Abstract
Background
Delayed heart-to-mediastinum ratio (HMR) has been associated with catecholamine levels and contractile reserve in dilated cardiomyopathy (DCM); however, there is scant evidence regarding the association between cardiac sympathetic activity and left ventricular reverse remodeling (LV-RR). We calculated the 123I-metaiodobenzylguanidine (123I-mIBG) HMR and washout rate (WR) in patients with DCM and investigated their associations with LV-RR.
Methods
From April 2003 to January 2020, in 120 patients with DCM who underwent 123I-mIBG scintigraphy. 66 patients undergoing follow-up echo and taking a beta-blocker from baseline were examined the relationship between 123I-mIBG and LV-RR. After that, this prognostic value for composite cardiac events was evaluated in the entire 120 patients.
Results
In LV-RR analysis, patients were 50.4 ± 12.2 years, with a mean left ventricular ejection fraction of 28.6%. Of 66 patients, 28 (42.4%) achieved LV-RR. Multiple logistic regression analysis of LV-RR revealed that not delayed HMR but the WR (cutoff value: 13.5%) was an independent predictor of LV-RR (odds ratio 6.514, p = 0.002). In the analysis for composite cardiac events, even though WR itself does not have the prognostic capacity, Kaplan–Meier survival curves divided by the cutoff value (delayed HMR = 2.0, WR = 13.5) showed that delayed HMR and WR values enabled the stratification of high-risk patients (log-rank p < 0.001).
Conclusions
The 123I-mIBG WR was associated with the prevalence of LV-RR in patients taking 100% of beta-blockers and 98.5% of renin-angiotensin system inhibitors. Reflecting the contractile reserve, the combined assessment of the delayed HMR and WR could be used to further precisely stratify the patients with DCM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The sympathetic nervous system innervating the heart is an important neurohumoral compensation mechanism for patients with chronic heart failure, and increased sympathetic activity and norepinephrine levels are present in patients with myocardial dysfunction [1,2,3,4,5]. Alternatively, prolonged norepinephrine overload can adversely affect the myocardial structure, as shown in ventricular remodeling, eventually leading to increased mortality and morbidity. The accumulation and extraction of 123I-metaiodobenzylguanidine (123I-mIBG) in the heart showed kinetics similar to those of norepinephrine, reflecting cardiac sympathetic activity.
Various reports have shown that the myocardial delayed heart-to-mediastinum ratio (HMR) is decreased in patients with heart failure and is associated with prognosis in patients with heart failure (HF) [6, 7] and dilated cardiomyopathy (DCM) [8] Although myocardial 123I-mIBG uptake has been associated with catecholamine levels and contractile reserve in DCM [9, 10], scant evidence regarding an association between cardiac sympathetic activity and left ventricular reverse remodeling (LV-RR) is available.
Because LV-RR has been regarded as a major prognostic phenomenon of DCM [11, 12], here, we calculated the 123I-mIBG HMR and washout rate (WR) in patients with DCM by standardization using the cross-calibration phantom method and investigated their associations with LV-RR and cardiac events.
Methods
Ethics statements
The study protocol was approved by our institutional committee on human clinical investigations (approval number: 2017-0031), and written informed consent was obtained from all enrolled patients.
Study population
From April 2003 to January 2020, hemodynamic parameters of patients with cardiomyopathy, excluding those with New York Heart Association Functional Class IV, were collected. DCM was defined as < 50% left ventricular ejection fraction (LVEF) and left ventricular end-diastolic dimension (LVDd) > 55 mm, or indexed LVDd > 33 mm/m2 (male) or 32 mm/m2 (female) determined by echocardiography [13]. Coronary artery disease diagnosed by coronary angiography or coronary multidetector computed tomography, and primary valvular heart disease were excluded. With the exception of one case of a transient complete atrioventricular block at the time of the procedure, all patients underwent endomyocardial biopsy to exclude secondary cardiomyopathy. For 120 patients with DCM who underwent 123I-mIBG scintigraphy, to estimate physical condition, we reviewed all patients’ laboratory measurements, echocardiography and electrocardiography findings, and medication records. When analyzing LV-RR, patients who were prescribed a beta-blocker within 1 month before 123I-mIBG imaging and/or those not taking a beta-blocker at 123I-mIBG imaging and patients undergoing cardiac resynchronization therapy implantation during the follow-up period were excluded to minimize the influence of new beta-blockers and cardiac resynchronization therapy introduction for both 123I-mIBG parameters and LV-RR. Finally, 66 patients with DCM who underwent 123I-mIBG scintigraphy fulfilled the inclusion criteria.
Laboratory examinations
Blood samples were collected to measure patients’ general biochemical data. Simultaneously, neurohormonal parameters (plasma adrenaline, noradrenaline, dopamine, and B-type natriuretic peptide levels) were measured.
Echocardiography
Standard M-mode and two-dimensional echocardiography, Doppler blood flow imaging, and tissue Doppler imaging were performed according to the guidelines of the American Society of Echocardiography [14] using a Vivid 7 ultrasound system (GE Healthcare, Milwaukee, WI, USA) within 1 week of the 123I-mIBG study. LVDd, left ventricular end-systolic diameter (LVDs), and LVEF were calculated using the Teichholz method. The peak flow velocities at the mitral level during rapid filling, atrial contraction, rapid filling/atrial contraction, and deceleration time were calculated using pulsed Doppler imaging. We recorded the tissue Doppler imaging wave of the mitral annulus from the septal side of the apical four-chamber view and analyzed the early diastolic filling velocity.
123I-mIBG protocol
Myocardial 123I-mIBG scintigraphy was performed in patients with a stable status of heart failure, and patients with symptoms of heart failure at rest or on intravenous therapy, such as inotropic drugs or vasodilators, were excluded. 111 MBq 123I-mIBG (Daiichi Radioisotope Laboratory, Tokyo, Japan) was intravenously injected into the patients, and anterior planar images were obtained 15 min (initial image) and 3 h (delayed image) after the injection using various gamma cameras equipped with a low-energy or low-medium-energy collimator. Myocardial 123I-mIBG uptake was quantified using a region of interest manually drawn to a suitable size for each patient. Before 2007, e.CAM and 9300A systems with a low-energy high-resolution collimator (Toshiba, Tochigi, Japan) were used (n = 40). After 2007, the Symbia S equipped with a low-medium-energy general-purpose (LMEGP) collimator (Siemens Japan Co., Ltd., Tokyo, Japan) (n = 72), Symbia T6 with an LMEGP collimator (Siemens Japan Co., Ltd.) (n = 10), Symbia T with an LMEGP collimator (Siemens Japan Co., Ltd.) (n = 2), and Symbia EVO with an LMEGP collimator (Siemens Japan Co., Ltd.) (n = 3) were used. The HMR was calculated by dividing the mean count/pixel in the left ventricle by that in the upper mediastinum. After 2007, the HMRs were calculated using the semiautomatic region of interest setting software “smart-MIBG” [15].
The average conversion coefficient (0.88) from the most common medium-energy group was used to calculate the standard HMR [16,17,18,19]. Standard HMR = 0.88/CCi (Conversion coefficient of the institutional camera/collimator system) × (institutional HMR − 1) + 1. Where CCi means the conversion coefficient of the institutional camera/collimator system (Supplemental Fig. 1). WR was calculated using the following formula [20]. Standard WR = (early HMR − delayed HMR)/early HMR × 100 (%).
Definition of LV-RR
LV-RR was defined as an absolute increase in the LVEF from ≥ 10% to a final value > 35% accompanied by a decrease in the LVDd ≥ 10% [21]. These values were obtained by echocardiography at baseline and within 12–18 months after registration.
Composite cardiac events
The composite cardiac events were evaluated in the entire 120 patients who underwent 123I-mIBG scintigraphy in our institution from April 2003 to January 2020. The follow-up duration was calculated from the date of the 123I-mIBG study to the date of the last clinical visit. Composite cardiac events were defined as sudden cardiac death, lethal arrhythmia, which is a potentially life-threatening arrhythmic event, including a documented episode of spontaneous sustained ventricular tachyarrhythmia, resuscitated cardiac arrest, appropriate implantable cardioverter defibrillator discharge (anti-tachycardia pacing or defibrillation), and hospitalization for worsening heart failure. Event data were collected from patient records at our hospital, telephone interviews, or correspondence by letter.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median with interquartile range and were compared using the Student t-test for parametric variables and Mann–Whitney U-test for non-parametric variables. Categorical variables are presented as number (%) and were compared using the chi-square test or Fisher exact test, as appropriate. Cox proportional hazard regression analysis was performed to compute the hazard ratios with 95% confidence intervals for the composite outcome. Univariate regression analyses were used to estimate LV-RR predictors, and odds ratios and 95% confidence intervals are presented with the logistic regression analysis. Receiver operating characteristic curve analysis was used to obtain the best prognostic predictor for composite outcomes and LV-RR. Kaplan–Meier curves were used to compare the composite outcomes according to sudden cardiac death, admission due to worsening heart failure, and ventricular tachycardia, and the differences were compared using the log-rank test.
All statistical analyses were performed using JMP Pro version 15.0 (SAS Institute, Cary, NC, USA). Figures were generated using Prism (GraphPad Software, San Diego, CA, USA).
Results
Baseline characteristics of patients with or without LV-RR
LV-RR analysis was performed for 66 patients who received a beta-blocker from the entry of the study (Table 1). After a mean 383 days of echocardiographic evaluation, 28 (42.4%) of 66 patients showed LV-RR. There were no significant differences between the groups with respect to age, sex, estimated glomerular filtration rate, LVEF, early and delayed HMR, and levels of B-type natriuretic peptide, noradrenaline, adrenaline, and dopamine. However, the QRS was significantly shorter and WR was significantly higher in patients with LV-RR than in those without it (p < 0.001 and p = 0.022, respectively) (Table 1, Fig. 1).
Box plot of the early HMR, delayed HMR and WR in the patients with and without LV-RR. Distribution of early and delayed HMR shows no significant difference in the patients with and without LV-RR; however, the WR is significantly higher in the LV-RR group than in the non-LV-RR group (p = 0.022). HMR heart-to-mediastinum ratio, WR washout rate, LV-RR left ventricular reverse remodeling
When receiver operating characteristic analysis was conducted using the WR, the receiver operating characteristic curve identified a WR cutoff value for LV-RR of 13.5% (area under the curve: 0.667; sensitivity, 64.3%; specificity, 73.7%) (Fig. 2).
Logistic regression analysis for predictors of LV-RR
In multiple logistic regression analysis for LV-RR (n = 66) (Table 2), the QRS and WR, not the delayed HMR, were independent predictors of LV-RR (odds ratio 0.931; 95% confidence interval 0.884–0.980; p < 0.001 and odds ratio, 6.514; 95% confidence interval 1.824–23.27; p = 0.002, respectively).
Relationship between MIBG and cardiac events
In order to estimate the relationship between 123I-mIBG and cardiac events, 120 patients with DCM who underwent 123I-mIBG scintigraphy in our institution were enrolled in additional analysis. Because delayed HMR has been considered a strong predictor of cardiac events in patients with cardiomyopathy, receiver operating characteristic curve analysis was performed to assess the ability of the delayed HMR to distinguish between patients with and without composite cardiac events. A delayed HMR ≥ 2 was predictive of cardiac events, with a sensitivity of 69.3% and specificity of 66.7% (area under the curve: 0.646, p = 0.014) (Fig. 3). The baseline clinical characteristics of the patients are presented in Supplemental Table 1. The mean age was 50.7 years, LVEF was 30.1%.
There were no significant differences between delayed HMR < 2.0 and delayed HMR ≥ 2.0 groups (n = 47 and 73, respectively) in sex, the body mass index, rate of atrial fibrillation, estimated glomerular filtration rate, and adrenaline, dopamine, and hemoglobin levels. The rates of diabetes mellitus, B-type natriuretic peptide and noradrenaline levels, LVDd, LVDs, and left atrial diameter were significantly higher in the delayed HMR < 2 group than in the delayed HMR ≥ 2.0 group, whereas age, mean arterial pressure, LVEF, and deceleration time were significantly lower in the delayed HMR < 2 group than in the delayed HMR ≥ 2.0 group. Aldosterone antagonists, diuretics, and amiodarone tended to be more frequently prescribed in the delayed HMR < 2 group than in the delayed HMR ≥ 2.0 group at both study entry and follow-up.
During the follow-up period (mean 6.0 years), sudden cardiac death, admission due to worsening heart failure, and lethal arrhythmia occurred in 3 (2.5%), 25 (20.8%), and 5 (4.2%) of patients, respectively.
The composite cardiac event-free survival rate was significantly lower in the delayed HMR < 2 group than in the delayed HMR ≥ 2 group (p < 0.001) (Fig. 4).
Kaplan–Meier analysis of the probability of cardiac events for inpatients with DCM divided into 2 groups according to the delayed HMR cutoff value of 2.0. The delayed HMR < 2 group (red) shows a significantly higher probability of a cardiac event than the delayed HMR ≥ 2 group (blue) (p < 0.001; log-rank test). DCM dilated cardiomyopathy, HMR heart-to-mediastinum ratio
Cox proportional hazards model for predictors of cardiac events
We examined the associations between patient characteristics and hemodynamic variables for composite cardiac events using Cox proportional hazards analysis (Supplemental Table 2) (n = 120). The mean arterial pressure, LVEF, and delayed HMR were identified in univariate analysis as predictors of cardiac events. In multivariate analysis, delayed HMR was an independent predictor of cardiac events (hazard ratio 0.326; 95% confidence interval 0.145–0.733; p = 0.007).
Risk stratification of patients with a delayed HMR and WR cutoff values
Kaplan–Meier survival curves divided by the cutoff value (delayed HMR = 2.0, WR = 13.5) showed that delayed HMR and WR values enabled the stratification of high-risk patients (log-rank p < 0.001) and patients with a delayed HMR ≥ 2.0 and WR ≥ 13.5 experienced subsequently no cardiac events (Fig. 5). Furthermore, in the analysis of patients who were taking beta-blockers during 123I-mIBG imaging and follow-up period (n = 89), WR was significantly lower in the patients with cardiac events in the delayed HMR ≥ 2 (n = 54) group, but this tendency was not detected in the group of patients with delayed HMR < 2 (n = 35) (Fig. 6). Representative cases of cardiac sympathetic nerve activity on 123I-mIBG scintigraphy are shown in Fig. 7.
Kaplan–Meier analysis of the probability of cardiac events for inpatients with DCM divided into 4 groups according to the HMR and WR. Kaplan–Meier survival curves for cardiac events reveals a significant difference divided into four groups according to the HMR and WR (p < 0.001). DCM dilated cardiomyopathy, HMR heart-to-mediastinum ratio, WR washout rate
Distribution of the WR in the HMR ≥ 2 and HMR < 2 patients with and without cardiac events. Distribution of WR was significantly lower in the patients with cardiac events in the delayed HMR ≥ 2 (n = 54) group; however, this difference was not detected in the group of patients with HMR < 2 (n = 35). HMR heart-to-mediastinum ratio, WR washout rate
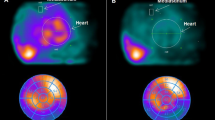
Representative cases of 123I-mIBG scintigraphy. A A 45-year-old man, and he was observed the delayed HMR was 2.27 and WR was 20.1. After 366 days later, LVEF was improved from 26.2% to 55.1%. B A 56-year-old man, and he was observed the delayed HMR was 1.39 and WR was 6.1. After 343 days later, LVEF was not significantly improved from 16.5 to 22.3%. C A 52-year-old man, and he was observed the delayed HMR was 2.28 and WR was decreased as 4.2. Even though delayed HMR ≥ 2, LVEF was not significantly improved from 18.4% to 23.1% after 354 days later. HMR heart-to-mediastinum ratio, WR washout rate, LVEF left ventricular ejection fraction
Discussion
This study showed that the cutoff value for the delayed HMR to predict cardiac events was a delayed HMR < 2, as in the previous report [7]. Furthermore, the WR and QRS were associated with LV-RR under 100% of beta-blocker and 98.5% of renin–angiotensin system inhibitors prescriptions and patients with both a delayed HMR ≥ 2.0 and WR ≥ 13.5 experienced the most favorable clinical outcomes. From these results, evaluation of not only delayed HMR but also WR values in DCM may enable a more detailed stratification of patients with not severe but mildly progressed myocardial impairment.
Myocardial 123I-mIBG scintigraphy is an analog of the adrenergic neuron blocking agent guanethidine, which is used to estimate myocardial presynaptic sympathetic innervation and activity, and reduced 123I-mIBG uptake reflects the severity of myocardial damage. In the normal myocardium, the WR value ranges from 0 to 1% [22, 23]. An increased WR reflects impaired sympathetic presynaptic activity, dysregulation of adrenoceptor signal transduction, and myocardial degeneration, and contributes to worsening heart failure [24,25,26,27], poor response to cardiac resynchronization therapy [28] and lethal arrhythmogenicity, leading to sudden cardiac death [29]. Imamura et al. reported that a WR ≤ 39% was the only significant independent predictor of LV-RR at 6 months post-left ventricular assist device implant [30] and the ADMIRE-HF study also showed that when prospectively evaluating symptomatic heart failure, the WR in patients with heart failure who experienced cardiac events was significantly higher than that in others [6]. Yong-Mei Cha et al. reported that non-responders to cardiac resynchronization therapy had a higher WR than responders (62% versus 37%, p = 0.003) [28], and another group reported that the WR can be used to predict the response to a beta-blocker [31]. However, Nakata, et al. reported that their results of the WR cannot be used as a prognostic index [32] and the WR did not have a relationship with hemodynamic indexes including peak oxygen consumption, nor did it have a prognostic value [33], Lee et al. and Hara et al. observed that the WR did not correlate with the percentage changes in LVEF after the initiation of beta-blocker therapy [20, 34]. Similar results were reported in a previous study in which beta-blockers were prescribed in all study participants [35].
The reason for the inconsistent usefulness of the WR as a predictor for cardiac events may be related to the biphasic change in the WR depending on the stage of heart failure and the course of treatment including beta-blockers [36].
Regarding the transition of WR value from early to late HF stage [37],
-
1.
Early stage: WR was elevated due to sympathetic nervous system hyperactivity and is expected to be highly reactive to cardioprotective drugs.
-
2.
Middle stage: WR was temporarily dropped due to sympathetic nervous system hypoactivity.
-
3.
Late stage: WR was re-elevated, reflecting myocardial damage itself, and is not expected to be highly reactive to cardioprotective drugs in this phase.
Actually, Verschure et al. reported that the WR of cardiac resynchronization therapy responders tended to be higher than that of non-responders in the stable stage [38]. Tamaki et al. reported the predictive value of the WR for sudden cardiac death in patients with heart failure, that patients with LVEF > 35% had a low rate of sudden cardiac death regardless of the WR values, and that there was no event except in 1 patient after 3 years of enrollment [39] and reported that patients with DCM (≥ 90% belonging to New York Heart Association class I or II) showing a high WR have a favorable outcome after the initiation of beta-blocker therapy regardless of LVEF [36]. Our results demonstrating that DCM patients with a high WR had better outcomes are partially in accordance with the aforementioned studies’ results.
The WR is thought to increase in the sympathetically hypersensitive state in the early heart failure phase and fluctuate with improvement in cardiac function. After myocardial degeneration progresses with the course of heart failure, the WR increases, reflecting myocardial degeneration, and when the damaged sympathetic nerve terminal relates to decreasing uptake-1, the WR function was finally decreased [36]. The patients with a high WR but subsequently declining WR had improved LVEF, suggesting that it varied not only with the degree of myocardial impairment but also with the therapeutic intervention and response [40,41,42,43]. Similar to our analysis, the QRS duration is a simple indicator that can be easily obtained from the ECG and has been reported to be related to the LV-RR [44]. Konishi et al. reported that QRS duration < 106 ms was associated with the rate of LV-RR in patients with non-ischemic cardiomyopathy [45]. WR alone is difficult to assess hemodynamics because it is expected to fluctuate depending on the stage of heart failure. We stratified patients using a combined assessment of the WR with the HMR. Just like us, Kasama et al. reported that the combined use of late ventricular potentials with the WR was useful to predict sudden cardiac death [46]. Recently, Chimura et al. reported that the combination of WR and late gadolinium enhancement on cardiac magnetic resonance is effective for risk stratification of DCM. The prognosis is good when WR is high but late gadolinium enhancement is negative, indicating that high WR is not a poor prognostic factor in itself in which myocardial damage is not advanced [47].
Deterioration of heart failure, arrhythmia, and sudden cardiac death results from multifactorial conditions and is related to various triggers. Although the biphasic nature of the WR makes it difficult to demonstrate its prognostic usefulness, it can be a powerful factor when combined with other factors. Further multicenter participation and prospective analyses of its prognostic value are needed to estimate the biphasic components of the WR.
Limitations
This study has some limitations. First, this was a single-center study with a small number of enrolled patients. Second, there were variations and deficiencies in the duration of the follow-up ultrasound sonographies in the retrospective study. Third, a detailed mechanistic analysis of the association between the WR and LV-RR was not performed in this retrospective analysis of clinical data. Finally, as a retrospective analysis, the effect of beta-blocker initiation time, titrated beta-blocker dose and length of HF on both delayed HMR and WR could not be examined and may have significant selection bias. In order to resolve these issues, further prospective validation is needed.
New knowledge gained
WR is difficult to predict cardiac events because it is variable depending on the stage of heart failure, influence of drug and device introduction. Combining the HMR and WR obtained by 123I-mIBG imaging could stratify patients with DCM precisely.
Conclusions
The standardized delayed HMR is a useful prognostic predictor of cardiac events. Furthermore, the WR was significantly related to LV-RR frequency. A combined assessment of the delayed HMR and WR may be useful for a more precise stratification of patients with DCM.
Clinical implications
-
The WR is an independent predictor of LV-RR in patients with DCM taking a beta-blocker.
-
Using both an HMR and WR would lead to more accurate stratification of patients with a low risk of cardiac events than using individual parameters.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 123I-mIBG:
-
123I-metaiodobenzylguanidine
- HMR:
-
Heart-to-mediastinum ratio
- DCM:
-
Dilated cardiomyopathy
- LV-RR:
-
Left ventricular reverse remodeling
- WR:
-
Washout rate
- LVEF:
-
Left ventricular ejection fraction
- HF:
-
Heart failure
- LVDd:
-
Left ventricular end-diastolic diameter
- LVDs:
-
Left ventricular end-systolic diameter
- LMEGP:
-
Low-medium-energy general purpose
- CCi:
-
Conversion coefficient of the institutional camera/collimator system
References
Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–21.
Cohn JN, Levine TB, Olivari MT, Garberg V, Lura D, Francis GS, et al. Plasma norepinephrine as a guide to prognosis in patients with chronic congestive heart failure. N Engl J Med. 1984;311:819–23.
Merlet P, Delforge J, Syrota A, Angevin E, Mazière B, Crouzel C, et al. Positron emission tomography with 11C CGP-12177 to assess beta-adrenergic receptor concentration in idiopathic dilated cardiomyopathy. Circulation. 1993;87:1169–78.
Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, et al. Decreased Catecholamine Sensitivity and β-Adrenergic-Receptor Density in Failing Human Hearts. N Engl J Med. 1982;307:205–11.
Tripsokiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. Physiology, pathophysiology and clinical implications. J Am Coll Cardiol. 2009;54:1747–62.
Jacobson AF, Senior R, Cerqueira MD, Wong ND, Thomas GS, Lopez VA, et al. Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure. Results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. 2010;55:2212–21.
Nakajima K, Nakata T, Yamada T, Yamashina S, Momose M, Kasama S, et al. A prediction model for 5-year cardiac mortality in patients with chronic heart failure using 123I-metaiodobenzylguanidine imaging. Eur J Nucl Med Mol Imaging. 2014;41:1673–82.
Momose M, Okayama D, Nagamatsu H, Kondo C, Hagiwara N, Sakai S. Long-term prognostic stratification by a combination of (123)I-metaiodobenzylguanidine scintigraphy and ejection fraction in dilated cardiomyopathy. Ann Nucl Med. 2011;25:419–24.
Ohshima S, Isobe S, Izawa H, Nanasato M, Ando A, Yamada A, et al. Cardiac sympathetic dysfunction correlates with abnormal myocardial contractile reserve in dilated cardiomyopathy patients. J Am Coll Cardiol. 2005;6(46):2061–8.
Kobayashi M, Izawa H, Cheng XW, Asano H, Hirashiki A, Unno K, et al. Dobutamine stress testing as a diagnostic tool for evaluation of myocardial contractile reserve in asymptomatic or mildly symptomatic patients with dilated cardiomyopathy. JACC Cardiovasc Imaging. 2008;1:718–26.
Merlo M, Pyxaras SA, Pinamonti B, Barbati G, Lenarda AD, Sinagra G. Prevalence and prognostic significance of left ventricular reverse remodeling in dilated cardiomyopathy receiving tailored medical treatment. J Am Coll Cardiol. 2011;29(57):1468–76.
Verdonschot JAJ, Hazebroek MR, Wang P, Sanders-van Wijk S, Merken JJ, Adriaansen YA, et al. Clinical phenotype and genotype associations with improvement in left ventricular function in dilated cardiomyopathy. Circ Heart Fail. 2018;11:e005220.
Kimura Y, Okumura T, Morimoto R, Kazama S, Shibata N, Oishi H, et al. A clinical score for predicting left ventricular reverse remodelling in patients with dilated cardiomyopathy. ESC Heart Fail. 2021;8(2):1359–68.
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
Okuda K, Nakajima K, Hosoya T, Ishikawa T, Konishi T, Matsubara K, et al. Semi-automated algorithm for calculating heart-to-mediastinum ratio in cardiac Iodine-123 MIBG imaging. J Nucl Cardiol. 2011;18:82–9.
Nakajima K, Matsubara K, Ishikawa T, Motomura N, Maeda R, Akhter N, et al. Correction of iodine-123-labeled meta-iodobenzylguanidine uptake with multi-window methods for standardization of the heart-to-mediastinum ratio. J Nucl Cardiol. 2007;14:843–51.
Nakajima K, Okuda K, Yoshimura M, Matsuo S, Wakabayashi H, Imanishi Y, et al. Multicenter cross-calibration of I-123 metaiodobenzylguanidine heart-to-mediastinum ratios to overcome camera-collimator variations. J Nucl Cardiol. 2014;21:970–8.
Nakajima K, Verschure DO, Okuda K, Verberne HJ. Standardization of 123I-meta-iodobenzylguanidine myocardial sympathetic activity imaging: phantom calibration and clinical applications. Clin Transl Imaging. 2017;5:255–63.
Okuda K, Nakajima K, Kitamura C, Kirihara Y, Hashimoto M, Kinuya S. Calibrated scintigraphic imaging procedures improve quantitative assessment of the cardiac sympathetic nerve activity. Sci Rep. 2020;10:21834.
Lee K, Daimon M, Kuwabara Y, Hasegawa R, Toyoda T, Sekine T, et al. Prediction of the response to beta-blocker therapy in patients with dilated cardiomyopathy: comparison of 123I-MIBG scintigraphy and low-dose dobutamine stress echocardiography. J Echocardiogr. 2009;7:74–9.
Morimoto R, Okumura T, Hirashiki A, Ishii H, Ichii T, Aoki S, et al. Myocardial contractile reserve predicts left ventricular reverse remodeling and cardiac events in dilated cardiomyopathy. J Cardiol. 2017;70:303–9.
Zhao C, Shuke N, Yamamoto W, Okizaki A, Sato J, Ishikawa Y, et al. Comparison of cardiac sympathetic nervous function with left ventricular function and perfusion in cardiomyopathies by (123)I-MIBG SPECT and (99m)Tc-tetrofosmin electrocardiographically gated SPECT. J Nucl Med. 2001;42:1017–24.
Currie GM, Iqbal B, Wheat JM, Wang L, Trifunovic M, Jelinek HF, et al. Risk stratification in heart failure using 123I-MIBG. J Nucl Med Technol. 2011;39:295–301.
Yamada T, Shimonagata T, Fukunami M, Kumagai K, Ogita H, Hirata A, et al. Comparison of the prognostic value of cardiac iodine-123 metaiodobenzylguanidine imaging and heart rate variability in patients with chronic heart failure: a prospective study. J Am Coll Cardiol. 2003;15(41):231–8.
Anastasiou-Nana MI, Terrovitis JV, Athanasoulis T, Karaloizos L, Geramoutsos A, Pappa L, et al. Prognostic value of iodine-123-metaiodobenzylguanidine myocardial uptake and heart rate variability in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2005;1(96):427–31.
Kuwabara Y, Tamaki N, Nakata T, Yamashina S, Yamazaki J. Determination of the survival rate in patients with congestive heart failure stratified by 123I-MIBG imaging: a meta-analysis from the studies performed in Japan. Ann Nucl Med. 2011;25:101–7.
Verberne HJ, Brewster LM, Somsen GA, van Eck-Smit BLF. Prognostic value of myocardial 123I-metaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J. 2008;29:1147–59.
Cha YM, Chareonthaitawee P, Dong YX, Kemp BJ, Oh JK, Miyazaki C, et al. Cardiac sympathetic reserve and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:339–44.
Kuramoto Y, Yamada T, Tamaki S, Okuyama Y, Morita T, Furukawa Y, et al. Usefulness of cardiac iodine-123 meta-iodobenzylguanidine imaging to improve prognostic power of Seattle heart failure model in patients with chronic heart failure. Am J Cardiol. 2011;15(107):1185–90.
Imamura T, Kinugawa K, Nitta D, Kinoshita O, Nawata K, Ono M. Preoperative iodine-123 meta-iodobenzylguanidine imaging is a novel predictor of left ventricular reverse remodeling during treatment with a left ventricular assist device. J Artif Organs. 2016;19:29–36.
Suwa M, Otake Y, Moriguchi A, Ito T, Hirota Y, Kawamura K, et al. Iodine-123 metaiodobenzylguanidine myocardial scintigraphy for prediction of response to beta-blocker therapy in patients with dilated cardiomyopathy. Am Heart J. 1997;133:353–8.
Nakata T, Nakajima K, Yamashina S, Yamada T, Momose M, Kasama S, et al. A pooled analysis of multicenter cohort studies of (123)I-mIBG imaging of sympathetic innervation for assessment of long-term prognosis in heart failure. JACC Cardiovasc Imaging. 2013;6:772–84.
Cohen-Solal A, Esanu Y, Logeart D, Pessione F, Dubois C, Dreyfus G, et al. Cardiac metaiodobenzylguanidine uptake in patients with moderate chronic heart failure: relationship with peak oxygen uptake and prognosis. J Am Coll Cardiol. 1999;33:759–66.
Hara Y, Hamada M, Ohtsuka T, Ogimoto A, Saeki H, Suzuki J, et al. Use of thallium-201 myocardial scintigraphy for the prediction of the response to beta-blocker therapy in patients with dilated cardiomyopathy. Circ J. 2002;66:1139–43.
Nishimura S, Izumi C, Himura Y, Kuroda M, Amano M, Harita T, et al. Very long-term follow-up data of non-ischemic idiopathic dilated cardiomyopathy after beta-blocker therapy: recurrence of left ventricular dysfunction and predictive value of 123I-metaiodobenzylguanidine scintigraphy. Heart Vessels. 2019;34:259–67.
Fujimoto S, Inoue A, Hisatake S, Yamashina S, Yamashina H, Nakano H, et al. Usefulness of 123I-metaiodobenzylguanidine myocardial scintigraphy for predicting the effectiveness of β-blockers in patients with dilated cardiomyopathy from the standpoint of long-term prognosis. Eur J Nucl Med Mol Imaging. 2004;31:1356–61.
Böhm M, Rosée KL, Schwinger RHG, Erdmann E. Evidence for reduction of norepinephrine uptake sites in the failing human heart. J Am Coll Cardiol. 1995;25:146–53.
Verschure DO, Poel E, Vincentis GD, Frantellizzi V, Nakajima K, Gheysens O, et al. The relation between cardiac 123I-mIBG scintigraphy and functional response 1 year after CRT implantation. Eur Heart J Cardiovasc Imaging. 2021;22:49–57.
Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53:426–35.
Fukuoka S, Hayashida K, Hirose Y, Shimotsu Y, Ishida Y, Kakuchi H, et al. Use of iodine-123 metaiodobenzylguanidine myocardial imaging to predict the effectiveness of blocker therapy in patients with dilated cardiomyopathy. Eur J Nucl Med. 1997;24:523–9.
Yamazaki J, Muto H, Kabano T, Yamashina S, Nanjo S, Inoue A. Evaluation of beta-blocker therapy in patients with dilated cardiomyopathy—clinical meaning of iodine 123-metaiodobenzylguanidine myocardial single-photon emission computed tomography. Am Heart J. 2001;141:645–52.
Fukuda K, Hasegawa S, Kawamura T, Waratani N, Hirata K, Higashimori A, et al. Changes in cardiac sympathetic nerve activity on 123 I-metaiodobenzylguanidine scintigraphy after MitraClip therapy. ESC Heart Fail. 2021;8:1590–5.
Kasama S, Toyama T, Hatori T, Sumino H, Kumakura H, Takayama Y, et al. Evaluation of cardiac sympathetic nerve activity and left ventricular remodelling in patients with dilated cardiomyopathy on the treatment containing carvedilol. Eur Heart J. 2007;28:989–95.
Marume K, Noguchi T, Tateishi E, Morita Y, Kamakura T, Ishibashi K, et al. Mortality and sudden cardiac death risk stratification using the noninvasive combination of wide QRS duration and late gadolinium enhancement in idiopathic dilated cardiomyopathy. Circ Arrhythm Electrophysiol. 2018;11(4):e006233.
Konishi S, Ohtani T, Mizuno H, Sera F, Nakamoto K, Chimura M. Simple electrocardiographic score can predict left ventricular reverse remodeling in patients with non-ischemic cardiomyopathy. Circ Rep. 2019;1(4):171–8.
Kasama S, Toyama T, Kaneko Y, Iwasaki T, Sumino H, Kumakura H, et al. Relationship between late ventricular potentials and myocardial 123I-metaiodobenzylguanidine scintigraphy in patients with dilated cardiomyopathy with mild to moderate heart failure: results of a prospective study of sudden death events. Eur J Nucl Med Mol Imaging. 2012;39:1056–64.
Chimura M, Yamada S, Taniguchi Y, Yasaka Y, Kawai H. Late gadolinium enhancement on cardiac magnetic resonance combined with 123I-metaiodobenzylguanidine scintigraphy strongly predicts long-term clinical outcome in patients with dilated cardiomyopathy. PLoS One. 2019;20(14):e0217865.
Acknowledgements
None.
Funding
This work was supported by the Japan Society for the Promotion of Science (JP) (KAKENHI grant number: JP22K08178 [to R.M]).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
T.O. received research grants from Ono Pharmaceutical Co. Ltd., Bayer Pharmaceutical Co. Ltd., Daiichi-Sankyo Pharma Inc., and Amgen Astellas BioPharma K.K. outside of the submitted work. T.O. received honoraria from Ono Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., and Medtronic Japan Co. Ltd. T.M. received lecture fees from Bayer Pharmaceutical Co. Ltd., Daiichi-Sankyo Co. Ltd., Sumitomo Pharma Co. Ltd., Kowa Co. Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K. K., Pfizer Japan Inc., Sanofi-Aventis K. K., and Takeda Pharmaceutical Co. Ltd. T.M. received an unrestricted research grant from the Department of Cardiology, Nagoya University Graduate School of Medicine, from Astellas Pharma Inc., Daiichi-Sankyo Co. Ltd., Sumitomo Dainippon Pharma Co. Ltd., Kowa Co. Ltd., MSD K. K., Mitsubishi Tanabe Pharma Co., Nippon Boehringer Ingelheim Co. Ltd., Novartis Pharma K. K., Otsuka Pharma Ltd., Pfizer Japan Inc., Sanofi-Aventis K. K., Takeda Pharmaceutical Co. Ltd., and Teijin Pharma Ltd. The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mizutani, T., Morimoto, R., Isobe, S. et al. Cardiac sympathetic activity and relationship to cardiac events and left ventricular reverse remodeling in patients with non-ischemic dilated cardiomyopathy. Ann Nucl Med 37, 451–461 (2023). https://doi.org/10.1007/s12149-023-01838-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12149-023-01838-9