Abstract
Photoperiodism is an adaptive, seasonal timing system that enables organisms to coordinate their development and physiology to annual changes in the environment using day length (photoperiod) as a cue. This review summarizes our knowledge of the physiological mechanisms underlying photoperiodism in spider mites. In particular, the two-spotted spider mite Tetranychus urticae is focussed, which has long been used as a model species for studying photoperiodism. Photoperiodism is established by several physiological modules, such as the photoreceptor, photoperiodic time measurement system, counter system, and endocrine effector. It is now clear that retinal photoreception through the ocelli is indispensable for the function of photoperiodism, at least in T. urticae. Visual pigment, which comprised opsin protein and a vitamin A-based pigment, is involved in photoreception. The physiological basis of the photoperiodic time measurement system is still under debate, and we have controversial evidence for the hourglass-based time measurement and the oscillator-based time measurement. Less attention has been centred on the counter system in insects and mites. Mite reproduction is possibly regulated by the ecdysteroid, ponasterone A. Prior physiological knowledge has laid the foundation for the next steps essential for the elucidation of the molecular mechanisms driving photoperiodism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Spider mites and photoperiodic regulation of diapause
Among arachnids, the Acari is the only group that feeds on plants. Approximately 7000 species of phytophagous mites are known, including the Tetranychidae, which is a large family with approximately 1300 species and 90 genera (Migeon and Dorkeld 2015). Species belonging to this family are termed ‘spider mites’ owing to their ability to produce silk webs. Spider mites are less than 1 mm in length and their life cycles include five developmental stages: egg, larva, protonymph, deutonymph, and adult. More than a hundred of these organisms are considered pests, and approximately ten are classified as major pests (Migeon and Dorkeld 2015). The most well-known and widespread of the pests is the two-spotted spider mite, Tetranychus urticae. This species attacks approximately 1100 species of plants (Migeon and Dorkeld 2015). In T. urticae, one generation is completed in less than 2 weeks when the temperature is between 21 and 23 °C and in only 7 days when the temperature is higher than 30 °C. Oviposition can begin within a few days after adult emergence. Each female may lay 100–180 eggs throughout her lifespan of a month (Tehri 2014). Because spider mites are economically important agricultural pests (Attia et al. 2013; Van Leeuwen et al. 2015), and owing to the simplicity and ease of rearing them in a laboratory, the biology of spider mites has been extensively studied. One of the topics that have been focussed on includes understanding the regulatory mechanism of diapause (Veerman 2001).
Diapause is the developmental arrest that occurs in recurring periods of adverse environmental conditions, and is most often observed in arthropods, especially in insects and mites. This is an adaptive strategy to synchronize the organism’s life cycle with favourable biotic and abiotic environmental conditions for development, reproduction, and survival (Tauber et al. 1986; Danks 1987). Diapause has been described in a large number of tetranychid species, and both the hibernal and aestival types of diapause (winter diapause and summer diapause, respectively) have been reported (Veerman 1985). In each species of spider mite, diapause occurs only at a specific stage, either the egg (embryonic diapause) or the adult female (reproductive diapause or adult diapause). For example, females of T. urticae enter diapause as adult and arrest ovarian development by terminating the transcription of vitellogenin, a precursor of a yolk protein, vitellin (Kawakami et al. 2009). The fruit tree red spider mite Panonychus ulmi enters diapause as egg. While summer eggs continually develop and do not undergo diapause, the winter eggs enter diapause at the blastoderm stage (Lees 1953a).
Diapause invokes a number of behavioural, physiological, morphological, and molecular modifications. In general, diapause is characterized by suppression of metabolism, changes in behaviour, and increased stress tolerance, often caused by the synthesis of cryoprotectants (Veerman 1985). For example, females of T. urticae in diapause alter expression of genes involved in digestion and detoxification, cryoprotection, carotenoid synthesis and the organization of the cytoskeleton (Bryon et al. 2013), suppress metabolism leading to a significant reduction in most amino acids and TCA cycle intermediates (Kohdayari et al. 2013), move to dark hibernacula for overwintering owing to loss of attraction to visible light and tendency to avoid UV light (Suzuki et al. 2013), and enhance tolerance to multiple stresses, including exposure to cold, heat, desiccation, anoxia, acaricides, and gamma irradiation (den Houter 1976; Ghazy and Suzuki 2014; Kohdayari et al. 2012; Lester and Petry 1995; Lester et al. 1997; Suzuki et al. 2015). In addition, diapause females change their body colour from yellow-green to orange, due to the accumulation of ketocarotenoids, especially astaxanthin, in their body, which is different from nondiapause females (Veerman 1974; Kawaguchi and Osakabe 2014). Astaxanthin acts as a scavenger of reactive oxygen species (Naguib 2000) and its accumulation is believed to confer higher tolerance to UV exposure in diapause individuals (Suzuki et al. 2009). Eggs of P. ulmi and Schizotetranychus schizopus in diapause also appear to accumulate higher amounts of carotenoids (see Veerman 1974).
Although diapause occurs as an obligatory phase of individual development (obligatory diapause) or in response to biotic and/or abiotic cues (facultative diapause), in general, facultative diapause is common in short-lived and multivoltine species, including mites. In temperate regions, the photoperiod is the major cue controlling diapause induction and termination, i.e., photoperiodism (Tauber et al. 1986; Danks 1987). For example, T. urticae adult females enter diapause when they experience short-day conditions during preimaginal development, whereas females in long-day conditions avert diapause and start oviposition immediately after adult emergence (Kawakami et al. 2009; Fig. 1). Diapause can be terminated only after completion of a physiological process called ‘diapause development’, of which the physiological mechanisms are still largely unknown (Hodek 1996). Diapause development proceeds at a slow rate spontaneously, but it can be accelerated by several environmental factors including photoperiod. For example, in T. urticae diapause development is accelerated under long-day conditions, but persists between several weeks to 2 or 3 months when mites experience short-day conditions (Koveos et al. 1993; Fig. 1).
Life cycle of Tetranychus urticae and its photoperiodism. A The life cycle of this species is regulated by photoperiod. Adult females enter diapause when they experience short-day conditions, whereas females in long-day conditions avert diapause and start oviposition. Diapause can be terminated spontaneously (horotelic process) in spider mites, but long-day conditions accelerate diapause development (tachytelic process). Thus, diapause can be terminated in a short period of time when diapause females were reared under long-day conditions, whereas it takes much longer time under short-day conditions. From Goto and Endo (2015). B Photoperiodic response curves of diapause induction (open circles) determined at 19 °C and diapause termination (closed circles) determined at 19 °C after cold storage at 4 °C, in the Leningrad (St. Petersburg) strain of T. urticae (Koveos et al. 1993)
A conceptual cascade involved in photoperiodism is shown in Fig. 2 (Saunders 2002). At the very beginning of the photoperiodic response, organisms must receive environmental light and/or dark signals through photoreceptors. The photoreceptors can be either retinal photoreceptors, a part of the visual system, or extraretinal photoreceptors, involved in the nonvisual system. The photic information is then sent to the photoperiodic time measurement system, which measures the length of day or night. A circadian clock is thought to play a pivotal role in the photoperiodic time measurement, which may rely on distinct photoreceptors. The counter system determines the number of light–dark cycles received and the endocrine effector directly regulates seasonal events, including diapause and seasonal morphs. The physiological mechanisms involved in these systems are reviewed in the following sections.
Various modules establishing photoperiodism. Light/dark signals are received by photoreceptors for photoperiodism and the circadian clock, which may not be identical (Veerman and Veenendaal (2003)). The photoperiodic time measurement system measures the length of day or night and involves the circadian clock. The counter system counts the number of light–dark cycles. When the number of cycles exceeds an internal threshold in the counter, the release/restraint of endocrine effectors is triggered and seasonal events occur (Goto and Numata (2014))
Photoreceptors
In insects, the photoperiodic signals can be received through retinal photoreceptors, extraretinal photoreceptors, or both, and there are no apparent phylogenetic constraints linked to their usage (Goto et al. 2010). For example, in the northern blow fly, Protophormia terraenovae, surgical removal of compound eyes from adult flies severely affected the induction of diapause in adults, underscoring the significance of retinal photoreceptors in this process (Shiga and Numata 1997). On the other hand, the urban bluebottle blow fly, Calliphora vicina, retained photoperiodic sensitivity for maternal induction of larval diapause even after removal of the optic lobe (a connective region between the central brain and compound eyes), indicating that their photoreceptors are extraretinal (Saunders and Cymborowski 1996). It should be noted that retinal and extraretinal photoreception are not mutually exclusive; in fact, the stink bug Plautia stali uses both for the photoperiodic induction of diapause (Morita and Numata 1999).
In mites, it has been suggested that extraretinal photoreception plays an important role in photoperiodism. This association has been made, for example, because the predacious eyeless mite Amblyseius potentillae, which does not possess eyes or ocelli-like structures, shows a clear photoperiodic response (McMurtry et al. 1976; van Houten and Veenendaal 1990). In contrast to eyeless phytoseiid mites, spider mites have distinct eyes on the dorsal side of the prodorsum. External morphology and internal structure of the eyes have been predominantly studied in T. urticae (McEnroe 1969; Mills 1973). Adult T. urticae possess two pairs of eyes (ocelli). The anterior eye faces dorsal-forward and the posterior eye faces dorsally. The anterior eye has a biconvex lens, whereas the posterior eye has a simple convex lens. Five or ten retinular cells form several rhabdomeres in the anterior and posterior eyes, respectively. Naegele et al. (1966) reported spectral sensitivity in the orientation and locomotor responses of T. urticae. Both these responses were demonstrated when mites were exposed to light with wavelengths ranging from 350 to 600 nm, but not to light >600 nm, with clear peaks observed in the spectral sensitivity in the UV region at 375 nm and green region at 525 nm. McEnroe and Dronka (1966, 1969) suggested that photoreception of UV and green light is performed by independent photoreceptor systems, and further concluded that the anterior eyes possess photoreceptors sensitive to UV and green light, whereas the posterior eyes possess photoreceptor sensitive only to UV light. However, physiological validation of these theories is still needed.
Hori et al. (2014) surgically removed the anterior and posterior eyes of T. urticae either bilaterally or unilaterally with a laser ablation system, to clarify whether the eyes play a role in the photoperiodic termination of diapause. A dye laser (coumarin 440) effectively focusses on the screening pigment of the eye of diapause females, and therefore the internal structure of the eyes, beside the external structure, was destroyed. Females in diapause were exposed to low temperatures and transferred to an environment characterized by long-day conditions, short-day conditions, or constant darkness (Fig. 3). Intact females terminate diapause in response to long-day conditions, whereas they remain in diapause when exposed to short-day conditions or constant darkness. Thus, long-day signals are required to terminate diapause. Bilateral and unilateral removal of the anterior eyes did not affect photoperiodic discrimination (i.e. the females terminate diapause under long-day conditions, whereas they maintain diapause under short-day conditions). The same holds true for bilateral and unilateral removal of the posterior eyes. In contrast, bilateral removal of both anterior and posterior eyes significantly affected the termination of diapause. Mites without eyes failed to discriminate photoperiods and maintained diapause, irrespective of the photoperiod (Fig. 3). Thus, in T. urticae, both anterior and posterior eyes function as photoreceptors for the photoperiodic termination of diapause. However, it is still unknown whether this finding is generally applicable to other spider mite species, since even closely related insect species use distinct photoreceptors (Goto et al. 2010). It is also noteworthy that unilateral removal of both anterior and posterior eyes also significantly reduced the incidence of diapause termination under long-day conditions (Fig. 3). A similar effect of unilateral removal of photoreceptors on the photoperiodic response was shown in P. terraenovae, P. stali, as well as the crickets Modicogryllus siamensis and Dianemobius nigrofasciatus (formerly known as Pteronemobius nigrofasciatus) (Shiga and Numata 1996, 1997; Morita and Numata 1999; Sakamoto and Tomioka 2007). These results suggest that photoperiodic machinery resides in both hemispheres in the brain (specifically in the case of mites, the synganglion), and photoperiodic information from photoreceptors on both sides must be integrated to fully discriminate the photoperiod. It is known in P. terraenovae that the circadian clock located in each optic lobe is causally involved in the photoperiodic response (Shiga and Numata 2009). Involvement of the circadian clock in photoperiodism will be discussed later in this review.
Photoperiodic termination of diapause in Tetranychus urticae and the role of the eyes. Mites in diapause were chilled at 5 °C for 20 days and then transferred to short-day conditions (S), long-day conditions (L), or constant darkness (DD) at 17 °C. No significant differences were detected between treatments with the same letter (Tukey-type multiple comparisons for proportions, P > 0.05; Zar 2010) in each panel. A Intact mites. B Mites whose both anterior and posterior eyes were removed unilaterally or bilaterally (Hori et al. 2014)
Although not statistically significant, the incidence of diapause termination under long-day conditions was still higher than that under short-day conditions, even when both anterior and posterior eyes were removed bilaterally (Fig. 3). Although it is possible that the eye-removal was not complete in some mites, these results may indicate supplemental involvement of extraretinal photoreception in photoperiodic termination of diapause in T. urticae, in addition to the principal role of retinal photoreception, as was found in P. stali (Morita and Numata 1999).
Suzuki et al. (2008a) investigated spectral sensitivity and light intensity required for photoperiodic induction of diapause in T. urticae (Fig. 4). When monochromatic light was used as a light source in a 8-h light: 16-h dark (LD 8:16 h) and intensity of the light was changed, the threshold intensity to induce diapause in 50 % of the individuals was the lowest for blue light (475 nm), intermediate for green light (572 nm), and highest for orange light (612 nm). On the other hand, T. urticae had no ability to respond to red light (658 nm). Thus, T. urticae can receive a broad range of light wavelengths and is highly sensitive to light with short wavelengths. Lees (1953a) also reported that P. ulmi is able to respond to a broad range of light wavelengths from the near UV (365 nm) to blue-green light (540 nm) to avert embryonic diapause, with maximal sensitivity of the blue (425 nm) region. This species had no ability to respond to the orange, red and infrared light (longer than 550 nm).
Diapause induction in Tetranychus urticae in cycles of 8-h of monochromatic light and 16-h of darkness at 18 °C. Various wavelengths of light (blue, 475 nm; green 572 nm; orange, 612 nm) at various intensities (50, 500, and 2500 mW/m2 for open circles, closed circles, and open squares, respectively) were used (Suzuki et al. 2008a)
Veerman (1980) investigated the photoperiodic response of four albino mutants isolated from wild T. urticae populations, in which uptake and oxidative metabolism of carotenoids were blocked. The original wild populations entered diapause in response to short-day conditions, whereas diapause incidence appeared to be lowered in the mutants. When a semisynthetic diet was used, no diapause was found in the albino mutant under short-day conditions on the standard diet. However, partial restoration of the photoperiodic response was obtained after addition of β-carotene to the diet, and full restoration was observed after the addition of vitamin A (Bosse and Veerman 1996). The significance of carotenoids in photoperiodic induction of diapause has also been clarified not only in the mites A. potentillae and Amblyseius cucumeris (Van Zon et al. 1981; Veerman et al. 1983; Overmeer et al. 1989), but also in several insect species (see Saunders 2012). Thus, vitamin A and its derivatives are prerequisites for the photoperiodic induction of diapause.
The requirement of vitamin A or its derivatives, and the broad range of the effective wavelength of light required for the photoperiodic response in T. urticae, is reminiscent of visual pigments. Visual pigments have been identified as photoreceptor molecules in various organisms, and comprised the opsin protein and a vitamin A-based pigment, which can be retinal or 3-hydroxyretinal. Each visual pigment generally exhibits a narrow range of spectral sensitivity; however, each species possesses multiple visual pigments with distinct spectral classes. Therefore, animals can perceive light across broad wavelengths (Henze and Oakley 2015). Tamaki et al. (2013) utilized RNAi to demonstrate that UV-, blue-, and long-wave-sensitive opsins are causally involved in photoperiodic photoreception in the nymphal diapause of M. siamensis. These results, together with those studies on mites, indicate that multiple types of visual pigments or a single type of visual pigment sensitive to a broad wavelength of light located in the anterior and posterior eyes function as photoreceptive molecules for the photoperiodic response in T. urticae.
Genome of T. urticae has been available as the first complete chelicerate genome (Grbić et al. 2011). Owing to the availability of next-generation sequencing methods, an increasing number of mite genomes and transcriptomes have been released in public database (Van Leeuwen and Dermauw 2016). In T. urticae, three putative opsin genes (tetur12g04340, tetur07g05150, tetur24g02280) and one peropsin gene (tetur04g04260) have been detected in the OrcAE database (Online Resource for Community Annotation of Eukaryotes; http://bioinformatics.psb.ugent.be/orcae/). Peropsin is a member of the opsin family and has characteristics of two functionally distinct opsin-groups, i.e. amino acid residues conserved among opsins involved in light-sensing and retinal-photoisomerase-like molecular properties. In a spider, peropsin is localized in nonvisual cells in the retina and acts as a photosensitive pigment with a nonvisual function (Nagata et al. 2010). In P. ulmi, five transcripts encoding putative opsin (GCAC01000911, GCAC01005648, GCAC01005363, GCAC01000911, GCAC01006475) and one transcript encoding putative peropsin (GCAC01001176) were found in the DDBJ/GenBank/EMBL database. Although the spectral sensitivity of these pigments in relation to the opsins and peropsin is still unknown, multiple opsin genes indicate that they are characterized by a broad sensitivity to light.
Photoperiodic time measurement and circadian clock
The relative importance of the light and dark components of the daily cycle have been investigated in various organisms by independently varying light and dark in overall cycle lengths close to 24 h in duration. It is now generally accepted that duration of the night is much more important than the duration of light not only in insects but also in mites (Saunders 2013). For example, Lees (1953b) combined various lengths of light and dark and found that diapause of P. ulmi is averted in photoperiods containing a night equal to or shorter than 8 h (e.g. LD 16:4 h, LD 24:4 h, LD 12:8 h, LD 16:8 h, and LD 24:8 h) but is clearly induced in photoperiods containing a night longer than 8 h (e.g. LD 4:12 h, LD 8:12 h, LD 12:12 h, LD 16:12 h), irrespective of the duration of the accompanying light component. Also in T. urticae, diapause incidence was low in photoperiods containing a short night (e.g. LD 12:8 h and 16:8 h) but approached 100 % in photoperiods containing a long night (e.g. LD 8:12 h and LD 12:12 h), regardless of the duration of the accompanying light component (Veerman 1977; Veerman and Veenendaal 2003). Nevertheless, terms focussing on day but not night, such as long-day conditions, short-day conditions and day length, have been commonly used in the literatures. These terms are used in this review.
Bünning (1936) first proposed the involvement of a circadian clock in photoperiodic time measurement. Bünning’s hypothesis posited that the 24-h circadian clock consisted of two 12-h half-cycles, which were termed the photophil and scotophil (light- and dark-loving phases, respectively). It also stated that short-day effects are observed when light is restricted to the photophil, while long-day effects are produced when light penetrates the scotophil. Although this idea is too simple to explain the range of photoperiodic responses, the basic concept of a circadian clock in photoperiodic time measurement is now widely accepted, not only in insects (Saunders and Bertossa 2011) but also in other organisms, ranging from fungi to mammals (Nelson et al. 2010).
Whether the circadian clock is involved in photoperiodic time measurement can be assessed by experiments revealing the known effects of environmental light pulses on the phase shifting and entrainment of circadian oscillations (Saunders 2002). For instance, a short-day photophase ranging from 10- to 12-h can be coupled with periods of scotophase varying from 4- to 72-h [known as the Nanda–Hamner protocol: Nanda and Hamner (1958)]. Alternatively, insects can be exposed to 48- or 72-h cycles consisting of a 12-h photophase with a light pulse systematically interrupting an extended period of perceived night [known as the Bünsow protocol: Bünsow (1960)]. In both types of experiments, these aberrant light cycles are repeated throughout the photoperiod-sensitive period, after which short-day effects are assessed for each condition. A circadian involvement is suspected when short-day effects occur in alternating peaks and troughs with an approximate 24-h periodicity in the extended scotophase. Conversely, the absence of this pattern is evidence of an hourglass-like timer, which is the case in the aphid Megoura viciae (Lees 1973). An hourglass is a mechanism that follows a set time course in darkness after being initiated at lights off and needs a minimum duration of light to restart the measurement process at the beginning of the next scotophase. This can be considered a non-circadian mechanism; however, it can also be considered a heavily dampened circadian oscillator, of which oscillation is easily dampened out below threshold in extended periods of darkness (Saunders 2010). Such dampened oscillator is able to measure only one night in the extended darkness, so that the accumulation of short-day information is lowered, so reducing the final incidence of diapause. This heavily dampened circadian oscillator has been shown to be important for photoperiodic timing even in M. viciae (Vaz Nunes and Hardie 1993). Thus, the functional role of a circadian clock in photoperiodic time measurement is now widely accepted, although some details are still under dispute (Bradshaw and Holzapfel 2007).
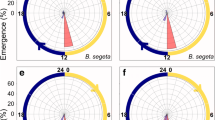
When T. urticae was reared in cycles consisting of 8-h of photophase and different durations of scotophase (the Nanda–Hamner protocol), peaks of high diapause incidence recurred with cycle lengths (duration of light plus dark) of approximately 24, 44, 64 and 84 h, which correspond to 16-, 36-, 56- and 76-h of scotophases, respectively. Diapause induction was completely averted in photoperiodic cycles consisting of 8-h photophase combined with scotophases ranging from 4 to 9, 24 to 28, 44 to 48 and 60 to 64-h (Veerman and Vaz Nunes 1980; Fig. 5). These results indicate a functional connection between the circadian system and photoperiodism in T. urticae. It is important to note that the resonance peaks are approximately 20-h apart. This indicates that period of the circadian clock involved in Tetranychus photoperiodism in the free-run state (free-running period) is 20-h.
Diapause incidence of Tetranychus urticae when exposed to cycles consisting of 8-h of light and various durations of scotophase (the Nanda–Hamner protocol) (Veerman and Vaz Nunes 1980)
Veerman and Vaz Nunes (1987) compared the response under a 12-h light:12-h dark (LD 12:12 h) with that from a 12-h light:36-h dark (LD 12:36 h) cycle. As the number of the light–dark cycles increased, diapause incidence increased in both conditions. However, the efficacy of diapause induction under the LD 12:36 h cycle was approximately half of that of LD 12:12 h, indicating that a night 36-h long in the environmental cycle of 48-h in LD 12:36 h is effectively one long night. If the clock measuring night length is an oscillator which is able to free-run under constant darkness, with a night 36-h long in an LD cycle of 12:36 h is measured as two long nights (Fig. 6). Thus, photoperiodic time measurement in the spider mite is either a true non-circadian hourglass or a heavily damping oscillator. Based on these results, Vaz Nunes proposed the double circadian oscillator model (Vaz Nunes 1998). This model assumes the presence of two independent circadian mechanisms where both play a role in the determination of the length of a night. One of the mechanisms is the long night (LN) system, which assigns scotophase a positive value when the night is long. The other is the short night (SN) system, which assigns scotophase a positive value when the night is short. The model successfully simulates the photoperiodic response in T. urticae with the assumption of a rapidly damping LN system and a non-damping SN system (Vaz Nunes 1998).
Different kinetics of photoperiodic time measurement between an oscillator and an hourglass and diapause incidence of Tetranychus urticae under Veerman-Vaz Nunes light–dark cycles. Open and closed horizontal bars indicate photophase and scotophase, respectively (Veerman and Vaz Nunes 1987). A Clocks based on the oscillator principle oscillate even under constant darkness, whereas clocks based on the hourglass stop after a single time measurement (a). Both the oscillator and hourglass can measure length of night under light–dark cycles (b). The oscillator can perform two acts of time measurement in nights 36-h long (c), compared to only one measurement when using an hourglass (d). Consequently, twice as many ‘inductive events’ can be counted during the same number of light (L) and dark (D) 12:36 cycles if the clock is an oscillator, as in the case of hourglass time measurement. B Diapause incidence of mites exposed to either constant darkness, LD 12:12 h, or LD 12:36 h during their development
In insects, interlocked positive and negative feedback loops based on transcription and translation are the essence of circadian clocks, and major players of the loops include period (per), timeless (tim), mammalian-type cryptochrome (cry-m; also known as cry2), cycle (cyc) and Clock (Clk) genes (Tomioka and Matsumoto 2015). Some insect species possess another type of cryptochrome; i.e. Drosophila-type cryptochrome (cry-d; also known as cry1). Its protein product acts as a photoreceptor molecule to reset the circadian clock (Tomioka and Matsumoto 2015). Knocking down these clock genes using RNAi revealed their causal involvement in photoperiodism in some insect species, (Numata et al. 2015). For example, RNAi of per, cry-m, cyc, and Clk disrupted the photoperiodic induction of reproductive diapause in the bean bug Riptortus pedestris (Ikeno et al. 2010, 2011a, b, 2013). NAi directed against per, tim, and cry-m, and pigment-dispersing factor (pdf), a putative output gene of the circadian clock, also disrupts the photoperiodic response in the mosquito Culex pipiens (Meuti et al. 2015). per RNAi disrupted photoperiodic induction of larval diapause in the jewel wasp Nasonia vitripennis (Mukai and Goto 2016) and photoperiodic induction of nymphal diapause as well as circadian locomotor rhythmicity in M. siamensis (Sakamoto et al. 2009). A genetic variant found in nature also supports causal involvement of the circadian clock in photoperiodism. A genetic variant of the drosophilid fly, Chymomyza costata, which is named the non-photoperiodic diapause (npd), showed an abnormal photoperiodic response (Riihimaa and Kimura 1988) and an arrhythmic pattern of adult eclosion (Lankinen and Riihimaa 1992). Daily oscillations in per and tim expression were clearly observed in wild-type flies, whereas per was expressed arrhythmically at low levels and tim mRNA was completely absent in the variant (Koštál and Shimada 2001, Pavelka et al. 2003), due to a large deletion in a crucial cis-regulatory element and minimal promoter (Kobelková et al. 2010). A genetic linkage analysis mapped the gene responsible for the abnormal photoperiodic phenotype to the locus containing tim (Pavelka et al. 2003). These results provide evidence for the role of tim in the photoperiodic induction of diapause in C. costata.
These studies raise the question: does malfunction of circadian clock genes affect photoperiodism by altering clock function, or does malfunction of circadian clock genes directly affect diapause? This question is a focal point in the discussion concerning the molecular basis of photoperiodism, with some studies in R. pedestris supporting the former possibility after analysing results from knocking down all major clock genes (Numata et al. 2015). Recently, Pegoraro et al. (2014) focussed on the photoperiodic response in chill-coma recovering time (CCRt) in D. melanogaster. Wild-type flies maintained under short-day conditions exhibited significantly shorter CCRt than flies under long-day conditions. Arrhythmic mutant strains, per 01, tim 01 and Clk Jrk, demonstrated a disrupted photoperiodic response in CCRt. It is of interest to note that mutants with long free-running periods consistently showed short-day-type responses in CCRt under both long and short photoperiods, compared with mutants with short free-running periods. The results aligned with those expected under Bünning’s hypothesis. In mutants with a long free-running period (where photophil is longer), various photophases consistently coincided with the photophil phase and were interpreted as short-day conditions (Pegoraro et al. 2014). The different photoperiodic phenotypes of the slow and fast clock mutants suggest a causative role for the circadian clock in the photoperiodic time measurement. Mohamed et al. (2014) focussed on the link connecting the circadian clock to a photoperiodic endocrine switch in the Chinese oak moth Antheraea pernyi, of which pupal diapause can be terminated by long-day conditions. Through employing RNAi, immunohistochemistry, radioimmunoassay (RIA) and radioenzymatic assay, they demonstrated that N-acetyltransferase (AA-NAT), a rate-limiting enzyme for the production of melatonin and one of the clock-controlled genes, regulates secretion of the prothoracicotropic hormone (PTTH), which stimulates the prothoracic gland to secrete ecdysteroids, and terminate diapause.
Although the causal involvement of the circadian clock in photoperiodism has been reported in these insect species, controversial evidence has accumulated in T. urticae. Veerman and colleagues focussed on the critical day length (CDL) to clarify the role of the circadian clock in photoperiodism in T. urticae. The CDL is the day length in which a half of population shows a long-day response with a distinct latitudinal cline (Goto and Numata 2014). Vaz Nunes et al. (1990) compared variation in CDL with variation in the free-running period of the Nanda–Hamner rhythm, which is involved in the photoperiodic response in T. urticae. If the circadian clock is indeed involved in the photoperiodic time measurement, there must be a correlation between them, as indicated by Pegoraro et al. (2014). However, only a very weak correlation between them was observed (Fig. 7), with little or no correlation between CDL and the circadian phenotype reported in Drosophila auraria (Pittencrigh et al. 1984), Drosophila littoralis (Lankinen and Forsman 2006), and the pitcher plant mosquito, Wyeomyia smithii (Bradshaw et al. 2003, 2006). Genetic analysis has also been completed in the spider mite. Reciprocal crosses were made between two strains of mites, which differed by 3-h in CDL and 2-h in the free-running period of the Nanda–Hamner rhythm. The crossing experiments showed that a short free-running rhythm is almost completely dominant over a long free-running rhythm, whereas CDL is inherited in an intermediate way (Vaz Nunes et al. 1990), indicating that these characteristics are governed by independent genetic elements. Moreover, in two strains of T. urticae, originating from the same latitude, CDL appeared to be the same, whereas the period of the free-running rhythm of the Nanda–Hamner experiments differed from 1 to 3-h among the strains, depending on temperature (Koveos and Veerman 1996). Veerman and Veenendaal (2003) revealed that the photoperiodic time measurement system is sensitive to light ranging from orange to red, whereas the Nanda–Hamner rhythm (of which free-running period is 20-h) is insensitive to the light, and therefore, it free-runs under the orange-red light photoperiod. These results do not provide evidence in favour of a circadian-based photoperiodic time measurement.
Relationship of the critical day length for induction of diapause and the period of the free-running rhythm under the Nanda–Hamner protocol in 10 Tetranychus urticae strains (Vaz Nunes et al. 1990). The regression line and r 2 value are also shown
Based on these results, Veerman (2001) emphasized that a clock role for the circadian system in mite photoperiodism is highly unlikely and photoperiodic time measurement in mites most likely is a non-circadian hourglass mechanism. In his idea, positive Nanda–Hamner and Bünsow results indicate that some subsystem(s) other than the photoperiodic time measurement system is affected by the circadian system, resulting in the rhythmic responses observed. Indeed, a wide array of physiological processes in an organism is expected to be fallen under circadian control (Allada and Chung 2010). Currently, there is a debate regarding which of the concepts (hourglass timer vs. circadian clock) in T. urticae is based only on classic physiology, and therefore, understanding of this process is still highly conceptual. It would be beneficial for new approaches to address the point to be proposed.
Homologues of clock and clock-related genes are also found in T. urticae (per, tetur11g03490; tim, tetur27g02370; cry-m, tetur09g05920; cyc, tetur08g07430; Clk, tetur08g07600; cry-d, tetur16g02770). pdf gene has not been found in T. tetranychus genome, but its putative receptor (tetur04g08940) was detected (Veenstra et al. 2012). Also in P. ulmi, transcripts of putative per (GCAC01001617 and very short sequence of GCAC01025045), cry-m (GCAC01005533 and rather short sequence of GCAC01001108), cyc (GCAC01002042), and Clk (GCAC01003834) and 2 cry-d (GCAC01000428 and GCAC01007196) were found, but tim transcript was not. However, the roles of these clock genes in the circadian clock and also in photoperiodism have not been investigated in any Acari species.
Counter
The photoperiodic counter registers successive cycles during the sensitive period until an internal threshold is reached, which triggers a physiological response mediated by endocrine effectors. In one model of photoperiodic summation, organisms accumulate a hypothetical diapause-inducing substance under short-day conditions in the counter system after processing photoperiodic information in the time measurement system (Gibbs 1975). Thus, a short-day response is elicited upon exceeding the internal threshold, whereas a long-day response is induced at subthreshold values (Gibbs 1975; see also Tagaya et al. 2010). In T. urticae, Koveos and Veerman (1994) found that the threshold for diapause termination in long-day conditions, expressed as the number of light–dark cycles required for 50 % diapause termination, is lower in the southern strain than the northern strain (Fig. 8). In addition, considerable differences in the number of light–dark cycles required for diapause termination were also observed among strains. These inter- and intra-strain variations would be derived from variation in the synthesis rate of the hypothetical substance or the threshold. However, their molecular and neural bases are still largely unknown. In A. pernyi, it has been suggested that the photoperiodic counter is driven by mutual inhibition between the melatonin and dopamine pathways (Wang et al. 2015). AA-NAT increased in expression level in response to long-day conditions, whereas dopa decarboxylase (DDC), the rate-limiting enzyme for the production of dopamine, decreased in expression level in response to changes in the photoperiod. Wang et al. (2013) also found in A. pernyi that expression of one type of serotonin receptors (5HTRB) decreases in response to long-day conditions, and RNAi directed against the receptor induces PTTH accumulation and results in early diapause termination. Injection of 5,7-dihydroxytryptamine (5,7-DHT), a pharmacological agent decreasing serotonin concentration, induces early emergence even under short-day conditions.
Number of long-day (LD 17:7 h) cycles required for diapause termination at 19 °C in three Tetranychus urticae strains after cold exposure at 4 °C. After experiencing the indicated number of long-day cycles, mites were transferred to continuous darkness at 19 °C. Percentages of diapause termination were determined 12 days after removal from cold storage. Closed circles Leningrad (St. Petersberg); open squares Voorne; open circles Thessaloniki-II (Koveos and Veerman 1994)
At least five (tetur22g00750, tetur12g02440, tetur01g00420, tetur07g07500, and tetur247g00020) and two (GCAC01002910 and GCAC01007129) sequences showing high similarity to DDC have been found in T. urticae and P. ulmi, respectively. Several genes and transcripts showing high similarity to the serotonin receptor are also found in them. Although genes homologous to AA-NAT have not been identified in Acari (Hiragaki et al. 2015), its activity and the action spectrum for suppression of the activity have been investigated in T. urticae (Suzuki et al. 2008b). It is of interest to measure the levels of these biogenic amines and the expression levels of these genes in mites under diapause-inducing short-day conditions and diapause-averting long-day conditions. They are the candidates of the diapause-inducing substances hypothesized by Gibbs(1975).
Endocrine effector
Although there is no conclusive evidence, it is reasonable to assume that embryonic and reproductive diapause in the spider mite is hormonally regulated, as reported previously in insects. In insects, embryonic diapause is regulated by diapause hormone (DH) or ecdysteroids, whereas reproductive diapause is regulated by juvenile hormone (JH) or ecdysteroids (Denlinger et al. 2012). DH, a member of the FXPRL-amid peptide family, is a crucial factor that directly regulates embryonic diapause of the silk moth Bombyx mori (Yamashita 1996). Although downstream cascade of DH has also been elucidated in B. mori, there is no evidence to suggest that the regulatory mechanisms documented in the species is applicable to embryonic diapause in other insect species. Ecdysteroids, a specific family of sterol derivatives, are essential for controlling insect development, including moulting, metamorphosis, and also diapause (Lafont et al. 2012). Embryonic diapause of the Australian plague locust Chortoicetes terminifera and the migratory locust Locusta migratoria is considered to be induced by the absence of ecdysteroids (Gregg et al. 1987; Tawfik et al. 2002a), whereas that of the gypsy moth Lymantria dispar is induced by an elevated ecdysteroid titre (Lee and Denlinger 1997). Low titre of JH, a family of acyclic sesquiterpenoids, is well-known to induce reproductive diapause in many insect species, such as the Colorado potato beetle Leptinotarsa decemlineata (de Kort 1990), the Northern house mosquito Culex pipiens (Readio et al. 1999), and P. stali (Kotaki et al. 2011). On the other hand, absence of ecdysteroids is considered to be the key in reproductive diapause in D. melanogaster (Richard et al. 1998) and L. migratoria (Tawfik et al. 2002b).
Genome analysis revealed that T. urticae has no ability to produce JH due to a lack of the CYP15A1 gene, which encodes the enzyme introducing the signature epoxide of insect JHs. Instead of JH, T. urticae produces methyl farnesoate (MF) (Grbić et al. 2011). The role of MF in spider mite physiology has not been verified, but Regev and Cone (1976) reported that females of T. urticae treated topically with farnesol laid more eggs than females. This result implies some role of MF in their reproduction. MF is also the final product in crustaceans, but there is a debate regarding its role. Laufer et al. (1998) revealed in the crayfish Procambarus clarkii that administration of MF stimulates ovarian maturation. However, recent studies have indicated that MF has no effects on vitellogenin (Vg) gene expression in the hepatopancreas of shrimp (Metapenaeus ensis), lobster (Homarus americanus), and crab (Charybdis feriatus) (Subramoniam 2011). In contrast, Marsden et al. (2008) indicated an inhibitory role of MF in the late stage of ovary development in black tiger prawn Penaeus monodon. In ticks, 20-hydroxyecdysone (20E) is responsible for the initiation of Vg synthesis, and ecdysteroids secreted by the epidermis and converted into 20E by the fat body (Cabrera et al. 2009). Vg is synthesized primarily in the fat body and midgut, and to a lesser extent in the ovary (Rosell and Coons 1992; Thompson et al. 2007). However, the source of extraovarian Vg has not been clearly determined for any mite species and it remains to be addressed, because in contrast to ticks, most mites lack the fat body (Cabrera et al. 2009). In Tetranychus, the midgut has been suggested to be a source of Vg (Shatrov 1997, 2002). The T. urticae genome lacks two P450 genes, CYP306A1 and CYP18A1, which encode C25 hydroxylase and a C26 hydroxylase/oxidase involved in hormone inactivation, respectively. The absence of CYP306A1 indicates that the spider mite uses the ecdysteroid, ponasterone A, as the moulting hormone instead of the typical arthropod 20E, which was confirmed by biochemical analysis of spider mite extracts (Grbić et al. 2011).
Cabrera et al. (2009) proposed a hypothesis to describe the regulation of vitellogenesis and female reproduction in Acari and theorized that ecdysteroids play an important role in acarine vitellogenesis. The synganglion synthesizes an ecdysiotropic hormone (EDTH) that initiates the production of ecdysteroids in the epidermis. The ecdysteroid then, possibly ponasterone A, induces Vg production in the midgut and ovary (Fig. 9). Reproductive diapause in mites is possibly induced and maintained by some process suppressing ponasterone A secretion. Kawakami and Numata (2013) found that topical application of a synthetic pyrethroid, cypermethrin (CyM), induces ovarian development in T. urticae undergoing diapause. In unengorged adult females of the soft tick Ornithodoros moubata, CyM induces Vg synthesis in the fat body and vitellin accumulation in the oocytes, whereas it does not induce oviposition (Chinzei et al. 1989; Taylor et al. 1991). Although the mechanism of action of the pyrethroid leading to the induction of vitellogenesis is unknown, Chinzei et al. (1989) suggested that the endocrinological steps required for vitellogenesis are induced by neurosecretory factors secreted artificially, due to the change in electrical activity of neurosecretory cells driven by the pyrethroid. In diapause adults of the beetle Henosepilachna vigintioctopunctata, pyrethroids also stimulate ovarian development and partially induce oocyte maturation and oviposition (Kono and Ozeki 1987). Diapause in mites is likely induced and maintained by suppression of neurosecretory secretion, although the identity of the neurosecretory factors remains unknown. Endocrinological mechanisms promoting embryonic diapause in spider mites are still unknown.
Model summarizing current knowledge on regulation of ovarian development in Tetranychus urticae. Anterior and posterior eyes function as photoreceptors in T. urticae. Visual pigments, which comprised opsin protein and a vitamin A-based pigment, would be involved in photoreception. The physiological basis of the photoperiodic time measurement system and the counter system is still largely unknown. Reproduction is possibly regulated by the ecdysteroid (E), ponasterone A. Cabrera et al. (2009) proposed that the synganglion synthesizes an ecdysiotropic hormone (EDTH) that initiates the production of ecdysteroids in the epidermis. The ecdysteroid then induces vitellogenin (Vg) production in the midgut and ovary. After incorporation into oocytes, Vg is stored in a crystalline form as vitellin (Vn), a reserve food source for the future embryo. Some process regulating ponasterone A synthesis would be involved in diapause induction. The role of methyl farnesoate (MF) in spider mite physiology has not been verified
Future prospectives
Although we have accumulated much data on the physiological mechanisms of mite photoperiodism, the molecular mechanisms underlying it, especially those related to photoperiodic time measurement and the counter, are still largely unknown (see Fig. 9 for a summarising model). Since genomic information is available for T. urticae (Grbić et al. 2011), now it is easy to clone genes of interest. Gene silencing is possible in T. urticae by injecting or feeding the organism double-stranded RNA (Khila and Grbić 2007; Kwon et al. 2013), although its efficacy seems to be very low. Silencing candidate genes considered to be involved in the photoperiodic cascade (for example, those involved in light perception, the circadian clock, and hormone syntheses) would be a valuable method to dissect molecular mechanisms underlying photoperiodism.
Recently, it has become relatively easier to access high-throughput technologies, including next-generation sequencing platforms and microarrays. Bryon et al. (2013) investigated essential physiological processes in T. urticae in diapause by studying genome-wide expression changes, using a custom-built microarray. Analysis of this dataset showed that 11 % of the total number of predicted T. urticae genes was differentially expressed. Similar experiments focussing on differential gene expression in diapause and nondiapause individuals have also been performed in various insect species (for example, Kankare et al. 2010; Kumar et al. 2014; Poelchau et al. 2013; Qi et al. 2015; Wadsworth and Dopman 2015). However, use of a high-throughput approach during the photoperiod-sensitive stage has been limited (Le Trionnaire et al. 2009; Poupardin et al. 2015; Zhang et al. 2011). Huang et al. (2015) utilized powerful RNA-seq technologies to elucidate gene expression in C. pipiens during its photoperiod-sensitive stage. This study found upregulation of tim, cry-d and JH-inducible proteins and activation of two amino acid metabolic pathways in non-blood-fed females under diapause-inducing short-day photoperiods. These genes and proteins are the candidates of the players in the photoperiodic time measurement and counting. The photoperiod-sensitive stage for diapause induction in T. urticae is predominantly restricted to deuronymphs with some sensitivity observed at the larval and protonymphal stages (Suzuki and Takeda 2009). It would be very interesting to compare gene expression between deutonymphs maintained in short-day and long-day conditions.
Geographic variation in diapause potential has been reported in T. urticae (Gotoh and Shinkaji 1981; Takafuji et al. 1991; Koveos et al. 1993; Vaz Nunes et al. 1990). Genetic crosses revealed that variation in diapause potential could be attributed to various genetic systems including the presence of dominant alleles at multiple loci (Kawakami et al. 2010), a recessive allele at a single locus (Kawakami et al. 2010; Ignatowicz and Helle 1986), and incompletely recessive alleles at multiple loci (Goka and Takafuji 1990, 1991; So and Takafuji 1992). Although several genes responsible for natural variation in the diapause phenotype have been elucidated in the model insect D. melanogaster (Schmidt et al. 2008; Tauber et al. 2007; Williams et al. 2006), such loci or genes responsible for the phenotype have not yet been revealed in T. urticae. Mapping the location of causal mutations using genetic crosses has traditionally been a complex and multistep procedure, but next-generation sequencing now allows for the rapid identification of causal mutations at the single-nucleotide resolution level even in complex genetic backgrounds (Schneeberger 2014). Recent advances of this mapping-by-sequencing approach include methods that are independent of reference genome sequences, genetic crosses or any type of linkage information. Van Leeuwen et al. (2012) adopted this methodology (bulk segregant analysis mapping method with high-throughput sequencing technology) to verify the locus responsible for the resistant phenotype to the acaricide etoxazole in the field-collected T. urticae population. Finally, they clarified a single amino acid change in the chitin synthase 1 as conferring target site resistance to etoxazole. These approaches could shed light on the molecular mechanisms underlying not only photoperiodism but also other physiological processes in spider mites (Van Leeuwen and Dermauw 2016).
References
Allada R, Chung BY (2010) Circadian organization of behaviour and physiology in Drosophila. Annu Rev Physiol 72:605–624. doi:10.1146/annurev-physiol-021909-135815
Attia S, Grissa KL, Lognay G, Bitume E, Hance T, Mailleux AC (2013) A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J Pest Sci 86:361–386. doi:10.1007/s10340-013-0503-0
Bosse ThC, Veerman A (1996) Involvement of vitamin A in the photoperiodic induction of diapause in the spider mite Tetranychus urticae is demonstrated by rearing an albino mutant on a semi synthetic diet with and without β-carotene or vitamin A. Physiol Entomol 21:188–192. doi:10.1111/j.1365-3032.1996.tb00854.x
Bradshaw WE, Holzapfel CM (2007) Evolution of animal photoperiodism. Ann Rev Ecol Evol Syst 38:1–25. doi:10.1146/annurev.ecolsys.37.091305.110115
Bradshaw WE, Quebodeaux MC, Holzapfel CM (2003) Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: adaptive response to the photic environment or correlated response to the seasonal environment? Am Nat 161:735–748. doi:10.1086/374344
Bradshaw WE, Holzapfel CM, Mathias D (2006) Circadian rhythmicity and photoperiodism in the pitcher-plant mosquito: can the seasonal timer evolve independently of the circadian clock? Am Nat 167:601–605. doi:10.1086/501032
Bryon A, Wybouw N, Dermauw W, Tirry L, Van Leeuwen T (2013) Genome wide gene-expression analysis of facultative reproductive diapause in the two-spotted spider mite Tetranychus urticae. BMC Genom 14:815. doi:10.1186/1471-2164-14-815
Bünning E (1936) Die endogene Tagesrhythmik als Grundlage der Photoperiodischen Reaktion. Ber Dtsch Bot Ges 54:590–607
Bünsow RC (1960) The circadian rhythm of photoperiodic responsiveness in Kalanchoë. Cold Spring Harb Symp Quant Biol 25:257–260. doi:10.1101/SQB.1960.025.01.027
Cabrera AR, Donohue KV, Roe RM (2009) Regulation of female reproduction in mites: a unifying model for the Acari. J Insect Physiol 55:1079–1090. doi:10.1016/j.jinsphys.2009.08.007
Chinzei Y, Itoh K, Ando K (1989) Cypermethrin induction of vitellogenesis and ovarian development in unfed adult female Ornithodoros moubata (Acari: Argasidae). Invertebr Reprod Dev 15:19–26. doi:10.1080/07924259.1989.9672017
Danks HV (1987) Insect dormancy: an ecological perspective. Biological Survey of Canada, Ottawa
de Kort CAD (1990) Thirty-five years of diapause research with the Colorado potato beetle. Entomol Exp Appl 56:1–13. doi:10.1111/j.1570-7458.1990.tb01376.x
den Houter JG (1976) The effect of acaricides on active and diapausing females of Tetranychus urticae Koch, the two-spotted spider mite. J Appl Entomol 81:248–252. doi:10.1111/j.1439-0418.1976.tb04233.x
Denlinger DL, Yocum GD, Rinehart JP (2012) Hormonal control of diapause. In: Gilbert LI (ed) Insect endocrinology. Academic Press, London, pp 430–463
Ghazy NA, Suzuki T (2014) Desiccation tolerance in diapausing spider mites Tetranychus urticae and T. kanzawai (Acari: Tetranychidae). Exp Appl Acarol 63:49–55. doi:10.1007/s10493-013-9760-0
Gibbs D (1975) Reversal of pupal diapause in Sarcophaga argyrostoma by temperature shifts after puparium formation. J Insect Physiol 21:1179–1186. doi:10.1016/0022-1910(75)90085-2
Goka K, Takafuji A (1990) Genetic studies on the diapause of the two-spotted spider mite, Tetranychus urticae Koch (1). Appl Entomol Zool 25:119–125. doi:10.1303/aez.25.119
Goka K, Takafuji A (1991) Genetic studies on the diapause of the two-spotted spider mite Tetranychus urticae Koch (2). Appl Entomol Zool 26:77–84. doi:10.1303/aez.26.77
Goto SG, Endo J (2015) Physiological and genetic mechanisms underpinning photoperiodism in the two-spotted spider mite. Comp Physiol Biochem 32:109–117 (in Japanese with English abstract)
Goto SG, Numata H (2014) Insect photoperiodism. In: Hoffmann KH (ed) Insect molecular biology and ecology. CRC Press, Boca Raton, pp 217–244
Goto SG, Shiga S, Numata H (2010) Photoperiodism in insects. Perception of light and the role of clock genes. In: Nelson RJ, Denlinger DL, Somers DE (eds) Photoperiodism: the biological calendar. Oxford University Press, Oxford, pp 258–286
Gotoh T, Shinkaji N (1981) Critical photoperiod and geographical variation of diapause induction in the two-spotted spider mite, Tetranychus urticae Koch (Acarina: Tetranychidae), in Japan. Jpn J Appl Entomol Zool 25:113–118. doi:10.1303/jjaez.25.113 (in Japanese with English abstract)
Grbić M et al (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479:487–492. doi:10.1038/nature10640
Gregg PC, Roberts B, Wentworth SL (1987) Levels of ecdysteroids in diapause and non-diapause eggs of the Australian plague locust, Chortoicetes terminifera (Walker). J Insect Physiol 33:237–242. doi:10.1016/0022-1910(87)90043-6
Henze MJ, Oakley TH (2015) The dynamic evolutionary history of pancrustacean eyes and opsins. Integr Comp Biol 55:830–842. doi:10.1093/icb/icv100
Hiragaki S, Suzuki T, Mohamed AAM, Takeda M (2015) Structures and functions of insect arylalkylamine N-acetyltransferase (iaaNAT); a key enzyme for physiological and behavioral switch in arthropods. Front Physiol 6:113. doi:10.3389/fphys.2015.00113
Hodek I (1996) Diapause development, diapause termination and the end of diapause. Eur J Entomol 93:475–487
Hori Y, Numata H, Shiga S, Goto SG (2014) Both the anterior and posterior eyes function as photoreceptors for photoperiodic termination of diapause in the two-spotted spider mite. J Comp Physiol A 200:161–167. doi:10.1007/s00359-013-0872-0
Huang X, Poelchau MF, Armbruster PA (2015) Global transcriptional dynamics of diapause induction in non-blood-fed and blood-fed Aedes albopictus. PLoS Negl Trop Dis 9:e0003724. doi:10.1371/journal.pntd.0003724
Ignatowicz S, Helle W (1986) Genetics of diapause suppression in the two-spotted spider mite, Tetranychus urticae Koch. Exp Appl Acarol 2:161–172. doi:10.1007/BF01213759
Ikeno T, Tanaka SI, Numata H, Goto SG (2010) Photoperiodic diapause under control of circadian clock genes in an insect. BMC Biol 8:116. doi:10.1186/1741-7007-8-116
Ikeno T, Numata H, Goto SG (2011a) Circadian clock genes period and cycle regulate photoperiodic diapause in the bean bug Riptortus pedestris males. J Insect Physiol 57:935–938. doi:10.1016/j.jinsphys.2011.04.006
Ikeno T, Numata H, Goto SG (2011b) Photoperiodic response requires mammalian-type cryptochrome in the bean bug Riptortus pedestris. Biochem Biophys Res Commun 410:394–397. doi:10.1016/j.bbrc.2011.05.142
Ikeno T, Ishikawa K, Numata H, Goto SG (2013) Circadian clock gene, Clock, is involved in the photoperiodic response of the bean bug Riptortus pedestris. Physiol Entomol 38:157–162. doi:10.1111/phen.12013
Kankare M, Salminen T, Laiho A, Vesala L, Hoikkala A (2010) Changes in gene expression linked with adult reproductive diapause in a northern malt fly species: a candidate gene microarray study. BMC Ecol 10:3. doi:10.1186/1472-6785-10-3
Kawaguchi S, Osakabe M (2014) Feeding is essential for body color change in diapausing females of the two-spotted spider mite Tetranychus urticae. Abstract book of the XIV International Congress of Acarology, p 113
Kawakami Y, Numata H (2013) Effects of a pyrethroid on ovarian development in diapause females of the two spotted spider mite. J Acarol Soc Jpn 22:45–47. doi:10.2300/acari.22.45
Kawakami Y, Goto SG, Ito K, Numata H (2009) Suppression of ovarian development and vitellogenin gene expression in the adult diapause of the two-spotted spider mite Tetranychus urticae. J Insect Physiol 55:70–77. doi:10.1016/j.jinsphys.2008.10.007
Kawakami Y, Ito K, Numata H, Goto SG (2010) Dominant and recessive inheritance patterns of diapause in the two-spotted spider mite, Tetranychus urticae. J Hered 101:20–25. doi:10.1093/jhered/esp085
Khila A, Grbić M (2007) Gene silencing in the spider mite Tetranychus urticae: dsRNA and siRNA parental silencing of the Distal-less gene. Dev Genes Evol 217:241–251. doi:10.1007/s00427-007-0132-9
Kobelková A, Bajgar A, Dolezel D (2010) Functional molecular analysis of a circadian clock gene timeless promoter from the drosophilid fly, Chymomyza costata. J Biol Rhythms 25:399–409. doi:10.1177/0748730410385283
Kohdayari S, Moharramipour S, Kamali K, Javaran MJ, Renault D (2012) Effects of acclimation and diapause on the thermal tolerance of the two-spotted spider mite Tetranychus urticae. J Therm Biol 37:41–423. doi:10.1016/j.jtherbio.2012.04.005
Kohdayari S, Moharramipour S, Larvor V, Hidalgo L, Renault D (2013) Deciphering the metabolic changes associated with diapause syndrome and cold acclimation in the two-spotted spider mite Tetranychus urticae. PLoS One 8:e54025. doi:10.1371/journal.pone.0054025
Kono Y, Ozeki N (1987) Induction of ovarian development by juvenile hormone and pyrethroids in Henosepilachna vigintioctopunctata (Coleoptera: Coccinellidae). Appl Entomol Zool 22:68–76. doi:10.1303/aez.22.68
Koštál V, Shimada K (2001) Malfunction of circadian clock in the non-photoperiodic diapause mutants of the drosophilid fly, Chymomyza costata. J Insect Physiol 11:1269–1274. doi:10.1016/S0022-1910(01)00113-5
Kotaki T, Shinada T, Kaihara K, Ohfune Y, Numata H (2011) Biological activities of juvenile hormone III skipped bisepoxide in last instar nymphs and adults of a stink bug, Plautia stali. J Insect Physiol 52:147–152. doi:10.1016/j.jinsphys.2010.10.003
Koveos DS, Veerman A (1994) Accumulation of photoperiodic information during diapause development in the spider mite Tetranychus urticae. J Insect Physiol 40:701–707. doi:10.1016/0022-1910(94)90097-3
Koveos DS, Veerman A (1996) Involvement of the circadian system in photoperiodic termination of diapause in the spider mite Tetranychus urticae. J Insect Physiol 42:681–691. doi:10.1016/0022-1910(96)00140-0
Koveos DS, Kroon A, Veerman A (1993) The same photoperiodic clock may control induction and maintenance of diapause in the spider mite Tetranychus urticae. J Biol Rhythms 8:265–282. doi:10.1177/074873049300800401
Kumar A, Congiu L, Lindström L, Piiroinen S, Vidotto M, Grapputo A (2014) Sequencing, de novo assembly and annotation of the Colorado potato beetle, Leptinotarsa decemlineata, transcriptome. PLoS One 9:e86012. doi:10.1371/journal.pone.0086012
Kwon DH, Park JH, Lee SH (2013) Screening of lethal genes for feeding RNAi by leaf disc-mediated systematic delivery of dsRNA in Tetranychus urticae. Pest Biochem Physiol 105:69–75. doi:10.1016/j.pestbp.2012.12.001
Lafont R, Dauphin-Villemant C, Warren JT, Rees H (2012) Ecdysteroid chemistry and biochemistry. In: Gilbert LI (ed) Insect endocrinology. Academic Press, London, pp 106–176
Lankinen P, Forsman P (2006) Independence of genetic geographical variation between photoperiodic diapause, circadian eclosion rhythm and Thr-Gly repeat region of the period gene in Drosophila littoralis. J Biol Rhythms 21:3–12. doi:10.1177/0748730405283418
Lankinen P, Riihimaa AJ (1992) Weak circadian eclosion rhythmicity in Chymomyza costata (Diptera: Drosophilidae), and its independence of diapause type. J Insect Physiol 38:803–811. doi:10.1016/0022-1910(92)90033-A
Laufer H, Biggers WJ, Ahl JSB (1998) Stimulation of ovarian maturation in the crayfish Procambarus clarkii by methyl farnesoate. Gen Comp Entodrinol 111:113–118. doi:10.1006/gcen.1998.7109
Le Trionnaire G et al (2009) Transcriptomic and proteomic analyses of seasonal photoperiodism in the pea aphid. BMC Genom 10:456. doi:10.1186/1471-2164-10-456
Lee K-Y, Denlinger DL (1997) A role for ecdysteroids in the induction and maintenance of the pharate first instar diapause of the gypsy moth, Lymantria dispar. J Insect Physiol 43:289–296. doi:10.1016/S0022-1910(96)00082-0
Lees AD (1953a) Environmental factors controlling the evocation and termination of diapause in the fruit tree red spider mite Metatetranychus ulmi Koch (Acarina: Tetranychidae). Ann Appl Biol 40:449–486
Lees AD (1953b) The significance of the light and dark phases in the photoperiodic control of diapause in Metatetranychus ulmi Koch. Ann Appl Biol 40:487–497. doi:10.1111/j.1744-7348.1953.tb02388.x
Lees AD (1973) Photoperiodic time measurement in the aphid Megoura viciae. J Insect Physiol 19:2279–2316
Lester PJ, Petry RJ (1995) Gamma irradiation for after harvest disinfestation of diapausing two spotted spider mite (Acari: Tetranychidae). J Econ Entomol 88:1361–1364. doi:10.1093/jee/88.5.1361
Lester PJ, Dentener PR, Bennett KV, Connolly PG (1997) Postharvest disinfestation of diapausing and non-diapausing twospotted spider mite (Tetranychus urticae) on persimmons: hot water immersion and coolstorage. Entomol Exp Appl 83:189–193. doi:10.1046/j.1570-7458.1997.00171.x
Marsden G, Hewitt D, Boglio E, Mather P, Richardson N (2008) Methyl farnesoate inhibition of late stage ovarian development and fecundity reduction in the black tiger prawn, Penaeus monodon. Aquaculture 280:242–246. doi:10.1016/j.aquaculture.2008.04.031
McEnroe WD (1969) Eyes of the female two-spotted spider mite, Tetranychus urticae I. Morphology. Ann Entomol Soc Am 62:461–466. doi:10.1093/aesa/62.3.461
McEnroe WD, Dronka K (1966) Color vision in the adult female two-spotted spider mite. Science 154:782–784. doi:10.1126/science.154.3750.782-a
McEnroe WD, Dronka K (1969) Eyes of the female two-spotted spider mite, Tetranychus urticae. II. Behavioral analysis of the photoreceptors. Ann Entomol Soc Am 62:466–469. doi:10.1093/aesa/62.3.466
McMurtry JA, Mahr DL, Johnson HG (1976) Geographic races in the predaceous mite, Amblyseius potentillae (Acari: Phytoseiidae). Int J Acalol 2:23–28. doi:10.1080/01647957608683752
Meuti ME, Stone M, Ikeno T, Denlinger DL (2015) Functional circadian clock genes are essential for the overwintering diapause of the Northern house mosquito, Culex pipiens. J Exp Biol 218:412–422. doi:10.1242/jeb.113233
Migeon A, Dorkeld F (2015) Spider mites web: a comprehensive database for the Tetranychidae. http://www.montpellier.inra.fr/CBGP/spmweb. Accessed 26 June 2016
Mills LR (1973) Structure of the visual system of the two-spotted spider-mite, Tetranychus urticae. J Insect Physiol 20:795–808. doi:10.1016/0022-1910(74)90171-1
Mohamed AAM, Wang Q, Bembenek J, Ichihara N, Hiragaki S, Suzuki T, Takeda M (2014) N-acetyltransferase (nat) is a critical conjunct of photoperiodism between the circadian system and endocrine axis in Antheraea pernyi. PLoS One 9:e92680. doi:10.1371/journal.pone.0092680
Morita A, Numata H (1999) Localization of the photoreceptor for photoperiodism in the stink bug, Plautia crossota stali. Physiol Entomol 24:189–195. doi:10.1046/j.1365-3032.1999.00130.x
Mukai A, Goto SG (2016) The clock gene period is essential for the photoperiodic response in the jewel wasp Nasonia vitripennis (Hymenoptera: Pteromalidae). Appl Entomol Zool 51:185–194. doi:10.1007/s13355-015-0384-1
Naegele JA, McEnroe WD, Soans AB (1966) Spectral sensitivity and orientation response of the two-spotted spider mite, Tetranychus urticae Koch, from 350 to 700 mμ. J Insect Physiol 12:1187–1195. doi:10.1016/0022-1910(66)90131-4
Nagata T, Koyanagi M, Tsukamoto H, Terakita A (2010) Identification and characterization of a protostome homologue of peropsin from a jumping spider. J Comp Physiol A 196:51–59. doi:10.1007/s00359-009-0493-9
Naguib YMA (2000) Antioxidant activities of astaxanthin and related carotenoids. J Agric Food Chem 48:1150–1154. doi:10.1021/jf991106k
Nanda KK, Hamner KC (1958) Studies on the nature of the endogenous rhythm affecting photoperiodic response of Biloxi soybean. Bot Gaz 120:14–25. doi:10.1086/335992
Nelson RJ, Denlinger DL, Somers DE (2010) Photoperiodism: the biological calendar. Oxford University Press, Oxford
Numata H, Miyazaki Y, Ikeno T (2015) Common features in diverse insect clocks. Zool Lett 1:10. doi:10.1186/s40851-014-0003-y
Overmeer WPJ, Nelis HJCF, De Leenheer AP, Calis JNM, Veerman A (1989) Effect of diet on the photoperiodic induction of diapause in three species of predatory mite, Amblyseius potentillae, A. cucumeris and Typhlodromus pyri. Exp Appl Acarol 7:281–287. doi:10.1007/BF01197922
Pavelka J, Shimada K, Koštál V (2003) TIMELESS: a link between fly’s circadian and photoperiodic clocks? Eur J Entomol 100:255–265. doi:10.14411/eje.2003.041
Pegoraro M, Gesto J, Kyriacou CP, Tauber E (2014) Role for circadian clock genes in seasonal timing: testing the Bünning hypothesis. PLoS Genet 10:e1004603. doi:10.1371/journal.pgen.1004603
Pittencrigh CS, Elliot J, Takamura T (1984) The circadian component in photoperiodic induction. In: Porter R, Collins GM (eds) Photoperiodic regulation of insect and molluscan hormones, Ciba Foundation Symposium No. 104. Pitman, London, pp 26–47
Poelchau MF, Reynolds JA, Elsik CG, Denlinger DL, Armbruster PA (2013) Deep sequencing reveals complex mechanisms of diapause preparation in the invasive mosquito, Aedes albopictus. Proc R Soc B 280:20130143. doi:10.1098/rspb.2013.0143
Poupardin R, Schöttner K, Korbelová J, Provazník J, Doležel D, Pavlinic D, Beneš V, Koštál V (2015) Early transcriptional events linked to induction of diapause revealed by RNAseq in larvae of drosophilid fly, Chymomyza costata. BMC Genom 16:720. doi:10.1186/s12864-015-1907-4
Qi X, Zhang L, Han Y, Ren X, Huang J, Chen H (2015) De novo transcriptome sequencing and analysis of Coccinella septempunctata L. in non-diapause, diapause and diapause-terminated states to identify diapause-associated genes. BMC Genom 16:1086. doi:10.1186/s12864-015-2309-3
Readio J, Chen M-H, Meola R (1999) Juvenile hormone biosynthesis in diapausing and nondiapausing Culex pipiens (Diptera: Culicidae). J Med Entomol 36:355–360
Regev S, Cone WW (1976) Evidence of gonadotropic effect of farnesol in the twospotted spider mite, Tetranychus urticae. Environ Entomol 5:517–519. doi:10.1093/ee/5.3.517
Richard DS, Watkins NL, Serafin RB, Gilbert LI (1998) Ecdysteroids regulate yolk protein uptake by Drosophila melanogaster oocytes. J Insect Physiol 44:637–644. doi:10.1016/S0022-1910(98)00020-1
Riihimaa AJ, Kimura MT (1988) A mutant strain of Chymomyza costata (Diptera: Drosophilidae) insensitive to diapause-inducing action of photoperiod. Physiol Entomol 13:441–445. doi:10.1111/j.1365-3032.1988.tb01128.x
Rosell M, Coons LB (1992) The role of the fat body, midgut and ovary in vitellogenin production and vitellogenesis in the female tick, Dermacentor variabilis. Int J Parasitol 22:341–349. doi:10.1016/S0020-7519(05)80012-8
Sakamoto T, Tomioka K (2007) Effects of unilateral compound-eye removal on the photoperiodic responses of nymphal development in the cricket Modicogryllus siamensis. Zool Sci 24:604–610. doi:10.2108/zsj.24.604
Sakamoto T, Uryu O, Tomioka K (2009) The clock gene period plays an essential role in photoperiodic control of nymphal development in the cricket Modicogryllus siamensis. J Biol Rhythm 24:379–390. doi:10.1177/0748730409341523
Saunders DS (2002) Insect clocks, 3rd edn. Elsevier, Amsterdam
Saunders DS (2010) Controversial aspects of photoperiodism in insects and mites. J Insect Physiol 56:1491–1502. doi:10.1016/j.jinsphys.2010.05.002
Saunders DS (2012) Insect photoperiodism: seeing the light. Physiol Entomol 37:207–218. doi:10.1111/j.1365-3032.2012.00837.x
Saunders DS (2013) Insect photoperiodism: measuring the night. J Insect Physiol 59:1–10. doi:10.1016/j.jinsphys.2012.11.003
Saunders DS, Bertossa RC (2011) Deciphering time measurement: the role of circadian ‘clock’ genes and formal experimentation in insect photoperiodism. J Insect Physiol 57:557–566. doi:10.1016/j.jinsphys.2011.01.013
Saunders DS, Cymborowski B (1996) Removal of optic lobes of adult blow flies Calliphora vicina leaves photoperiodic induction of larval diapause intact. J Insect Physiol 42:807–811. doi:10.1016/0022-1910(96)00007-8
Schmidt PS, Zhu C-T, Das J, Batavia M, Yang L, Eanes WF (2008) An amino acid polymorphism in the couch potato gene forms the basis for climatic adaptation in Drosophila melanogaster. Proc Natl Acad Sci USA 105:16207–16211. doi:10.1073/pnas.0805485105
Schneeberger K (2014) Using next-generation sequencing to isolate mutant genes from forward genetic screens. Nat Rev Genet 15:662–676. doi:10.1038/nrg3745
Shatrov AB (1997) Vitellogenesis and egg-shell formation in ovipositing females of the trombiculid mite Hirsutiella zachvatkini (Schluger) (Acariformes: Trombiculidae). Acarologia 38:141–151
Shatrov AB (2002) Oogenesis in ovipositing females of the microtrombidiid mite Platytrombidium fasciatum (C. L. Koch) (Acariformes: Microtrombidiidae). Invertebr Reprod Dev 42:1–15. doi:10.1080/07924259.2002.9652504
Shiga S, Numata H (1996) Effects of compound eye-removal on the photoperiodic response of the band-legged ground cricket, Pteronemobius nigrofasciatus. J Comp Physiol A 179:625–633. doi:10.1007/BF00216127
Shiga S, Numata H (1997) Induction of reproductive diapause via perception of photoperiod through the compound eyes in the adult blow fly, Protophormia terraenovae. J Comp Physiol A 181:35–40. doi:10.1007/s003590050090
Shiga S, Numata H (2009) Roles of PER immunoreactive neurons in circadian rhythms and photoperiodism in the blow fly, Protophormia terraenovae. J Exp Biol. doi:10.1242/jeb.027003
So PM, Takafuji A (1992) Local variation in diapause characteristics of Tetranychus urticae Koch (Acarina: Tetranychidae). Oecologia 90:270–275. doi:10.1007/BF00317185
Subramoniam T (2011) Mechanisms and control of vitellogenesis in crustaceans. Fish Sci 77:1–21. doi:10.1007/s12562-010-0301-z
Suzuki T, Takeda M (2009) Diapause-inducing signals prolong nymphal development in the two-spotted spider mite Tetranychus urticae. Physiol Entomol 34:278–283. doi:10.1111/j.1365-3032.2009.00688.x
Suzuki T, Fukunaga Y, Amano H, Takeda M, Goto E (2008a) Effects of light quality and intensity on diapause induction in the two-spotted spider mite, Tetranychus urticae. Appl Entomol Zool 43:213–218
Suzuki T, Takashima T, Izawa N, Watanabe M, Takeda M (2008b) UV radiation elevates arylalkylamine N-acetyltransferase activity and melatonin content in the two-spotted spider mite, Tetranychus urticae. J Insect Physiol 54:1168–1174. doi:10.1016/j.jinsphys.2008.06.005
Suzuki T, Watanabe M, Takeda M (2009) UV tolerance in the two-spotted spider mite, Tetranychus urticae. J Insect Physiol 55:649–654. doi:10.1016/j.jinsphys.2009.04.005
Suzuki T, Kojima T, Takeda M, Sakuma M (2013) Photo-orientation regulates seasonal habitat selection in the two-spotted spider mite, Tetranychus urticae. J Exp Biol 15:977–983. doi:10.1242/jeb.079582
Suzuki T, Wang C-H, Gotoh T, Amano H, Ohyama K (2015) Deoxydant-induced anoxia as a physical measure for controlling spider mites (Acari: Tetranychidae). Exp Appl Acarol 65:293–305. doi:10.1007/s10493-015-9881-8
Tagaya J, Numata H, Goto SG (2010) Sexual difference in the photoperiodic induction of pupal diapause in the flesh fly Sarcophaga similis. Entomol Sci 13:311–319. doi:10.1111/j.1479-8298.2010.00394.x
Takafuji A, So PM, Tsuno N (1991) Inter-population and intra-population variations in diapause attribute of the two-spotted spider mite, Tetranychus urticae Koch, in Japan. Res Popul Ecol 33:331–344. doi:10.1007/BF02513558
Tamaki S, Takemoto S, Uryu O, Kamae Y, Tomioka K (2013) Opsins are involved in nymphal photoperiodic responses in the cricket Modicogryllus siamensis. Physiol Entomol 38:163–172. doi:10.1111/phen.12015
Tauber MJ, Tauber CA, Masaki S (1986) Seasonal adaptations of insects. Oxford University Press, Oxford
Tauber et al (2007) Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316:1895–1898. doi:10.1126/science.1138412
Tawfik AI, Tanaka Y, Tanaka S (2002a) Possible involvement of ecdysteroids in embryonic diapause of Locusta migratoria. J Insect Physiol 48:743–749. doi:10.1016/S0022-1910(02)00099-9
Tawfik AI, Tanaka Y, Tanaka S (2002b) Possible involvement of ecdysteroids in photoperiodically induced suppresion of ovarian development in a Japanese strain of the migratory locust, Locusta migratoria. J Insect Physiol 48:411–418. doi:10.1016/S0022-1910(02)00058-6
Taylor D, Chinzei Y, Ito K, Higuchi N, Ando K (1991) Stimulation of vitellogenesis by pyrethroids in mated and virgin female adults, male adults, and fourth instar females of Ornithodoros moubata (Acari: Argasidae). J Med Entomol 28:322–329. doi:10.1093/jmedent/28.3.322
Tehri K (2014) A review on reproductive strategies in two spotted spider mite, Tetranychus Urticae Koch 1836 (Acari: Tetranychidae). J Entomol Zool Stud 2:35–39
Thompson DM, Khalil SMS, Jeffers LA, Sonenshine DE, Mitchell RD, Osgood CJ, Roe RM (2007) Sequence and the developmental and tissue-specific regulation of the first complete vitellogenin messenger RNA from ticks responsible for heme sequestration. Insect Biochem Mol Biol 37:363–374. doi:10.1016/j.ibmb.2007.01.004
Tomioka K, Matsumoto A (2015) Circadian molecular clockworks in non-model insects. Curr Opin Insect Sci 7:58–64. doi:10.1016/j.cois.2014.12.006
van Houten YM, Veenendaal RL (1990) Effect of photoperiod, temperature, food and relative humidity on the induction of diapause in the predatory mite Amblyseius potentillae. Exp Appl Acarol 10:111–128. doi:10.1007/BF01194087
Van Leeuwen T, Dermauw W (2016) The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu Rev Etnomol 61:475–498. doi:10.1146/annurev-ento-010715-023907
Van Leeuwen T, Demaeght P, Osborne EJ, Dermauw W, Gohlke S, Nauen R, Grbić M, Tirry L, Merzendorfer H, Clark RM (2012) Population bulk segregant mapping uncovers resistance mutations and the mode of action of a chitin synthesis inhibitor in arthropods. Proc Natl Acad Sci USA 190:4407–4412. doi:10.1073/pnas.1200068109
Van Leeuwen T, Tirry L, Yamamoto A, Nauen R, Dermauw W (2015) The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pest Biochem Physiol 121:12–21. doi:10.1016/j.pestbp.2014.12.009
Van Zon AQ, Overmeer WPJ, Veerman A (1981) Carotenoids function in photoperiodic induction of diapause in a predacious mite. Science 213:1131–1133. doi:10.1126/science.213.4512.1131
Vaz Nunes M (1998) A double circadian oscillator model for quantitative photoperiodic time measurement in insects and mites. J Theor Biol 194:299–311. doi:10.1006/jtbi.1998.0767
Vaz Nunes M, Hardie J (1993) Circadian rhythmicity is involved in photoperiodic time measurement in the aphid Megoura viciae. Experientia 49:711–713. doi:10.1007/BF01923957
Vaz Nunes M, Koveos DS, Veerman A (1990) Geographical variation in photoperiodic induction of diapause in the spider mite (Tetranychus urticae): a causal relation between critical nightlength and circadian period? J Biol Rhythms 5:47–57. doi:10.1177/074873049000500105
Veenstra JA, Rombauts S, Grbić M (2012) In silico cloning of genes encoding neuropeptides, neurohormones and their putative G-protein coupled receptors in a spider mite. Insect Biochem Mol Biol 42:277–295. doi:10.1016/j.ibmb.2011.12.009
Veerman A (1974) Carotenoid metabolism in Tetranychus urticae Koch (Acari: Tetranychidae). Comp Biochem Physiol B 47:101–116. doi:10.1016/0305-0491(74)90095-9
Veerman A (1977) Aspects of the induction of diapause in a laboratory strain of the mite Tetranychus urticae. J Insect Physiol 23:703-711. doi:10.1016/0022-1910(77)90087-7
Veerman A (1980) Functional involvement of carotenoids in photoperiodic induction of diapause in the spider mite, Tetranychus urticae. Physiol Entomol 5:291–300. doi:10.1111/j.1365-3032.1980.tb00237.x
Veerman A (1985) Diapause. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies and control, vol 1A. Elsevier, Amsterdam, pp 279–316
Veerman A (2001) Photoperiodic time measurement in insects and mites: a critical evaluation of the oscillator–clock hypothesis. J Insect Physiol 47:1097–1109. doi:10.1016/S0022-1910(01)00106-8
Veerman A, Vaz Nunes M (1980) Circadian rhythmicity participates in the photoperiodic determination of diapause in spider mites. Nature 287:140–141. doi:10.1038/287140a0
Veerman A, Vaz Nunes M (1987) Analysis of the operation of the photoperiodic counter provides evidence for hourglass time measurement in the spider mite Tetranychus urticae. J Comp Physiol A 160:421–430. doi:10.1007/BF00615076
Veerman A, Veenendaal RL (2003) Experimental evidence for a non-clock role of the circadian system in spider mite photoperiodism. J Insect Physiol 49:727–732. doi:10.1016/S0022-1910(03)00097-0
Veerman A, Overmeer WPJ, Van Zon AQ, de Boer JM, de Waard ER, Huisman HO (1983) Vitamin A is essential for photoperiodic induction of diapause in an eyeless mite. Nature 302:248–249. doi:10.1038/302248a0
Wadsworth CB, Dopman EB (2015) Transcriptome profiling reveals mechanisms for the evolution of insect seasonality. J Exp Biol 218:3611–3622. doi:10.1242/jeb.126136
Wang Q, Mohamed AAM, Takeda M (2013) Serotonin receptor B may lock the gate of PTTH release/synthesis in the chinese silk moth, Antheraea pernyi; a diapause initiation/maintenance mechanism? PLoS One 8:e79381. doi:10.1371/journal.pone.0079381
Wang Q, Egi Y, Takeda M, Oishi K, Sakamoto K (2015) Melatonin pathway transmits information to terminate pupal diapause in the Chinese oak silkmoth, Antheraea pernyi, and through reciprocated inhibition of dopamine pathway functions as a photoperiodic counter. Entomol Sci 18:193–198. doi:10.1111/ens.12083
Williams KD, Busto M, Suster ML, So AK-C, Ben-Shahar Y, Leevers SJ, Sokolowski MB (2006) Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc Natl Acad Sci USA 103:15911–15915. doi:10.1073/pnas.0604592103
Yamashita O (1996) Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J Insect Physiol 42:669–679. doi:10.1016/0022-1910(96)00003-0
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, Upper Saddle River
Zhang Q, Lu Y-X, Xu W-H (2011) Integrated proteomic and metabolomic analysis of larval brain associated with diapause induction and preparation in the cotton bollworm, Helicoverpa armigera. J Proteome Res 11:1042–1053. doi:10.1021/pr200796a
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Goto, S.G. Physiological and molecular mechanisms underlying photoperiodism in the spider mite: comparisons with insects. J Comp Physiol B 186, 969–984 (2016). https://doi.org/10.1007/s00360-016-1018-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-016-1018-9