognosis in patients with DCM and to clarify the predictive value of 123I-MIBG scintigraphic findings.
Patients and methods
Study population
Relevant data of 81 consecutive patients (mean age 59 ± 11 years) with DCM who had undergone 123I-MIBG scintigraphy before starting β-blockers (carvedilol or bisoprolol fumarate) from 1993 to 2005 in Tenri Hospital were retrospectively investigated. DCM was defined by the following criteria: (1) LV dysfunction with left ventricular ejection fraction (LVEF) < 40% according to transthoracic echocardiography (TTE) and (2) no significant coronary artery disease according to coronary angiography or coronary multidetector computed tomography. Patients whose LV dysfunction was caused by acute myocarditis, stress-induced cardiomyopathy, cardiac amyloidosis, sarcoidosis, valvular heart disease, or sepsis were also excluded. To distinguish the patients with DCM from those with tachycardia-induced cardiomyopathy, we also excluded patients with atrial fibrillation which may become the cause of tachyarrhythmia. The study protocol was approved by the institutional ethics committee of Tenri Hospital (Nara, Japan).
TTE variables and classification of study patients
During the acute phase of heart failure, patients were treated with standard therapy such as diuretics. After control of heart failure had been achieved and before starting β-blocker therapy, baseline TTE was performed. Follow-up TTE was routinely performed 6 months, and 1, 2 and 3 years after initiating β-blocker therapy. TTE variables included left atrial dimensions, left ventricular end-diastolic diameter (LVDd), left ventricular end-systolic diameter and thickness of left interventricular septum and posterior wall measured by M-mode echocardiography. LVEF was measured using a modified Simpson’s method. These comprehensive echocardiographic assessments were conducted by experienced sonographers using commercially available ultrasound systems. TTE performed during long-term follow-up [10.4 (IQR 6.3–13.6) years after initiating β-blocker therapy] was also evaluated.
Study patients were classified according to chronological changes in LVEF as in a previous study [8]. First, study patients were divided into initial recovery and non-recovery groups by comparing LVEF before and early after (from 6 months to 3 years) initiating β-blocker therapy. Initial recovery was defined as recovery of LVEF to > 40% with a net increase in LVEF of ≥ 10% from baseline. Non-recovery was defined as not meeting the above criteria. Patients who died within 3 years without recovery of LVEF were classified into the non-recovery group. The time to achieve initial recovery was also evaluated in the initial recovery group, time to initial recovery being defined as time from baseline TTE to first TTE showing initial recovery.
Next, patients with initial recovery were divided into two subgroups: namely, sustained recovery and recurrence groups, by comparing LVEF early and long-term after initiating β-blocker therapy. Recurrence was defined as showing a decrease in LVEF ≥ 10% at long-term follow-up TTE compared with early after initiating β-blocker therapy, whereas sustained recovery was defined as showing sustained improvement in LVEF from early after initiating β-blocker therapy to long-term follow-up TTE.
123I-MIBG scintigraphy
123I-MIBG scintigraphy was performed at rest in the fasting state after stabilization of heart failure and before starting β-blockers. A large-field gamma camera (Toshiba GCA9300A/HG; Toshiba, Tokyo, Japan) was used to acquire anterior planar images of the chest 15 min and 4 h after intravenous injection of 111 MBq of 123I-MIBG. The heart to mediastinum ratio (HMR) was determined by measuring the average counts in each region of interest after a nuclear cardiologist, who was blinded to the patient’s data, had manually outlined regions of interest in the heart, upper mediastinum and right lung. The 123I-MIBG washout rate (WR) from the heart was calculated as the difference between early and delayed images.
Clinical outcomes and their predictors
Clinical follow-up data were obtained from the patients’ medical records. The outcome measure was cardiac death, which was defined as sudden death or death from congestive heart failure (CHF). To identify predictors of clinical outcomes, the patients’ clinical characteristics, laboratory and cardiac catheterization data and TTE and 123I-MIBG scintigraphic findings at diagnosis were investigated. Clinical characteristics and cardiac catheterization data were collected from the medical records. Clinical characteristics included age, sex, underlying disorders, and medications. Underlying disorders included hypertension, dyslipidaemia and diabetes mellitus. Hypertension was defined as systolic blood pressure of ≥ 140 mmHg and/or a diastolic pressure of ≥ 90 mmHg or use of antihypertensive medications. Dyslipidaemia was defined as serum cholesterol concentration ≥ 220 mg/dL or use of cholesterol-lowering medications. Diabetes mellitus was defined as hyperglycaemia requiring medication. Medications included angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), aldosterone antagonists, diuretics and amiodarone. Laboratory data included estimated glomerular filtration rate, and serum concentrations of haemoglobin, creatinine, sodium and brain natriuretic peptide (BNP), the last being available only for 65 patients (80%). All study patients had undergone cardiac catheterization at diagnosis. Cardiac catheterization data included resting heart rate, systolic and diastolic arterial pressure, mean pulmonary arterial pressure, mean pulmonary capillary wedge pressure and cardiac output. TTE and 123I-MIBG scintigraphic findings are shown above.
Furthermore, to determine predictors of recurrence of LV dysfunction for patients in the initial recovery group, clinical characteristics, laboratory data, TTE findings at both baseline and initial recovery and 123I-MIBG scintigraphic findings were evaluated.
Statistical analysis
Statistical analysis was performed using JMP version 8. Categorical variables are presented as numbers and percentages, and were compared using Fisher’s exact test. Continuous variables are presented as mean (standard deviation) or median [interquartile range (IQR)]. Continuous variables were compared using unpaired t test or the Wilcoxon rank-sum test between two groups. Event-free survival rates from cardiac death are presented in Kaplan–Meier curves and were compared by a log-rank test. Relative risks and 95% confidence intervals were calculated using Cox proportional-hazards analysis. A p value of < 0.05 was considered to denote statistical significance.
Results
Baseline patient characteristics and initial recovery
The study cohort comprised 59 men and 22 women with a mean age of 59 ± 11 years. LVDd and LVEF at diagnosis were 66 ± 7.4 mm and 28 ± 7.5%, respectively. Median duration of follow-up was 11.5 (IQR 5.8–15.0) years. The baseline characteristics of all 81 patients are shown in Tables 1 and 2. Early after initiating β-blocker therapy, 47 patients (58%) showed initial recovery (initial recovery group), and 34 patients (42%) did not (non-recovery group). LVDd and LVEF at early follow-up TTE were 56 ± 5.6 mm and 54 ± 9.0% in the initial recovery group, and 66 ± 9.9 mm and 30 ± 9.1% in the non-recovery group. The non-recovery group tended to be older and had a larger LVDd at diagnosis than the initial recovery group, but these were not statistically significant. The non-recovery group more often received amiodarone and implantable cardioverter-defibrillator than the initial recovery group. All patients had received β-blocker. ACEIs or ARBs had been administered to 60% of the initial recovery and 62% of the non-recovery group. There were no significant differences in treatment strategy at diagnosis between the initial recovery and non-recovery groups.
Chronological changes in LVEF during long-term follow-up
Long-term follow-up TTE was performed 10.4 (IQR 6.3–13.6) years after initiating β-blocker therapy. Four of the 34 patients in the non-recovery group showed cardiac death within 3 years of diagnosis without recovery of LVEF. The remaining 30 patients in the non-recovery group did not have recovery of LVEF at long-term follow-up (LVEF 29 ± 8.7%). No patient experienced recovery of LVEF more than 3 years after initiating β-blocker therapy. Sustained recovery of LVEF occurred in 36 of 47 patients (77%) in the initial recovery group (sustained recovery group). However, LV dysfunction recurred in 11 of 47 patients (23%) in the initial recovery group (recurrence group). In the sustained recovery group, LVEF was 27 ± 6.8% at diagnosis, 53 ± 9.0% at early and 57 ± 8.8% at long-term after initiating β-blocker therapy. In the recurrence group, LVEF was 25 ± 8.0% at diagnosis, 57 ± 8.9% at early and 33 ± 9.7% at long-term after initiating β-blocker therapy. Recurrence of LV dysfunction developed 6.0 (IQR 4.1–6.6) years after initiating β-blocker therapy in the recurrence group. Chronological changes in LVEF are shown in Fig. 1.
At the last follow-up, β-blockers and ACEIs or ARBs were being administered to 94% and 61% of the sustained recovery group, 100% and 55% of the recurrence group, and 100% and 62% of the non-recovery group, respectively. There were no significant differences between groups in treatment strategy at the last follow-up.
Predictors of recurrence of LV dysfunction
Clinical characteristics, laboratory data and scintigraphic and TTE variables both before initiating β-blockers and at initial recovery were compared between the sustained recovery and recurrence groups to determine predictors of recurrence of LV dysfunction. The time to initial recovery was longer, mean pulmonary arterial pressure and mean papillary wedge pressure at diagnosis were higher in the recurrence than the sustained recovery group. There were no significant differences in 123I-MIBG scintigraphic findings at diagnosis or other TTE findings at both baseline and initial recovery (Tables 3, 4).
Cardiac events and their predictors
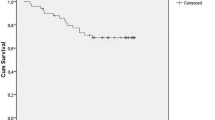
Cardiac death-free rates for all the study patients were 85% at 10 years and 73% at 15 years (Fig. 2a). The cardiac death-free rate was significantly better in the sustained recovery group than in the other two groups (cardiac death-free rate at 15 years: 100% vs. 86% vs. 39%, respectively; p < 0.01) (Fig. 2b). There were no cardiac deaths in the sustained recovery group, whereas there were 14 deaths (sudden death, 10; death due to CHF, 4) in the non-recovery group and 2 deaths (sudden death, 1; death due to CHF, 1) in the recurrence group. Univariate Cox proportional-hazard analysis identified LVDd and HMR as predictors of cardiac death (Table 5). Focusing on sudden death, HMR tended to be lower in 11 patients with sudden death than those without sudden death (HMR at 15 min, 1.68 vs. 1.82, p = 0.08; HMR at 4 h, 1.53 vs. 1.64, p = 0.15). Cardiac death-free rates tended to be higher in patients with a HMR at 4 h of ≥ 1.63 (n = 41) than in those with a HMR at 4 h of < 1.63 (n = 40) (91% vs. 80% at 10 years, 82% vs. 66% at 15 years; p = 0.17) (Fig. 3). This cut-off value of HMR at 4 h was determined by the median value.
Discussion
In the present study, we examined chronological changes in LV function and responsiveness to β-blocker therapy in patients with DCM and clarified the very long-term clinical outcomes, prognostic factors and predictive value of 123I-MIBG scintigraphy findings.
Chronological changes in LV function and prognosis of subgroups
In the present study, 36 patients (44%) had sustained recovery of LVEF, 11 (14%) had recurrence of LV dysfunction, and 34 (42%) had no recovery of LVEF. Previous studies have reported that LVEF recovered in 30–50% of patients with DCM [8, 9, 14, 15] and that there is a 15–39% incidence of recurrence of LV dysfunction after initial recovery [7, 8]. Regarding the timing of recurrence, Gupta et al. [8] reported that 32% of patients had recurrences by 5 years after diagnosis and that 68% had recurrences thereafter. Although the duration of follow-up in our study was much longer than in these previous studies (11.5 years vs. 4.2 years), the incidences of recovery and recurrence, and timing of recurrence in our study are consistent with those of previous studies. Additionally, we documented chronological changes in LV function during very long-term follow-up, and no patients who failed to show recovery of LV function within 3 years after initiating β-blocker therapy experienced improvement in LVEF thereafter. These findings suggest that further treatment, such as cardiac resynchronization therapy or cardiac transplantation, should be considered for patients with no evidence of recovery of LVEF by 3 years after initiating β-blocker therapy.
Regarding prognosis, the event-free rate was significantly greater in the sustained recovery group than in the non-recovery and recurrence groups. Previous studies have also shown that patients who recover LV function have better prognoses; however, there are few data on prognosis of patients with recurrence of LV dysfunction [7,8,9, 14]. In this study, the prognosis of patients with recurrence of LV dysfunction is similar to that of those with sustained recovery up to 10 years; however, once LV dysfunction has recurred, the prognosis became poorer than that of patients with sustained recovery.
Predictors of recurrence
As to predictors of recurrence, time to initial recovery was longer in the recurrence than the sustained recovery group. Both patients with recurrence and sustained recovery showed similar LVEF at initial recovery, but some later developed recurrences whereas others showed sustained recovery. These findings suggest that recovery of LVEF early after initiating β-blocker therapy cannot be regarded as complete recovery. It is possible that ultrastructural damage to the myocardium may persist after initial recovery and be related to recurrence; however, we did not confirm this possibility by endomyocardial biopsy in all patients [16]. The prolonged time to initial recovery in the recurrence group may reflect persistence of ultrastructural damage to the myocardium. Therefore, careful follow-up is needed, keeping in mind the possibility of recurrence, even if LVEF once improved, especially in patients whose time to initial recovery was longer.
Predictive value of 123I-MIBG scintigraphy
Reduced HMR is a predictor of prognosis, and 123I-MIBG scintigraphic findings can predict cardiac death. In the current study, the main cause of cardiac deaths was not CHF (5 patients, 31%), but sudden death (11 patients, 69%), and HMR tended to be lower in patients with sudden death. 123I-MIBG scintigraphic findings may be able to predict sudden death in patients with DCM, as has been shown for those with chronic heart failure [17]. Increased sympathetic activity can modulate arrhythmogenic mechanism of re-entry, automaticity, and triggered activity, provoking lethal arrhythmias [18]. However, 123I-MIBG scintigraphic findings did not predict improvement in cardiac function. The explanation for this discrepancy is unknown; however, several studies have reported similar results to ours. Lee et al. have reported that MIBG imaging does not predict increase in LVEF after β-blocker therapy in patients with DCM [19]. Yamazaki et al. have also reported no significant differences in 123I-MIBG scintigraphic findings, including WR and reduced HMR, between responders and non-responders to β-blockers [20].
Limitations
The present study has several limitations, mainly because of its retrospective nature. First, not all patients were followed-up systematically during long-term follow-up, and selection bias was inevitable. Second, biomarkers such as BNP were not available for all patients because this study included old data and BNP was unavailable before 1994. Third, a relatively small percentage of patients had received ACEIs or ARBs compared with previous studies. This discrepancy may be attributable to adverse reactions to ACEIs and ARBs, such as cough, hypotension and renal dysfunction. East Asian ethnicity is known to be a risk factor for ACEI-induced cough, whereas African–American ethnicity is not [21]. Furthermore, the indication of chronic heart failure for ARBs was first approved in Japan in 2005. Patients enrolled in the present study were treated between 1993 and 2005, at least in part explaining the low rate of administration of ACEIs or ARBs. The same tendency was observed in another study evaluating the clinical characteristics and outcomes of hospitalized patients with CHF that was conducted in Japan between 2001 and 2002, in which 62.5% of participants received ACEIs or ARBs [22]. Although the percentages of patients receiving ACEIs or ARBs were similar in the three subgroups in this study, they may have affected our results. Fourth, the limited number of patients and cardiac events did not allow us to perform multivariable analysis. Finally, we excluded patients with atrial fibrillation but there remains a possibility that our study cannot completely exclude patients with tachycardia-induced cardiomyopathy.
Conclusions
With β-blocker therapy, 42% of patients did not improve and 14% had recurrence of LV dysfunction; both groups of patients had poor prognoses. Careful follow-up is needed, even after restoration of LV dysfunction. Furthermore, 123I-MIBG scintigraphy can provide clinicians with additional information regarding prognosis.
References
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines (2013) 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62(16):e147–e239
Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P (2016) Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 37(23):1850–1858
MERIT-HF Study Group (1999) Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 353(9169):2001–2007
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, Shusterman NH (1996) The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 334(21):1349–1355
Fukuoka S, Hayashida K, Hirose Y, Shimotsu Y, Ishida Y, Kakuchi H, Eto T (1997) Use of iodine-123 metaiodobenzylguanidine myocardial imaging to predict the effectiveness of beta-blocker therapy in patients with dilated cardiomyopathy. Eur J Nucl Med 24(5):523–529
Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, Suzuki T, Kurabayashi M (2007) Evaluation of cardiac sympathetic nerve activity and left ventricular remodelling in patients with dilated cardiomyopathy on the treatment containing carvedilol. Eur Heart J 28(8):989–995
Park JS, Kim JW, Seo KW, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, Shin JH (2014) Recurrence of left ventricular dysfunction in patients with restored idiopathic dilated cardiomyopathy. Clin Cardiol 37(4):222–226
Gupta A, Goyal P, Bahl A (2014) Frequency of recovery and relapse in patients with nonischemic dilated cardiomyopathy on guideline-directed medical therapy. Am J Cardiol 114(6):883–889
Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Di Lenarda A, Sinagra G (2011) Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol 57(13):1468–1476
Wieland DM, Wu J, Brown LE, Mangner TJ, Swanson DP, Beierwaltes WH (1980) Radiolabeled adrenergic neuron-blocking agents: adrenomedullary imaging with [131I]iodobenzylguanidine. J Nucl Med 21(4):349–353
Schofer J, Spielmann R, Schuchert A, Weber K, Schluter M (1988) Iodine-123 meta-iodobenzylguanidine scintigraphy: a noninvasive method to demonstrate myocardial adrenergic nervous system disintegrity in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol 12(5):1252–1258
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, Agostini D, Weiland F, Chandna H, Narula J, ADMIRE-HF Investigators (2010) Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol 55(20):2212–2221
Kamiyoshi Y, Yazaki Y, Urushibata K, Koizumu T, Kasai H, Izawa A, Kinoshita O, Hongo M, Ikeda U (2008) Risk stratification assessed by combined lung and heart iodine-123 metaiodobenzylguanidine uptake in patients with idiopathic dilated cardiomyopathy. Am J Cardiol 101(10):1482–1486
Choi JO, Kim EY, Lee GY, Lee SC, Park SW, Kim DK, Oh JK, Jeon ES (2013) Predictors of left ventricular reverse remodeling and subsequent outcome in nonischemic dilated cardiomyopathy. Circ J 77(2):462–469
Ikeda Y, Inomata T, Iida Y, Iwamoto-Ishida M, Nabeta T, Ishii S, Sato T, Yanagisawa T, Mizutani T, Naruke T, Koitabashi T, Takeuchi I, Nishii M, Ako J (2016) Time course of left ventricular reverse remodeling in response to pharmacotherapy: clinical implication for heart failure prognosis in patients with idiopathic dilated cardiomyopathy. Heart Vessels 31(4):545–554
Ishii S, Inomata T, Fujita T, Iida Y, Ikeda Y, Nabeta T, Yanagisawa T, Naruke T, Mizutani T, Koitabashi T, Takeuchi I, Ako J (2016) Clinical significance of endomyocardial biopsy in conjunction with cardiac magnetic resonance imaging to predict left ventricular reverse remodeling in idiopathic dilated cardiomyopathy. Heart Vessels 31(12):1960–1968
Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, Masuda M, Okuda K, Iwasaki Y, Yasui T, Hori M, Fukunami M (2009) Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol 53(5):426–435
Brunner-La Rocca HP, Esler MD, Jennings GL, Kaye DM (2001) Effect of cardiac sympathetic nervous activity on mode of death in congestive heart failure. Eur Heart J 22(13):1136–1143
Lee K, Daimon M, Kuwabara Y, Hasegawa R, Toyoda T, Sekine T, Kawata T, Komuro I (2009) Prediction of the response to beta-blocker therapy in patients with dilated cardiomyopathy: comparison of 123I-MIBG scintigraphy and low-dose dobutamine stress echocardiography. J Echocardiogr 7(4):74–79
Yamazaki J, Muto H, Kabano T, Yamashina S, Nanjo S, Inoue A (2001) Evaluation of beta-blocker therapy in patients with dilated cardiomyopathy—clinical meaning of iodine 123-metaiodobenzylguanidine myocardial single-photon emission computed tomography. Am Heart J 141(4):645–652
Morimoto T, Gandhi TK, Fiskio JM, Seger AC, So JW, Cook EF, Fukui T, Bates DW (2004) Development and validation of a clinical prediction rule for angiotensin-converting enzyme inhibitor-induced cough. J Gen Intern Med 19(6):684–691
Kawashiro N, Kasanuki H, Ogawa H, Matsuda N, Hagiwara N (2008) Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC-HF registry. Circ J 72(12):2015–2020
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nishimura, S., Izumi, C., Himura, Y. et al. Very long-term follow-up data of non-ischemic idiopathic dilated cardiomyopathy after beta-blocker therapy: recurrence of left ventricular dysfunction and predictive value of 123I-metaiodobenzylguanidine scintigraphy. Heart Vessels 34, 259–267 (2019). https://doi.org/10.1007/s00380-018-1245-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-1245-y