Abstract
To explore the effects of biochar application on CO2 and CH4 emissions as well as the temperature response of CO2 emissions, a 1-year experiment was conducted with three treatments (control; CF, chemical fertilizer only; BCF, biochar combined with chemical fertilizer) in a vegetable field. The results showed that (1) compared with CF, short-term application of biochar significantly enhanced the cumulative CO2 emissions by 27.5% from a soil–plant system by increasing the soil microbial biomass (e.g., MBC) and C substrates (e.g., SOC); (2) lowest emissions of CH4 were observed in the BCF treatment, and an increase in CH4 consumption and reduced competition with NH4+ may be responsible for the significant reduction in CH4 source strength in biochar-amended soil; and (3) activation energy (Ea) was identified as an important factor influencing the temperature sensitivity (Q10) of CO2 emissions. Fertilization (CF and BCF) reduced the average Q10 and Ea values of CO2 emissions by 9.0–26.7% and 23.5–10.1%, respectively, relative to the control. In addition, the average Ea value in the BCF treatment (51.9 kJ mol−1) was significantly higher than those in the control and CF treatments. The increase in Q10 and Ea values following biochar application possibly contributed to the supplementation of limited labile C and nutrients but highly resistant C following biochar application. Soil pH and crop cultivation may play key roles in influencing the change in Ea. Our study concludes that biochar amendment increased CO2 emissions and temperature response of CO2 emission from the soil–plant system while reducing CH4 emissions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The changing climate was mainly induced by greenhouse gas (GHG) emissions, including carbon dioxide (CO2) and methane (CH4). In the last 20 years, CH4 emission around the world increased by 10% (Jackson et al. 2020), and the emissions of GHG from agricultural ecosystems were about 5.24 Gt CO2 equivalents year−1, which contributed 11% of the total global anthropogenic emissions (Pearson et al. 2017). Hence, altering agricultural management schemes is warranted to reduce GHG emissions and mitigate climate change (Tang et al. 2021).
Turnover of soil organic carbon (SOC) was found as an important factor that largely influences the global climate change (Pan et al. 2004). For example, sequestration and mineralization of SOC are closely related to the GHG emissions (Lee et al. 2020). Fang et al. (2017) reported that global warming may lower the C sequestration potential. During the mineralization of SOC, temperature plays a vital role, which results in variability in the C pool (Criscuoli et al. 2019; Kan et al. 2020; Wang et al. 2019). The response to temperature changes, such as temperature sensitivity (Q10, defined as the rate of change of soil CO2 emission as a consequence of temperature increase of 10 ℃) (Kirschbaum 1995) and activation energy (Ea, defined as the necessary energy for reacting molecules to break and form new bonds after a collision) (Thiessen et al. 2013), could be used to evaluate the feedback intensity between CO2 emission and global warming (Zhou et al. 2009), as well as the response of SOC to global warming (Fang et al. 2014). Generally, the value of Q10 increased with recalcitrance of decomposed substrates (Craine et al. 2010; Wang et al. 2019).
Biochar, as a soil amendment, has been incorporated into soil to improve soil properties and soil structure, increase nutrient availability, and microbial activities (Anderson et al. 2011; Criscuoli et al. 2014; Duan et al. 2020; Dai et al. 2021). As a stable amendment, biochar currently has been an attractive measure to enhance C sequestration on a long-term field scale (Singh et al. 2015; Kan et al. 2020). Therefore, there has been growing call to add biochar into soil to promote C sequestration and improve soil quality. However, in short periods of time (i.e., months), biochar will undergo structural changes, primarily the oxidation of surface, and can be utilized by microbes as a C source (Cheng et al. 2006; Zavalloni et al. 2011). As a result, biochar could be an ecosystem C source, instead of a sink, within a short-term period in soil. For example, Ameloot et al. (2013) determined that the increases in short-term CO2 and N2O emissions (117 days) were observed in biochar-amended soils due to the rapid degradation of labile compounds in the biochar (Zimmerman et al. 2011). Alternatively, new substrate addition would stimulate the “priming effects,” defined as the changes in the mineralization of native soil organic matter (Kuzyakov et al. 2000; Kuzyakov 2010). The negative priming effects, such as reduced N2O production and CH4 oxidation, have been reported in soil treated with biochar (Spokas and Reicosky 2009; Wu et al. 2019; Duan et al. 2020) due to biochar’s porous native and high affinity for natural organic matter (Kasozi et al. 2010; Zimmerman et al. 2011). However, biochar could also promote the mineralization of soil C due to the positive priming effect (Dong et al. 2018; Kan et al. 2020; Dai et al. 2021). Meanwhile, biochar incorporation can increase the root biomass, net photosynthesis, and grain yield, and then influence the net CO2 emissions from the soil–plant system (Masto et al. 2013; Sun et al. 2017). Hence, the short-term response of greenhouse gas emissions to the biochar application in agricultural systems should receive more attention.
Exogenous C input (e.g., biochar) may alter the chemical recalcitrance of organic matter and environmental conditions, and result in a change in the temperature response of CO2 emissions (Fang et al. 2014, 2017; Wang et al. 2019). According to the fundamental enzymatic kinetic theory, organic compounds with higher molecular weights showed lower rates of decomposition and higher values of Q10 and Ea relative to organic compounds with lower molecular weights. However, the decreases and increases in Q10 and Ea were observed in biochar-added soils (He et al. 2016; Fang et al. 2017; Pei et al. 2017; Wang et al. 2019). The contradictory results may be caused by the interactions of physical–chemical protection and substrate C quality change (Conant et al. 2011). Biochar application in a short-term period may introduce more C, including stable and labile C, which is related to the temperature response. However, most of previous studies on the temperature response to C emission were conducted in laboratory incubation, and more field works are necessarily needed.
Here, we hypothesized that biochar incorporated into soil would increase the gaseous C loss and temperature sensitivity of CO2 emissions, especially in a short time period. In this study, we conducted a short-term vegetable cultivation experiment (approximately 1 year) to investigate the response of CO2 and CH4 emissions as well as the temperature sensitivity of CO2 emissions to biochar amendment. The objectives of this study were (1) to explore the effects of biochar amendment on the soil CO2 and CH4 emissions, (2) to determine the temperature response of CO2 emissions in biochar-amended soil, and (3) to try to identify key factors that influence C emissions and the temperature response of CO2 emissions.
Materials and methods
Study site description
The experiment was conducted in the National Monitoring Station of Soil Fertility and Fertilizer Efficiency on Purple Soils (30°26′N, 106°26′E) in the Beibei District of Chongqing, southwestern China. The in situ soil is classified as Regosol in the Food and Agriculture Organization classification scheme (FAO 1988). The details of this trial site were described in the study of Huang et al. (2018, 2019). The basic property of soil is shown in Table 1.
Experimental design
Nine 2 m × 1 m plots were selected for this study from 2016 to 2017. Three treatments (one treatment per plot), including no fertilizer (control), chemical fertilizer only (CF), and biochar combined with chemical fertilizer (BCF), were arranged in a completely randomized design with three replicates (total 9 plots). The same amount of total nitrogen (N), phosphorus (P), and potassium (K) was applied in the CF and BCF treatments. Chemical fertilizers were applied as urea (N-eq, 46%), single superphosphate (P2O5-eq, 12%), and muriate of potash (K2O-eq, 60%). Biochar derived from rape straw was purchased from Sichuan Jiusheng Agricultural Technology Development Co. Ltd., China. The property of biochar is given in Table 1.
Four vegetable crops were grown in rotation during the experimental period from November 2016 to November 2017. The cultivated vegetable crops were lettuce (Lactuca sativa L. var. angustana Irish, November 2016 to January 2017), cabbage (Brassica oleracea L. var. capitata L., January 2017 to May 2017), chili (Capsicum annuum L., May 2017 to September 2017), and lettuce (Lactuca sativa L. var. angustana Irish, September 2017 to November 2017). In the CF treatment, the amount of chemical fertilizer was applied according to the Fertilization Guide for Major Crops in China (Zhang et al. 2009), as shown in our previous study (Huang et al. 2019). In the BCF treatment, 10 t hm−2 biochar was applied to soil before transplanting lettuce (October 20, 2016) and chili (May 5, 2017) for each addition according to our previous study (Huang et al. 2019). The deficient nutrients in the BCF treatment were supplemented with chemical fertilizer based on the same amount of total N, P, and K. Chemical fertilizers in the CF and BCF treatments were applied through basal fertilization and topdressing. The fertilization procedures were described in our previous study (Huang et al. 2019). The time schedule for fertilization and vegetable cultivation for different vegetables is described in Table S1.
Measurement of CO 2 and CH 4
The gases of CO2 and CH4 were sampled using the static closed chamber method during the experimental period. The setup of the chamber and the method of gas collection were given in the study of Huang et al. (2019). Briefly, gas samples were collected once every week (between 9:00 and 11:00) and every 2 or 3 days for 1 week following basal fertilizer and topdressing. After gas sample collection, the fluxes of CO2 and CH4 were measured simultaneously via the gas chromatography facility (Agilent 7890A; Agilent, Inc., USA). During the entire experiment, gas samples were collected 63 times in total. The calculations used to determine CO2 and CH4 fluxes and cumulative CO2 and CH4 emissions were similar to the study reported by Huang et al. (2019). Air and soil temperature (5 cm depth in soil) and the soil moisture content were recorded at the beginning and the end of sampling, and average of the two values was calculated. Because the greenhouse gas chamber measurements cannot exclude CO2 emissions from plant roots, the CO2 emissions in this study were the net CO2 emissions from vegetable fields, which integrated soil respiration, belowground greenhouse gas emissions, and CO2 assimilated by plants.
Soil sampling and measurements
Topsoil (0–20 cm) was sampled on November 23, 2017. In each plot, five soil cores were randomly sampled and mixed to form a pooled sample. The pooled samples were placed in the sterile plastic bags and transported to the laboratory. Meanwhile, soil bulk density was obtained via the cutting ring method. Sampled soil was thoroughly mixed and passed through a 2-mm sieve after all the visible roots and stones had been removed. Fresh soil was used for the analysis of soil dissolved organic carbon (DOC) and microbial biomass carbon (MBC), and the final concentrations of DOC and MBC were normalized by the dry mass of soil. The remaining soil was air-dried to measure the total SOC and soil pH.
Soil water–filled pore space (WFPS) was calculated according to the following equation (Li et al. 2013): WFPS = (gravimetric moisture × soil bulk density × 100) / [1 − (soil bulk density / 2.65)], with 2.65 g cm−3 of particle density.
Soil DOC content was extracted with a soil-to-water ratio of 1:10 (w/w), and the extracted solution was centrifuged and filtered through prewashed 0.45-µm cellulose acetate filters. All filtered solutions were measured via the Multi N/C® 2100 Analyzer (Analytik Jena, Germany) (Ghani et al. 2003). After being extracted by chloroform fumigation with 0.5 mol L−1 K2SO4, the extracts were used to measure the soil MBC content through the method of K2Cr2O7 external heating with titrating FeSO4 (Yang et al. 2008).
Temperature response
Temperature sensitivity (Q10) and activation energy (Ea) of CO2 emission were used to describe the relationship between temperature and CO2 emission.
The Q10 was calculated with the following equation (Zhou et al. 2007; Chen et al. 2016):
where y is the flux of CO2 over time (mg m−2 h−1), and a and b are the exponential fit parameters. Parameter a is the intercept of CO2 flux when the temperature is 0 ℃. T is the soil temperature (℃).
The activation energy was calculated using the exponential Arrhenius function according to Thiessen et al. (2013):
where y is the flux of CO2 over time (mg m−2 h−1), A is the constant, Ea is the activation energy (J mol−1), R is the universal gas constant (8.314 J mol−1 K−1), and T is the soil temperature in Kelvin (K). In chemical kinetics, Ea is defined as the necessary energy for reacting molecules to break and form new bonds after a collision. To calculate the daily Ea, a maximum likelihood estimate of the slope of the linear regression of the natural logarithms of CO2 flux against the reciprocal of absolute soil temperature was obtained. To estimate the average Ea during the experimental period, we multiplied the slope values by the gas constant R.
Statistical analysis
The data were statistically analyzed using SPSS 23.0 and Origin 8.5 software. The Kolmogorov–Smirnov test was used to test the normality of all data. Both parametric and nonparametric approaches were used to test the differences. For the normal distributed data, comparisons of data among treatments were performed by one-way analysis of variance analysis (ANOVA) in combination with the least significant difference (LSD) test. For non-normally distributed data, comparisons of data were performed by the Kruskal–Wallis test. After Bartlett’s test of sphericity (p < 0.05), the variables related to soil properties, Q10, Ea, and cumulative CO2 and CH4 emissions were subjected to principal component analysis (PCA) to identify key factors for Q10, Ea, and cumulative CO2 and CH4 emissions using Origin 8.5. Automatic linear modeling was performed at the 95% confidence level using SPSS 18.0. Spearman’s coefficient was used in the nonparametric correlation analysis. Statistical significance was determined at p = 0.05 and p = 0.01.
Results
CO 2 and CH 4 emissions
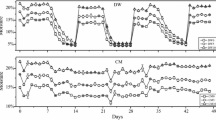
As shown in Fig. 1a, there were two peaks of CO2 flux during the experimental period, which were observed in April and August, respectively. The highest CO2 fluxes with the values of 3254.8 mg m−2 h−1 and 3201.9 mg m−2 h−1 were both found in the BCF treatment on April 13 and August 9, respectively. Compared with the control, fertilization (CF and BCF) increased the flux of CO2, except for the period of higher air temperature (from July to August). Higher CO2 fluxes were observed in the BCF treatment than in the CF treatment when the air temperature was over 18 ℃. Additionally, the second peak of CO2 flux in the BCF treatment (on August 9) was later than that in the CF treatment (on July 26). During the experimental period (Fig. 1b), BCF significantly increased the cumulative CO2 emission by 27.5% and 37.1%, relative to the control and CF treatments, respectively.
CO2 and CH4 fluxes with time (a, c) and cumulative CO2 and CH4 (b, d) in different treatments. Control, no fertilizer; CF, chemical fertilizer only; BCF, biochar combined with chemical fertilizer. Different lowercase letters indicate that the differences are significant (p < 0.05). Red arrows in scatters indicate the time of biochar application
In contrast to the CO2 flux, the variation in the CH4 flux during the experimental period was not significant (Fig. 1c). However, after the application of biochar, a significant fluctuation in CH4 flux was observed, especially after the second time of biochar application. Compared with the control, CF and BCF both reduced the cumulative CH4 emission, and the cumulative CH4 emission in the BCF treatment was − 1.09 kg hm−2 (Fig. 1d).
Temperature sensitivity (Q 10 ) and activation energy (E a ) of CO 2 emission
Because of the negative value of the CH4 flux, only the temperature sensitivity (Q10) and activation energy (Ea) of CO2 emission were calculated in this study. The flux of CO2 has an exponential relationship with the soil temperature (Fig. S1a–c). The dynamic of Q10 over time is shown in Fig. 2a. Fertilizer application (CF and BCF) reduced the Q10 values during the experimental period. When the first biochar application was applied, BCF reduced the Q10 values relative to the CF treatment, but increased the values when the second biochar application was applied. In each season of vegetable growing, the peak of Q10 values was observed, especially in April. As shown in Fig. 2b, the lowest value of average Q10 was observed in the CF treatment, which was significantly reduced by 29.2% relative to the control. However, there were no significant differences between the CF and BCF treatments, even if a higher value of average Q10 (Q10 = 2.1) was observed in the BCF treatment.
Temperature sensitivity (Q10) (a, b) and activation energy (Ea) (c, d) of CO2 emissions in different treatments. Control, no fertilizer; CF, chemical fertilizer only; BCF, biochar combined with chemical fertilizer. Different lowercase letters indicate that the differences are significant (p < 0.05). Red arrows in scatters indicate the time of biochar application
Similar to the Q10 dynamic of CO2 emission, peaks of Ea value were all found in each vegetable growing season, especially in the initial time of vegetable growing (Fig. 2c). Compared with CF, BCF increased the Ea values by 33.7–49.5%, regardless of the number of biochar applications. In addition, the average Ea value in BCF treatment (51.9 kJ mol−1) was significantly higher than those in the control (60.4 kJ mol−1) and CF (36.2 kJ mol−1) treatments (Fig. 2d).
Soil property
Compared with CF, BCF increased the contents of DOC, MBC, and SOC by 800.7% (p < 0.05), 33.3% (p < 0.05), and 68.9% (p > 0.05), respectively (Table 2). In addition, the highest values of soil pH and WFPS were both found in the control, followed by those in the BCF treatment.
Correlation of soil properties, Q 10 , E a , and carbon emissions
The first two principal components (PC1 and PC2) accounted for 50.0% and 31.3% of the total variation in PCA, respectively (Fig. 3). The variation in cumulative CO2 emissions has a positive relationship with SOC but a negative relationship with cumulative CH4 emissions (Fig. 3). Soil DOC was the key factor influencing the variation in Q10 and Ea according to the results of PCA. Correlations among soil properties, Q10, Ea, and carbon emissions (CO2 and CH4) are listed in Table S2. The cumulative CO2 and CH4 emissions were both significantly associated with SOC (r = 0.887 and r = − 0.888, respectively). The Q10 value was correlated with Ea (r = 0.837), soil DOC (r = 0.732), and pH (r = 0.765) (p < 0.05 or 0.01). The value of Ea has a significant relationship with soil DOC (r = 0.933), pH (r = 0.873), and WFPS (r = 0.792). In addition, automatic linear modeling revealed that soil SOC, together with MBC, was the primary factor associated with the cumulative CO2 emissions, as well as SOC and pH associated with the cumulative CH4 emissions (Fig. 3). Activation energy (Ea) and soil DOC were the key factors influencing Q10 and Ea, respectively.
Discussion
Biochar application influencing the carbon emission
Biochar, as a soil amendment, plays a key role in C utilization and in decreasing greenhouse gas emissions. In general, biochar reduces the CO2 emissions through the expansion of the soil C pool (Kavitha et al. 2018). In the present study, however, biochar application increased the CO2 emissions from the soil–plant system during the short-term experiment, relative to the no-biochar (control and CF) treatments (Fig. 1b). The observation of increased cumulative CO2 emissions in the biochar (BCF) treatment was inconsistent with the previous literature (Lu et al. 2014; Bending et al. 2014; Chen et al. 2017), which demonstrated that biochar application significantly decreased soil CO2 emissions during short-term incubations. Similarly, the studies of Zhou et al. (2017) and Ge et al. (2020) showed that biochar (produced from bamboo) addition decreased the cumulative soil CO2 emissions in the field experiments. The inconsistent results may be caused by the different biochar feedstocks, pyrolysis temperatures, and addition rates (Ameloot et al. 2013; Lu et al. 2014; Bending et al. 2014). First, the pyrolysis temperature of 450–500 °C in this study was incomplete oxidization, which may increase volatile matter content and then promote the abiotic release of inorganic C in biochar (Ameloot et al. 2013; Yang et al. 2018). In addition, a greater positive priming effect of biochar was observed immediately at low pyrolysis temperatures (Zimmerman et al. 2011). Second, short-term application of biochar may induce priming effects, causing native soil organic C or labile compounds in biochar to readily decompose by microorganisms (Zimmerman 2010; Wang et al. 2016; Yang et al. 2018). Meanwhile, the combined application of biochar and N fertilization could stimulate CO2 release from biochar with an increased value of 28.3% (Lu et al. 2014). Third, biochar application in a short period of time provided labile C for soil microbes (especially for the “r-strategist” microbes that are adapted to respond quickly to newly available C sources) and then stimulated soil respiration (Paul and Clark 1989; Zimmerman et al. 2011; Teutscherova et al. 2017; Duan et al. 2020). This hypothesis is supported by the higher contents of soil DOC and MBC in the biochar treatment (Table 2). In addition, the results of automatic linear modeling also verified that the enhanced microbial biomass (e.g., MBC) and C substrates (e.g., SOC) in soils may lead to greater CO2 emissions (Fig. 3). Although the adsorption and/or encapsulation of biochar can protect native soil labile C from microbial utilization and inhibit the decomposition of native SOC (Zimmerman et al. 2011; Lu et al. 2014; Bending et al. 2014; Chen et al. 2017), the colocation of microorganisms and various nutrients on biochar surfaces and/or in pores may provide a highly suitable habitat for microbes and increase microbial C use efficiency, and subsequently higher CO2 emissions (Lehmann et al. 2011; Zavalloni et al. 2011). It is worth noting that CO2 emissions in this study were the net CO2 emissions from the soil–plant system, which integrated soil respiration, root respiration, and the CO2 assimilated by plants. The significant negative relationship between total vegetable yield and cumulative CO2 emissions may indicate the key roles of root respiration and plant photosynthesis in CO2 emissions (Table S2), especially root respiration. Additionally, biochar application obtained higher total vegetable yields than no-biochar (Table S3). Therefore, short-term biochar and N combined application cannot offset, at least partly, the negative effect of biochar or plant photosynthesis on CO2 emissions.
It is well known that dryland soil under oxic conditions has the capacity of CH4 sink due to the soil methanotrophic bacteria oxidizing CH4 to CO2 (Suwanwaree and Robertson 2005; Criscuoli et al. 2019). The flux of soil CH4 is controlled by the production of CH4 by methanogens and consumption of CH4 by methanotrophs, as well as the soil conditions that can impact the growth of methanogens and methanotrophs (Le Mer and Roger 2001; Conrad 2007). Consistent with the reported literature (Jeffery et al. 2011; Feng et al. 2012; Qin et al. 2016; Liu et al. 2016b), biochar application in this study significantly reduced the cumulative CH4 emissions relative to the control and CF treatments (Fig. 1d). A potential explanation is the fact that enhanced soil aeration would increase the activity of methanotrophs due to the biochar’s large surface area and pore volume (Wang et al. 2018), which was supported by the negative relationship of cumulative CH4 emissions and CO2 emissions (Fig. 3 and Table S2). This result suggested that increased soil CH4 consumption rather than decreased CH4 production dominated the influence of biochar in mitigating CH4 emission from dryland soil–plant system. Another potential explanation, as discussed above, is that the progressive protection of biochar may prevent SOC from being used by methanogens (Zimmerman et al. 2011), resulting in decreased CH4 production. The higher contents of SOC observed in the BCF treatment may be attributed to the protection of biochar in this study (Table 2). Soil pH plays a key role in affecting both methanogenesis and methanotrophy (Hanson and Hanson 1996; Jeffery et al. 2016). Generally, a pH ranging from 6 to 8 is optimal for most methanogens (Garcia et al. 2000), and high acidity does not favor an increase in the microbial habitability of methanogens (e.g., reducing the abundance of methanogens) (Jeffery et al. 2016). Therefore, a significant increase in CH4 sink strength was observed in biochar-treated soil with a pH of 5.0, which is consistent with the findings of Jeffery et al. (2016). However, we observed a CH4 source in the CF treatment, even if the soil pH was lower than that in the BCF treatment (Table 2). Except for the negative effect of biochar, a possible explanation is that there was more N fertilizer amount in the CF (1200 kg ha−1 N fertilizer) treatment than in the BCF (1120 kg ha−1 N fertilizer) treatments. The NH4+-containing or NH4+-delivering fertilizers will compete with CH4 at the binding sites, consequently decreasing the oxidation of CH4 (Htun et al. 2017; Huang et al. 2020). Besides, the incorporation of biochar with a high C/N ratio of 142.2 may increase the immobilization of inorganic N (e.g., NH4+) and reduce the competitive exclusion of CH4 (Huang et al. 2020). Meanwhile, in this study, a lower content of NH4+ was observed in the BCF (100.7 mg kg−1) treatment than that in the CF (112.3 mg kg−1) treatment. Therefore, short-term application of biochar showed a significant increase in CH4 sink strength/reduction in CH4 source strength.
Biochar application influencing the temperature response of CO 2 emissions
In this study, fertilization incorporation reduced the temperature response of CO2 emissions (expressed as Q10 or Ea), compared to the control (Fig. 2a, b). This may be caused by the fact that nutrients (e.g., N and P) from fertilizers changed the substrate C quality, which is linked to soil C emissions (Guo et al. 2017). Previous studies determined that the N addition potentially increased those microbial abundance using labile C and elevated cellulose-decomposing enzyme activity (Carreiro et al. 2000; Keeler et al. 2009). Thus, increased Q10 was observed following fertilization or artificial N deposition in previous studies (Liu et al. 2016a; Guo et al. 2017; Ge et al. 2020). The inconsistency of the literature with this study is likely attributed to the different fertilization times (e.g., long-term fertilization (> 10 years) in the study of Guo et al. (2017) and short-term fertilization (approximately 1 year) in this study). Long-term N inputs may change the substrate quality characterized by C complexities and increase the recalcitrant C, leading to an enhanced Q10 value (Guo et al. 2017). Generally, the temperature sensitivity of resistant C was higher than that of labile C due to the former needing more activation energy (Ea) and time, according to the enzyme kinetic theory (Davidson and Janssens 2006; Conant et al. 2011). Our observation of the positive relationship between Ea and Q10 (Fig. 3 and Table S2) possibly supported the enzyme kinetic hypothesis. Therefore, the reduced Q10 under short-term fertilizer inputs may be well explained by the lower Ea in the CF and BCF treatments.
Compared with the CF treatment, biochar addition increased the Q10 and Ea, especially after the second application, which is consistent with the report of Wang et al. (2019). Multiple reasons may be responsible for this increase in Q10 and Ea. For example, the biochar-induced increase in temperature sensitivity may be attributed to the accumulation of resistant C pools in soil organic matter due to biochar aromatic properties (Zhou et al. 2017; Wang et al. 2019). On the other hand, the increase in Q10 and Ea values following biochar application may contribute to enhanced nutrient availability and microbial activities, leading to the decomposition of soil organic matter (Lehmann et al. 2011; Criscuoli et al. 2014), as evidenced by the increased MBC (Table 2), CO2 flux (Fig. 1a), and N (or P, K) fertilizer utilization efficiency (unpublished data) in the BCF treatment. The increased nutrient availability may reduce the degradability of resistant C, possibly by decreasing the affinity of microbial enzymes (such as phenol oxidase and peroxidase) to substrates (Guo et al. 2017), and thus increase Q10 and Ea following biochar application (Fig. 2). In addition, resistant C pools might increase in dry farmland (as in our study) under high microbial activities after biochar addition, contributing to an increase in Q10 values (Wang et al. 2019). However, the fact that biochar applications reduced Q10 values was also reported in some studies (Pei et al. 2017). These discrepancies may be attributed to the high rate of biochar application in the study of Pei et al. (2017) (i.e., 40–100 t ha−1), which is significantly higher than the rates used in the studies of Zhou et al. (2017) (i.e., 10–30 t ha−1), Kan et al. (2020) (i.e., 1.8–7.2 t ha−1), and our study (i.e., 10–20 t ha−1). More biochar incorporated into soil can increase the non-biochar labile dissolvable C of native soil, which would be entrapped in the porous structure of biochar (Bending et al. 2014). The colocation of microorganisms and entrapped C, as mentioned above, may enhance the availability of soil decomposable C, thus reducing the Q10 values (Pei et al. 2017). Although a higher DOC content was observed in soil treated with biochar (Table 2), the low ratio of DOC to SOC (i.e., 1.25%) may indicate that more resistant C remained in soil treated with biochar in the short-term period. Meanwhile, more recalcitrant C with a higher Ea dominated in the soil since the limited labile C was depleted quickly, especially after the second biochar addition.
The temperature response of CO2 emissions is directly affected by external factors that limit decomposition, except for direct factors (such as substrate availability and microbial enzyme affinity) (Davidson and Janssens 2006; von Lützow and Kögel-Knabner 2009; Fang et al. 2017). Soil pH played a key role in the temperature response of CO2 emissions in this study due to the significant association of soil pH with Q10 and Ea (Table S1 and Fig. 3). Acidifying soil caused by fertilization is characterized by high osmotic pressures, low soil minerals, and high aluminum toxicity, which would reduce microbial activity and consequently decrease the temperature response (Treseder 2008; Liu and Greaver 2010). Thus, the higher soil pH in the BCF treatment may be partly responsible for the higher temperature response, relative to the CF. In addition, the peak of Ea with time was observed within 1 week of crop transplantation in each growing season, regardless of treatment (Fig. 2c). We speculate that crop cultivation measures may influence Ea possibly by inducing changes in the external and/or direct factors (e.g., root biomass). Unfortunately, the soil indexes with time were not detected in this study. However, the significant relationship of Ea and vegetable yields may indicate the important effect of vegetable cultivation on the temperature response of CO2 emissions (Table S2). As mentioned above, biochar application may impact CO2 emissions due to root respiration. Overall, short-term application of biochar increased the temperature response of CO2 emissions in the soil–plant system.
Conclusion
Short-term application of biochar significantly increased CO2 emissions from the soil–plant system. However, biochar addition showed a significant reduction in CH4 source strength in dryland soil, possibly by increasing CH4 consumption and reducing competition with NH4+. Fertilization reduced the temperature sensitivity (Q10) of CO2 emissions by decreasing the activation energy (Ea). In addition, biochar significantly increased the temperature response (Q10 and Ea) of CO2 emission, relative to solely chemical fertilizer application, which is related to the supplementation of limited labile C and nutrients but highly resistant C following biochar application. External factors (e.g., pH and crop cultivation) play key roles in influencing the change in Ea. Thus, our study suggests that the short-term response of biochar to C gas emissions and temperature should be considered to better understand the long-term effect of biochar on C release and sequestration.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anderson CR, Condron LM, Clough TJ, Fiers M, Stewart A, Hill RA, Sherlock RR (2011) Biochar induced soil microbial community change: implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 54(5–6):309–320
Ameloot N, De Neve S, Jegajeevagan K, Yildiz G, Buchan D, Funkuin YN, Prins W, Bouckaert L, Sleutel S (2013) Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol Biochem 57:401–410
Bending GD, Baeyens J, Prayogo C, Jones JE (2014) Impact of biochar on mineralisation of C and N from soil and willow litter and its relationship with microbial community biomass and structure. Biol Fertil Soils 50:695–702
Carreiro MM, Sinsabaugh RL, Repert DA, Parkhurst DF (2000) Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81(9):2359–2365
Chen J, Zhou X, Wang J, Hruska T, Shi W, Cao J, Zhang B, Xu G, Chen Y, Luo Y (2016) Grazing exclusion reduced soil respiration but increased its temperature sensitivity in a meadow grassland on the Tibetan Plateau. Ecol Evol 6(3):675–687
Chen J, Li S, Liang C, Xu Q, Li Y, Qin H, Fuhrmann JJ (2017) Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: effect of particle size and addition rate. Sci Total Environ 574:24–33
Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH (2006) Oxidation of black carbon by biotic and abiotic processes. Org Geochem 37(11):1477–1488
Conrad R (2007) Microbial ecology of methanogens and methanotrophs. Adv Agron 96(07):1–63
Craine JM, Fierer N, McLauchlan KK (2010) Widespread coupling between the rate and temperature sensitivity of organic matter decay. Nat Geosci 3:854–857
Criscuoli I, Alberti G, Baronti S, Favilli F, Martinez C, Calzolari C, Pusceddu E, Rumpel C, Viola R, Miglietta F (2014) Carbon sequestration and fertility after centennial time scale incorporation of charcoal into soil. PLoS One 9(3):e91114
Criscuoli I, Ventura M, Sperotto A, Panzacchi P, Tonon G (2019) Effect of woodchips biochar on sensitivity to temperature of soil greenhouse gases emissions. Forests 10(7):1–14
Conant RT, Ryan MG, Agren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvonen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Steinweg JM, Wallenstein MD, Wet- terstedt, J.A.M., Bradford, M.A., (2011) Temperature and soil organic matter decomposition rates-synthesis of current knowledge and a way forward. Global Change Biol 17:3392–3404
Dai Z, Xiong X, Zhu H, Xu GJ, Leng P, Li JH, Tang C, Xu MG (2021) Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 3:239–254
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Dong XL, Singh BP, Li GT, Lin QM, Zhao XR (2018) Biochar application constrained native soil organic carbon accumulation from wheat residue inputs in a long-term wheat-maize cropping system. Agric Ecosyst Environ 252:200–207
Duan M, Wu F, Jia Z, Wang S, Cai Y, Chang SX (2020) Wheat straw and its biochar differently affect soil properties and field-based greenhouse gas emission in a Chernozemic soil. Biol Fertil Soils 56:1023–1036
Fao F (1988) UNESCO soil map of the world, revised legend. World Resources Report 60:138
Fang Y, Singh BP, Matta P, Cowie AL, Van Zwieten L (2017) Temperature sensitivity and priming of organic matter with different stabilities in a Vertisol with aged biochar. Soil Biol Biochem 115:346–356
Fang Y, Singh BP, Singh B (2014) Temperature sensitivity of biochar and native carbon mineralisation in biochar-amended soils. Agric Ecosyst Environ 191:158–167
Feng Y, Xu Y, Yu Y, Xie Z, Lin X (2012) Mechanisms of biochar decreasing methane emission from Chinese paddy soils. Soil Biol Biochem 46:80–88
Garcia JL, Patel BKC, Olivier B (2000) Taxonomic, phylogenetic, and ecological diversity of methanogenic archaea. Anaerobe 6(4):205–226
Ge X, Cao Y, Zhou B, Xiao W, Tian X, Li MH (2020) Combined application of biochar and N increased temperature sensitivity of soil respiration but still decreased the soil CO2 emissions in moso bamboo plantations. Sci. Total Environ. 730:139003
Ghani A, Dexter M, Perrott KW (2003) Hot-water extractable carbon in soils: a sensitive measurement for determining impacts of fertilisation, grazing and cultivation. Soil Biol Biochem 35(9):1231–1243
Guo H, Ye CL, Zhang H, Pan S, Ji YG, Li Z, Liu MQ, Zhou XH, Du GZ, Hu F, Hu SJ (2017) Long-term nitrogen & phosphorus additions reduce soil microbial respiration but increase its temperature sensitivity in a Tibetan alpine meadow. Soil Biol Biochem 113:26–34
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol Rev 60(2):439–471
He X, Du Z, Wang Y, Lu N, Zhang Q (2016) Sensitivity of soil respiration to soil temperature decreased under deep biochar amended soils in temperate croplands. Appl Soil Ecol 108:204–210
Htun YM, Tong YN, Gao PC, Ju XT (2017) Coupled effects of straw and nitrogen management on N2O and CH4 emissions of rain fed agriculture in Northwest China. Atmos Environ 157:156–166
Huang R, Tian D, Liu J, Lv S, He XH, Gao M (2018) Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric Ecosys Environ 265:576–586
Huang R, Wang YY, Liu J, Li JC, Xu GX, Luo M, Xu C, Ci E, Gao M (2019) Variation in N2O emission and N2O related microbial functional genes in straw- and biochar-amended and non-amended soils. Appl Soil Ecol 137:57–68
Huang R, Liu J, He X, Xie D, Ni J, Xu C, Zhang Y, Ci E, Wang Z, Gao M (2020) Reduced mineral fertilization coupled with straw return in field mesocosm vegetable cultivation helps to coordinate greenhouse gas emissions and vegetable production. J Soils Sediment 20(4):1834–1845
Jackson JR, Saunois M, Bousquet P, Candaell JG, Poulter B, Stavert AR, Bergamaschi P, Niwa Y, Segers A, Tsuruta A (2020) Increasing anthropogenic methane emissions arise equally from agricultural and fossil fuel sources. Environ Res. Lett. 15(7):071002
Jeffery S, Verheijen FG, van der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosyst Environ 144:175–187
Jeffery S, Verheijen FG, Kammann C, Abalos D (2016) Biochar effects on methane emissions from soils: a meta-analysis. Soil Biol Biochem 101:251–258
Kan ZR, Liu QY, Wu G, Ma ST, Virk AL, Qi JY, Zhao X, Zhang HL (2020) Temperature and moisture driven changes in soil carbon sequestration and mineralization under biochar addition. J Clean Prod 265:121921
Kasozi GN, Zimmerman AR, Nkedi-Kizza P, Gao B (2010) Catechol and humic acid sorption onto a range of laboratory-produced black carbons (biochars). Environ Sci Technol 44:6189–6195
Kavitha B, Reddy PVL, Kim B, Lee SS, Pandey SK, Kim KH (2018) Benefits and limitations of biochar amendment in agricultural soils: a review. J Environ Manage 227:146–154
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kirschbaum MUF (1995) The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biol Biochem 27:753–760
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Friedel JK, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37:25–50
Lee JH, Lee JG, Jeong ST, Gwon HS, Kim PJ, Kim GW (2020) Straw recycling in rice paddy: trade-off between greenhouse gas emission and soil carbon stock increase. Soil and Tillage Research 199:104598
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota – a review. Soil Biol Biochem 43:1812–1836
Li LJ, Han XZ, You M, Horwath WR (2013) Nitrous oxide emissions from Mollisols as affected by long-term applications of organic amendments and chemical fertilizers. Sci Total Environ 452–453:302–308
Liu B, Mou C, Yan G, Xu L, Jiang S, Xing Y, Wang Q (2016a) Annual soil CO2 efflux in a cold temperate forest in northeastern China: effects of winter snowpack and artificial nitrogen deposition. Sci Rep 6(1):18957
Liu S, Zhang Y, Zong Y, Hu Z, Wu S, Zhou J, Jin Y, Zou J (2016b) Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: a meta-analysis. GCB Bioenergy 8(2):392–406
Liu L, Greaver TL (2010) A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13(7):819–828
Lu W, Ding W, Zhang J, Li Y, Luo J, Bolan N, Xie Z (2014) Biochar suppressed the de- composition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol Biochem 76:12–21
Masto RE, Kumar S, Rout TK, Sarkar P, George J, Ram LC (2013) Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. CATENA 111:64–67
Paul EA, Clark FE (1989) Soil microbiology and biochemistry. Academic Press, San Diego
Pan GX, Li LQ, Wu L, Garcia XH (2004) Storage and sequestration potential of topsoil organic carbon in China’s paddy soils. Glob Change Biol 10:79–92
Pei JM, Zhuang S, Cui J, Li JQ, Li B, Wu JH, Fang CM (2017) Biochar decreased the temperature sensitivity of soil carbon decomposition in a paddy field. Agric Ecosyst Environ 249:156–164
Pearson TRH, Brown S, Murray L, Sidman G (2017) Greenhouse gas emissions from tropical forest degradation: an underestimated source. Carbon Bal Manage 12:3
Qin X, Li YE, Wang H, Liu C, Li J, Wan Y, Gao Q, Fan F, Liao Y (2016) Long-term effect of biochar application on yield-scaled greenhouse gas emissions in a rice paddy cropping system: a four-year case study in south China. Sci. Total Environ. 569:1390–1401
Singh BP, Fang Y, Boersma M, Collins D, Van Zwieten L, Macdonald LM (2015) In situ persistence and migration of biochar carbon and its impact on native carbon emission in contrasting soils under managed temperate pastures. PLoS One 10:e0141560
Spokas KA, Reicosky DC (2009) Impacts of sixteen different biochars on soil greenhouse gas production. Annals of Environmental Science 3:179–193
Sun CX, Chen X, Cao MM, Li MQ, Zhang YL (2017) Growth and metabolic responses of maize roots to straw biochar application at different rates. Plant Soil 416:487–502
Suwanwaree P, Robertson GP (2005) Methane oxidation in forest, successional, and no-till agricultural ecosystems: effects of nitrogen and soil disturbance. Soil Sci Soc Am J 69(6):1722–1729
Tang Y, Gao W, Cai K, Chen Y, Li C, Lee X, Cheng H, Zhang Q, Cheng J (2021) Effects of biochar amendment on soil carbon dioxide emission and carbon budget in the karst region of Southwest China. Geoderma 385:114895
Teutscherova N, Vazquez E, Masaguer A, Navas M, Scow KM, Schmidt R, Benito M (2017) Comparison of lime- and biochar-mediated pH changes in nitrification and ammonia oxidizers in degraded acid soil. Biol Fertil Soils 53:811–821
Thiessen S, Gleixner G, Wutzler T, Reichstein M (2013) Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass – an incubation study. Soil Biol Biochem 57:739–748
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
von Lützow M, Kögel-Knabner I (2009) Temperature sensitivity of soil organic matter decomposition ne what do we know? Biol Fertil Soils 46:1–15
Wang C, Liu J, Shen J, Chen D, Li Y, Jiang B, Wu J (2018) Effects of biochar amendment on net greenhouse gas emissions and soil fertility in a double rice cropping system: a 4-year field experiment. Agric Ecosyst Environ 262:83–96
Wang J, Xiong Z, Kuzyakov Y (2016) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8(3):512–523
Wang XJ, Chen GH, Wang SY, Zhang LY, Zhang RD (2019) Temperature sensitivity of different soil carbon pools under biochar addition. Environ Sci Pollut Res 26:4130–4140
Wu Z, Song Y, Shen H, Jiang X, Li B, Xiong Z (2019) Biochar can mitigate methane emissions by improving methanotrophs for prolonged period in fertilized paddy soils. Environ Pollut 253:1038–1046
Yang JH, Wang CL, Dai HL (2008) Agricultural soil analysis and environmental monitoring (in Chinese). China Land Press, Beijing
Yang X, Wang D, Lan Y, Meng J, Jiang L, Sun Q, Cao D, Sun Y, Chen W (2018) Labile organic carbon fractions and carbon pool management index in a 3-year field study with biochar amendment. J Soils Sediment 18(4):1569–1578
Zhang FS, Chen XP, Duan BW (2009) Guide to fertilization of major crops in China. China Agricultural University Press, Beijing ((in Chinese))
Zhou GY, Zhou XH, Zhang T, Du ZG, He YH, Wang XH, Shao JJ, Cao Y, Xue SG, Wang HL, Xu CY (2017) Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. Forest Ecol Manag 405:339–349
Zhou T, Shi P, Hui D, Luo Y (2009) Global pattern of temperature sensitivity of soil heterotrophic respiration (Q10) and its implications for carbon-climate feedback. J Geophys Res Biogeo 114(2):1–9
Zhou X, Wan SQ, Luo YQ (2007) Source components and interannual variability of soil CO2 efflux under experimental warming and clipping in a grassland ecosystem. Glob Chang Biol 13:761–775
Zavalloni C, Alberti G, Biasiol S, Vedove GD, Fornasier F, Liu J, Peressotti A (2011) Microbial mineralization of biochar and wheat straw mixture in soil: a short-term study. Appl Soil Ecol 50(1):45–51
Zimmerman AR (2010) Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ Sci Technol 44:1295–1301
Zimmerman AR, Gao B, Ahn MY (2011) Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol Biochem 43:1169–1179
Acknowledgements
This work was supported by the Chongqing Technology Innovation and Application Demonstration Special Key R & D Project (cstc2018jscx-mszdX0061), Chongqing Key Laboratory of Soil Multiscale Interfacial Process, Scientific Research Project for the Follow-up Work of the Three Gorges Project from the State Major Water Conservancy Project (5001022019CF50001), National Natural Science Foundation of China (42107247), and Key Program of China National Tobacco Corporation Sichuan (CYC202004).
Author information
Authors and Affiliations
Contributions
Rong Huang: Conceptualization, methodology, investigation, and writing of the original draft.
Zifang Wang: Conceptualization, methodology, and validation.
Yi Xiao: Investigation, methodology, and validation.
Luo Yu: Methodology and validation.
Xuesong Gao: Formal analysis and writing including review and editing.
Changquan Wang: Supervision and funding acquisition.
Bing Li: Formal analysis and writing including review and editing.
Qi Tao: Methodology and writing including review and editing.
Qiang Xu: Methodology and validation.
Ming Gao: Supervision and funding acquisition.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Zhihong Xu
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Combination of biochar and N cannot offset the negative effect of biochar on soil CO2 emissions.
• Short-term application of biochar showed a significant increase in CH4 sink strength/reduction in CH4 source strength.
• A lower value of Ea is responsible for the lower Q10 in soil treated with fertilizer.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, R., Wang, Z., Xiao, Y. et al. Increases in temperature response to CO2 emissions in biochar-amended vegetable field soil. Environ Sci Pollut Res 29, 50895–50905 (2022). https://doi.org/10.1007/s11356-022-19011-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-022-19011-6