Abstract
A chorioallantoic membrane artery in embryos of the red-footed tortoise, Chelonoidis carbonaria was occlusively cannulated for measurement of blood pressure and injection of drugs. Two age groups of embryos in the final 10 % of incubation were categorized by the ratio of embryonic body to yolk mass. All embryos first received cholinergic and β-adrenergic blockade. This revealed that β-adrenergic control was established in both groups whereas cholinergic control was only established in the older group immediately prior to hatching. The study then progressed as two series. Series one was conducted in a subset of embryos treated with histamine before or after injection of ranitidine, the antagonist of H2 receptors. Injection of histamine caused an initial phasic hypertension which recovered, followed by a longer lasting hypertensive response accompanied by a tachycardia. Injection of the H2 receptor antagonist ranitidine itself caused a hypotensive tachycardia with subsequent recovery of heart rate. Ranitidine also abolished the cardiac effects of histamine injection while leaving the initial hypertensive response intact. In series, two embryos were injected with histamine after injection of diphenhydramine, the antagonist to H1 receptors. This abolished the whole of the pressor response to histamine injection but left the tachycardic response intact. These data indicate that histamine acts as a non-adrenergic, non-cholinergic factor, regulating the cardiovascular system of developing reptilian embryos and that its overall effects are mediated via both H1 and H2 receptor types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies on control of the cardiovascular system of reptiles have explored changes in heart rate and blood flow, including the role of cardio-respiratory interactions associated with temperature change, activity levels, and feeding (Clark et al. 2005; Galli et al. 2004; Hicks et al. 2000; Wang and Hicks 1996a, b; Wang et al. 1997, 2001a, b). The role of the autonomic nervous system in instigating these changes has been investigated using pharmacological techniques. In the rattlesnake, Crotalus durrisus, vagal cholinergic tone on the heart predominates over adrenergic tone in inactive and active animals. Changes in heart rate in this species are largely due to withdrawal of a vagal tonus and sympathetic regulation of the systemic circulation (Wang et al. 2001b). During digestion, snakes such as pythons and boa constrictors can more than double their heart rate after ingestion (Secor and Diamond 1998; Secor et al. 2000). However, in the Boa constrictor, while the increase in heart rate during activity is predominantly due to withdrawal of vagal tone, this mechanism does not account for all of the increase during digestion, implying involvement of non-adrenergic, non-cholinergic (NANC) factors in controlling the response (Wang et al. 2001a).
While neural control of the cardiovascular system has traditionally been assigned to the two arms of the autonomic nervous system (Taylor et al. 1999), there are a number of other neural, endocrine, and autocrine factors now known to play a role in the integration of cardiovascular responses. The role of these NANC factors in the maintenance of cardiovascular homeostasis in adult vertebrates has been recognized for some time (Bult et al. 1990; Morris and Nilssen 1994). More recently, the cardiovascular regulatory capacity for factors such as neurogenic peptides and nitric oxide has been investigated in reptiles (Galli et al. 2005a, b; Skovgaard et al. 2005a, b). The NANC factor histamine is known to be produced by enterochromaffin-like cells in the stomach and released by the gut following a meal (Hakanson et al. 1986). It recently has been suggested to play an important part in the cardiovascular responses associated with specific dynamic action in digesting reptiles (Reite 1970; Skovgaard et al. 2009; Wang et al. 2001a). Histamine acts as a NANC factor exerting a positive chronotropic effect on the heart of the python during the first 24 h after ingestion of a meal (Skovgaard et al. 2009). Cross perfusion of blood plasma taken from recently fed snakes into unfed snakes demonstrated that a factor initiating this response was present in the blood following a meal (Enok et al. 2012). These studies illustrate that histamine is an important regulator of cardiovascular function in adult reptiles but its functional significance during embryonic development of reptiles remains unknown.
During development in some reptilian species, autonomic nervous system control of cardiovascular function is absent until shortly before hatching. It has previously been suggested that developing birds and reptiles have increased dependence on humoral regulators such as angiotensin II, catecholamines, and nitric oxide to alter cardiovascular function during early development (Crossley and Altimiras 2000; Crossley et al. 2003a, b). For example, in embryos of the American alligator, Alligator mississippiensis, cholinergic tone on heart rate is absent throughout the majority of development while β-adrenergic control is due to circulating catecholamines (Crossley and Altimiras 2005; Eme et al. 2011a, b). The relative absence of these classic autonomic regulatory receptor tones on cardiovascular function in embryos could increase the relative contribution of regulatory factors such as NANC to both maintain basal function and mediate change to meet the convective transport demands placed on the system.
The present study explored the cardiovascular responses of an embryonic reptile, the red-footed tortoise, to injections of histamine plus H1 and H2 receptor antagonists following muscarinic cholinergic and β-adrenergic blockade. There are four distinct subtypes of histamine receptors characterized in mammals and designated H1–H4 (Jutel et al. 2009) and similar subtypes may be present in reptiles. Our focus was on late embryonic development in order to investigate the differences in cardiovascular regulatory repertoire between the embryonic and the adult phenotype. Given the identified function of histamine as a regulator of the mammalian and adult reptilian cardiovascular systems, we hypothesized that it could be a NANC factor in the developing embryo, given the relative immaturity of CNS-regulatory mechanisms during embryonic development of some reptiles (Crossley and Altimiras 2005; Crossley et al. 2003c).
Materials and methods
Embryo acquisition and incubation
Eggs of the red-footed tortoise (Chelonoidis carbonaria, Spix, 1824) were collected from a captive bred population held at the Jacarezário, an animal holding and rearing facility in the Department of Zoology at the São Paulo State University (UNESP), located in Rio Claro, SP, Brazil. Eggs from seven clutches were labeled with the laid date as well as clutch number and then placed on a layer of vermiculite in plastic boxes (38 cm long, 28.5 cm wide, and 6.5 cm deep) held in an incubator (101M, Eletrolab) at 32 °C. The water content of the vermiculite was maintained close to saturation by spraying it with dechlorinated water twice weekly via a hand-operated misting device. All embryos used in this study were assumed to be in the final 10 % of their period of incubation based on predicted incubation dates and the presence of hatched siblings.
Instrumentation and data acquisition
Immediately prior to an experiment each egg was removed from the incubator, weighed, and candled to determine orientation of the embryo and location of an accessible tertiary chorioallantoic membrane (CAM) artery. A portion of the eggshell was removed at the site chosen for surgery and the egg was viewed under a dissection microscope (Wild, Heerbrugg M5-48225, Switzerland) held at room temperature (~27 °C) while an occlusive catheter was inserted into the artery. The catheter consisted of heat-pulled polyethylene 50 tubing, and was filled with heparinized saline. At completion of the procedure, the catheter was used for measurement of arterial pressure and for injection of drugs, as previously described (Crossley and Altimiras 2005). These surgical procedures were minimally invasive and durable, with preparations lasting for the duration of the study of up to 6 h. Two preparations from the less mature group of embryos that did not stabilize post-surgery were not included in the analyses.
Following catheterization, all embryos were transferred to a water-jacketed, multi-chamber experimental apparatus (~700 cm3 per chamber, two embryos per chamber placed on cotton wool) and allowed to recover until cardiovascular parameters stabilized (∼1 h). Temperature in the chambers was maintained at 30 °C by recirculating water from a constant temperature water bath (TE-2005, Tecnal, Brazil). This temperature was selected to enable comparison with previous studies on embryonic reptilian cardiovascular function. Each chamber was fitted with a lid with three ports that allowed the catheter and airlines to enter the chamber and was flushed continuously with room air supplied by a gas-line at a flow rate of ∼100 ml min−1. To prevent changes in chamber temperature due to air inflow, all incoming air traversed a section of polyethylene (PE) 50 tube coiled inside the individual chamber. Each arterial catheter was attached via saline-filled PE 50 tubing to a disposable blood pressure transducer (ADInstruments model MLT0699) held 1–3 cm above the egg. This was connected to an amplifier (Quad Bridge Amp ADinstruments CO, USA), and the pressure signal acquired at 40 Hz using a PowerLab 16/35® data recording system (ADInstruments) connected to a computer running LabChart Pro® software (v 7.2.5 ADInstruments). Pressure transducers were calibrated prior to each measurement period with a vertical column of saline, and heart rate was determined from the arterial pressure trace using a software tachograph. Egg position relative to the zero line set at the level of the transducer was measured at the completion of each study and added to the pressure values during analysis to account for the position below zero.
Protocol for drug injections
Drugs were injected via a T connector in the arterial catheter line as previously described (Crossley and Altimiras 2005). Total injection volumes were normalized to 5 % of an estimated total blood volume based on an embryonic yolk free mass of 30 g. This mass was based on preliminary sampling of embryos and 1-day-old hatchlings. In all cases, one-third of the injected volume consisted of the administered drug and two-third of the volume was a saline flush to ensure the drug had entered the CAM artery. To account for potential effects of the injected volume, all embryos received a control injection of heparinized saline with the volume identical to each injection volume.
In this study, subsequent to a control saline injection, all embryos were injected with cholinergic and β-adrenergic receptor antagonists in the following sequence: atropine (1 mg kg−1, SIGMA) followed by propranolol (2 mg kg−1, SIGMA). Following each injection, the animals were allowed to reach stable values for both arterial pressure (P m) and heart rate (f H) and maintained that level for 20 min before the next injection was given. Complete blockade was verified in two embryos, from the younger age group, using acetylcholine (10−5 M). β-adrenergic receptor blockade was verified in an embryo from the younger age group using epinephrine (10−3 M) after propranolol injection. A total of 15 embryonic tortoises received this blockade regime. Cholinergic and adrenergic tonus on the heart was calculated from cardiac interval(s) measured from the peak arterial pressures, which are analogous to the R–R interval (Altimiras et al. 1997).
In the first series of the study, a subset of the embryonic tortoises (n = 4) that had responded to both atropine and propranolol injections received an injection of histamine (300 nM kg−1). A second group (n = 5) received the H2 receptor blocking compound ranitidine (∼40 mg kg−1) followed by an injection of histamine (300 nM kg−1). All animals were allowed a stabilization period of approximately 1 h prior to the next injection.
In the second series of the study, the H1 receptor antagonist diphenhydramine (40 mg kg−1) was injected into a subset of embryos (n = 2) following cholinergic and adrenergic receptor blockade. Following stabilization each embryo received an injection of histamine (300 nM kg−1). This part of the protocol was limited by the death of two additional embryos following injection of the H1 antagonist. Cardio-toxic effects resulting from H1 receptor antagonists have been reported previously (Llenas et al. 1999; Skovgaard et al. 2009).
Mass determination
At the completion of the study embryos were killed with an injection of Propofol (0.2 ml, 10 mg/ml, B.Braun Mesulgen, Denmark) into the arterial catheter. After 30 min, f H and P m had declined to zero, the yolk-free embryonic mass, residual yolk mass, and heart mass were determined.
Age determination
Based on hatching clutch-mates, all embryonic tortoises were estimated to be in the final 10 % of incubation. During this period of reptilian development, mass has previously been suggested to be the most accurate index on embryonic age (Crastz 1982; Ferguson 1985; Tokita and Kuratani 2001). However, given the differing egg masses between the embryos used in this study and a prior study (Yntema 1968), the ratio between embryonic and yolk masses was chosen as a more useful index of developmental stage. The relative mass of yolk has previously been used as a characteristic of morphological development in chelonian reptiles (Crastz 1982; Tokita and Kuratani 2001). For this reason, all embryos were indexed for age based on the ratio of embryonic mass to residual yolk mass.
Statistical analysis
Differences in egg mass were analyzed using a one-way ANOVA with age index as the independent factor. Differences in embryonic mass and residual yolk mass were analyzed with an ANCOVA with age index as the independent factor and egg mass as the covariate. Differences in heart mass were analyzed using an ANCOVA with age index as the independent factor and embryonic mass as the covariate.
Differences in baseline f H and P m between age groups were analyzed using a one-way ANOVA with age index as the independent factor. Responses of f H and P m to saline, atropine, and propranolol injections were analyzed using a paired t test compared to the pre-injection values for each drug. Due to the multifaceted response of f H and P m to ranitidine injections in series one all the effects of each drug injection group were analyzed using a one-way repeated measures ANOVA. A two-way repeated measures ANOVA was used to analyze the effect of histamine on f H and P m in a group subjected to cholinergic and adrenergic blockade alone in comparison to a group that was treated with cholinergic, β-adrenergic, and H2 receptor blockade. In series two, the response to histamine post H1 blockade was not statistically analyzed due to limited sample size. In all cases, data are presented as mean ± SE, with significant differences between groups or injections identified when the P < 0.05.
Results
Mass, baseline P m, and f H
Two groups of embryos were discriminated on the basis of their different ratios of embryonic to yolk mass of 3.3 ± 0.8 and 8.8 ± 0.6, taken as indicators of their respective incubational ages. These wet mass ratios for embryonic and yolk tissue are similar to that previously reported for embryonic American alligators (Alligator mississipiensis) (Crossley and Altimiras 2005) as well as embryonic snapping turtles (Chelydra serpentina) at 90 % of incubation (Crossley unpublished). Embryonic wet mass significantly increased by 15 % between the two groups while residual yolk mass significantly decreased by 50 % (Table 1).
Cholinergic and β-adrenergic blockade
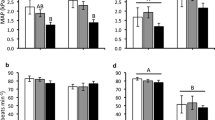
Saline injection with the volume equivalent to each drug injection volume had no affect on P m and f H in the 3.3 and the 8.8 age-indexed tortoise embryos. An embryonic cholinergic tone on f H (+16 beats min−1) was evident in tortoises that had reached the 8.8 index of development (Fig. 1b). This increase yielded a calculated cholinergic tonus of 28 % on f H (Table 2). Notably, cholinergic blockade had no impact on either f H or P m in the younger group of embryonic tortoises studied (Fig. 1a, b). In preliminary studies, the concentration of atropine (1 mg kg−1) used in this study, resulting in the tachycardia demonstrated in the older embryos eliminated the bradycardic response to acetylcholine in younger embryos. This verifies that the while cholinergic receptors are present in younger embryos; cholinergic tone on the heart is absent.
Control (white column), atropine (1 mg/kg) response (black column) and propranolol (2 mg/kg) response (gray column) of (a) mean arterial pressure (P m) and (b) heart rate (f H) in embryonic red-footed tortoises at two indexed ages of development. Sample size age index 3.3 (n = 5) and age index 8.8 (n = 10). Data are presented as mean ± SEM. An asterisk indicates significant (P < 0.05) differences from control values with the control for the injection of propranolol being the atropine responses
At both indices of development studied β-adrenergic blockade with propranolol significantly decreased f H (−13 and −17 min−1, respectively) without altering P m (Fig. 1a, b). The proportional reduction in f H was similar at both indices (−25 % at 3.3 and −27 % at 8.8) (Fig. 1b). Again in preliminary studies, the concentration of propranolol (2 mg kg−1) used in this study eliminated the tachycardic response to epinephrine in a young age embryo.
Histamine response with or without H2 receptor blockade
In embryonic tortoises that had been cholinergically and β-adrenergically blocked, histamine injections caused a complex cardiovascular reaction. As illustrated in the representative trace (Fig. 2a, b), upon initial injection of histamine P a increased (∼1.4 kPa) rapidly to 50 % above the pre-injection control value, with a peak response at 37 ± 16 s (indicated by Hist 1). Meanwhile f H gradually increased (∼13 beats min−1), peaking at a rate approximately 30 % higher than control levels at ∼2.0 min post injection (Hist 2) while P m returned transiently to control values (Fig. 3a, b). After 5.0 ± 1.5 min (Hist 3) P m secondarily increased significantly above pre-injection (∼0.7 kPa) while f H remained elevated (Fig. 3a, b). All embryos returned to pre-histamine injection values at approximately 35 min (33.5 ± 6.0 min) after the initial injection.
A representative trace of the a arterial pressure (P a) and b heart rate (f H) response to an injection of histamine (300 nM kg−1) in an embryonic red-footed tortoise at the age index of 8.8, following cholinergic and β-adrenergic blockade. The bracket is the point of drug injection. The three identified phases of the response described in the text are labeled as Hist 1, Hist 2, and Hist 3
The control and three part (Hist 1, Hist 2, Hist 3) mean injection responses to histamine alone (white column) or histamine injection after treatment with ranitidine (black column) of (a) mean arterial pressure (P m) and (b) heart rate (f H) in embryonic red-footed tortoises at the age index of 8.8, following cholinergic and β-adrenergic blockade. Data are presented as mean ± SE. An asterisk indicates significant (P < 0.05) response to histamine injection with or without pretreatment with ranitidine. The labels on the horizontal axis refer to the time course of the histamine response as described in the text and labeled on Fig. 2
Pretreatment with the H2 receptor antagonist ranitidine (∼40 mg kg−1) resulted in the cardiovascular response previously described (Fig. 3a, b). Following H2 receptor blockade, subsequent injections of histamine had no significant impact on f H but P m was significantly elevated, again by 50 % of control (∼0.7 kPa) during the Hist 1 response period (Fig. 3a).
Histamine H2 receptor tone
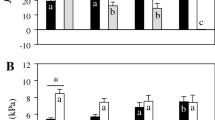
Injections of the H2 receptor antagonist ranitidine (40 mg kg−1) in embryonic animals cholinergically and β-adrenergically blocked resulted in a bimodal response that consisted of an initial significant hypotensive tachycardia recorded as a 41 % decrease in P m (−0.9 kPa) and 36 % increase in f H (+14 beats min−1), respectively (denoted as H2P1), after which f H returned to its pre-injection value while P m remained significantly reduced (denoted as H2P2, see Fig. 4a, b).
The P m (a) and f H (b) values following cholinergic and β-adrenergic receptor blockade either before (white column) or after (black column) injection of saline or the H2 receptor antagonist ranitidine (40 mg kg−1) in embryonic red-footed tortoises at the age index of 8.8, n = 5. Data are presented as mean ± SE. An asterisk indicates significant (P < 0.05) differences from control values of the H2 Pt. 1 (black column) and H2 Pt. 2 (gray column) of the blockade response. Control (pre-injection) values are presented for both saline injection (Sal) and pre-H2 blockade (H2 con). See text for explanation
Histamine post H1 receptor blockade
In two embryonic tortoises that had been subjected to cholinergic and β-adrenergic blockade, pretreatment with the H1 receptor antagonist diphenhydramine (40 mg kg−1) altered the general cardiovascular response to histamine injection (Fig. 5c, d). In both embryonic animals, diphenhydramine eliminated both the rapid and the delayed hypertensive (Fig. 5a) responses to histamine characterized as Hist 1 and Hist 3 in Fig. 2a while the progressive tachycardia, although much reduced, persisted (Fig. 6b) and is indicated at Hist 2 on Fig. 2a, though the apparent increase in f H cannot be tested for significance as the data are from two animals.
Representative traces of the: arterial pressure (P a) and heart rate (f H) responses to an injection of histamine (300 nM kg−1) following injection of the H2 antagonist ranitidine (a, b) or after pretreatment with the H1 antagonist diphenhydramine (40 mg kg−1) (c, d) in an embryonic red-footed tortoise at the age index of 8.8, following cholinergic and β-adrenergic blockade. The arrow represents the time of injection. The recorded changes should be compared with those presented in Fig. 2
Control (white column) and injection response (black column) to histamine after treatment with diphenhydramine (40 mg kg−1) of (a) mean arterial pressure (P m) and (b) heart rate (f H) in embryonic red-footed tortoises at the age index of 8.8 (n = 2), following cholinergic and β-adrenergic blockade. Data are presented as mean ± SE
Discussion
In egg-laying amniotes studied to date, embryonic cardiovascular regulatory capacity is limited relative to the adult phase of life, with an increased dependence on local and humoral regulatory mechanisms (Altimiras and Crossley 2000; Crossley and Altimiras 2000, 2005; Crossley et al. 2003a, b, c, 2010; Crossley and Burggren 2009; Eme et al. 2011a). Specifically many species either lack or have limited central nervous system regulatory capacity even late in the embryonic phase of life (Altimiras et al. 2009; Crossley and Altimiras 2005; Crossley and Burggren 2009; Crossley et al. 2003b, c, 2010; Eme et al. 2011c). This feature by default places increased dependence on humoral mechanisms. Recent investigations have delved into the possible non-CNS-mediated regulatory components during embryonic development, and have identified an increased dependence on systemic regulatory factors such as catecholamines and angiotensin II (Crossley et al. 2010; Eme et al. 2011a; Tate et al. 2012). Our investigation indicates that like other non-mammalian vertebrates, the embryonic red-footed tortoise lacks cholinergic tone on the heart until just prior to hatching (Fig. 1a, b). This appears to amplify the relative contribution of β-adrenergic tone (Fig. 1a, b) as well as the NANC factor histamine as tonic regulators of cardiovascular function.
Studies of embryonic birds, represented by the chicken, Gallus domesticus (Crossley and Altimiras 2000, 2012; Crossley et al. 2003a, b), the emu Dromaius novaehollandiae (Crossley et al. 2003a, b) and migratory waterfowl (Crossley unpublished data) revealed that circulating catecholamines mediate an excitatory adrenergic tone on cardiovascular function at 50 % of development. In contrast, inhibitory vagal control of heart rate is present only at the later stages of development in emu and after hatching in the some chicken breeds (Crossley and Altimiras 2012; Crossley et al. 2003a, b). In the green iguana (Iguana iguana) embryo, adrenergic tone is present throughout development but cholinergic tone appears immediately prior to hatching (Taylor, Santori, Leite, Crossley and Abe, in preparation). Our findings in the red-footed tortoise confirm this relationship as there was a clear β-adrenergic tone on f H (Fig. 1b) at both points of development studied while cholinergic tone on f H was restricted to the group studied immediately before hatching (embryonic mass/yolk mass index = 8.8).
The present investigations were extended to include the role of the nonadrenergic noncholinergic (NANC) factor histamine as a component of cardiovascular regulation in embryonic tortoises during the final 10 % of incubation time (Fig. 3a, b). Injection of the H2 receptor antagonist ranitidine into embryonic animals in the 8.8 age-indexed age following autonomic blockade resulted in an initial hypotensive tachycardia after which f H returned to its pre-injection value while P m remained significantly reduced (Fig. 4a, b). While the mechanism for the transient tachycardia was not investigated the data imply that red-footed tortoise embryos have a histaminergic tonus on the peripheral circulation. Acute histamine injection after autonomic blockade resulted in a general hypertensive tachycardic response, which was separable into two phases, an initial immediate increase in pressure that recovered back toward the pre-injection level, followed by a secondary increase, accompanied by a developing tachycardia that recovered over a prolonged period (Fig. 3a, b). Injection of the H2 receptor antagonist ranitidine, with subsequent injection of histamine caused an initial hypertensive response but no change in heart rate, demonstrating that the maintained pressor response and associated tachycardia on injection of histamine was mediated by H2 receptors (Fig. 3a, b). This response may be attributed to direct action on the heart as prior studies of isolated atria and ventricles from embryonic chicken hearts reported a positive inotropic and chronotropic response to histamine treatment that was antagonized by H2 blockade (Tanaka et al. 1995). Histamine has also been shown to stimulate a postprandial tachycardia by a direct effect on cardiac H2 receptors in unfed pythons, Python regius (Skovgaard et al. 2009). Thus, the current finding of an in vivo H2 histaminergic tonus on the cardiovascular system may represent a common feature across amniotic egg-laying vertebrates and the capacity to react is retained into adulthood in reptiles. The persistence of the hypertensive response to histamine following the combination of cholinergic, adrenergic, and H2 receptor blockade can be attributed to the possible involvement of other histamine receptors acting on both cardiac tissue as well as the peripheral vasculature (Fig. 3a, b). Following injection of the H1 receptor antagonist diphenhydramine into red-footed tortoise embryos, subsequent injection of histamine no longer induced an initial hypertensive response but a developing tachycardia was retained (Fig. 6a, b). Placental vessels have been previously reported to exhibit an H1 receptor dependent contraction (Mills et al. 2007) so injection of the antagonist may have blocked this mechanism in the embryonic red-footed tortoise. These data imply that the H1 and H2 receptors mediate separate components of the response to histamine, with the initial phasic hypertensive response mediated by H1 receptor stimulation while the developing tachycardia and associated hypertension was mediated by H2 receptor stimulation.
Having identified histamine as a factor in the control of cardiovascular function during late development of a reptilian embryo it is of interest to speculate on the nature of its effects. Histamine released from cardiac mast cells induces an increase in heart rate in mammals (Genovese and Spadaro 1997). Consequently, it seems reasonable to attribute the role of histamine to peripheral effects on individual tissues. Histamine is co-released with norepinephrine by nerve endings in the cardiac ganglion of the guinea pig (Li et al. 2006) and histamine receptors have been detected on target organs such as the heart. Studies on guinea pig hearts indicated that H1 receptors are present at the atrioventricular node and coronary vessels, and stimulation may cause vasodilatation while the H2 receptor is present in the sinoatrial node and ventricular fibers, with histamine promoting atrial contraction (Levi and Kuye 1974). However, histamine has also been detected in neurons and nerve fibers in the central nervous system of mammals, where it possesses neurotransmitter properties (Schwartz et al. 1991). All four types of histamine receptor are known to be expressed in the central nervous system (Brown et al. 2001). Central injection of histamine into the forebrain of mammals causes changes in both blood pressure and heart rate. These responses appear to be mediated by stimulation of H1 receptors. Histamine containing neurons project from the hypothalamus to the nucleus tractus solitarius (NTS) that plays an important role as a relay center for baroreceptor inputs and is important in regulating arterial blood pressure. Injection of histamine or an H1 agonist into the NTS of anaesthetized rats caused mean arterial pressure and heart rate to significantly increase (Bhuiyan et al. 2011). The effects were dose-dependent and blocked by an H1 receptor antagonist. Gene expression for H1 receptors was detected in neurons in the NTS suggesting that regulation of cardiovascular homeostasis is via activation of H1 receptors expressed in neurons in the NTS (Bhuiyan et al. 2011). In contrast injection of histamine into the rostral ventro-lateral medulla in anaesthetized rats produced a dose-dependent hypertension and bradycardia, due to stimulation of H2 receptors (Bealer 1999; Granata and Reis 1987). So in mammals, there is abundant evidence of a role for histamine in central control of the cardiovascular system with different receptor types localized in specific areas of the brain (Brown et al. 2001).
In reptiles, histamine has been generally described as causing a depression of blood pressure (Reite 1972), but some turtles (Emys orbicularis, Pseudemys scripta, Clemmys caspica leprosa) show a biphasic response, starting with a rise in pressure and then a decrease as shown in the initial response obtained in the present study. Antihistamines were reported to uncover the depressor phase (Reite 1972), and in the present study ranitidine caused a chronic hypotension. Previous studies have shown no marked effect of histamine on the heart of lizards, snakes, and crocodilians (Reite 1972) but chelonians showed a rise in tension and contractile force, data that are somewhat confirmed by the increase in heart rate and blood pressure recorded in this study. The sites of release and of action of histamine in reptiles are not as yet clear. Although cross perfusion of blood plasma taken from recently fed snakes into unfed snakes demonstrated that the NANC factor was present in the blood, its release during digestion was not stimulated by the gastric hormones gastrin or cholecystokinin nor was it released from mast cells (Enok et al. 2012). The present data certainly imply that histamine has a role in controlling the cardiovascular system of embryonic red-footed tortoise and we can speculate that the cardiac chronotropic response may possibly be due to stimulation of H2 receptors on the heart while the pressor responses may relate to histamine H1 and H2 receptors located on peripheral vessels or in the CNS. Further experiments to investigate these possibilities are planned. While the origin of the histamine is currently unknown there is a clear histaminergic tonus on the cardiovascular system prior to hatching in embryonic red-footed tortoises possibly compensating for limited autonomic tone in these animals.
References
Altimiras J, Crossley DA II (2000) Control of blood pressure mediated by baroreflex changes of heart rate in the chicken embryo (Gallus gallus). Am J Physiol Regul Integr Comp Physiol 278:R980–R986
Altimiras J, Aissaoui A, Tort L, Axelsson M (1997) Cholinergic and adrenergic tones in the control of heart rate in teleosts. How should they be calculated? Comp Biochem Physiol Physiol 118:131–139
Altimiras J, Crossley DA II, Villamor E (2009) Prenatal development of cardiovascular regulation in avian species. In: Mogens GL, Wood SC (eds) Cardio-respiratory control in vertebrates: comparative and evolutionary aspects. Springer, New York, pp 397–427
Bealer SL (1999) Central neuronal histamine contributes to cardiovascular regulation. News Physiol Sci 14:100–105
Bhuiyan ME, Waki H, Gouraud SS, Takagishi M, Kohsaka A, Maeda M (2011) Histamine receptor H1 in the nucleus tractus solitarii regulates arterial pressure and heart rate in rats. Am J Physiol Heart Circ Physiol 301:H523–H529
Brown RE, Stevens DR, Haas HL (2001) The physiology of brain histamine. Prog Neurobiol 63:637–672
Bult H, Boeckxstaens GE, Pelckmans PA, Jordaens FH, Van Maercke YM, Herman AG (1990) Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345(6273):346–347
Clark TD, Wang T, Butler PJ, Frappell PB (2005) Factorial scopes of cardio-metabolic variables remain constant with changes in body temperature in the varanid lizard, Varanus rosenbergi. Am J Physiol Regul Integr Comp Physiol 288:R992–R997
Crastz F (1982) Embryological stages of the marine turtle Lepidochelys olivacea (Eschscholtz). Rev Biol Trop 30:113–120
Crossley DA II, Altimiras J (2000) Ontogeny of cholinergic and adrenergic cardiovascular regulation in the domestic chicken (Gallus gallus). Am J Physiol Regul Integr Comp Physiol 279:R1091–R1098
Crossley DA II, Altimiras J (2005) Cardiovascular development in embryos of the American alligator Alligator mississippiensis: effects of chronic and acute hypoxia. J Exp Biol 208:31–39
Crossley DA II, Altimiras J (2012) Effect of selection for commercially productive traits on the plasticity of cardiovascular regulation in chicken breeds during embryonic development. Poult Sci 91:2628–2636
Crossley DA II, Burggren WW (2009) Development of cardiac form and function in ectothermic sauropsids. J Morphol 270:1400–1412
Crossley DA II, Bagatto BP, Dzialowski EM, Burggren WW (2003a) Maturation of cardiovascular control mechanisms in the embryonic emu (Dromiceius novaehollandiae). J Exp Biol 206:2703–2710
Crossley DA II, Burggren WW, Altimiras J (2003b) Cardiovascular regulation during hypoxia in embryos of the domestic chicken Gallus gallus. Am J Physiol 284:R219–R226
Crossley DA II, Hicks JW, Altimiras J (2003c) Ontogeny of baroreflex regulation in the American alligator Alligator mississippiensis. J Exp Biol 206:2895–2902
Crossley DA II, Jonker SS, Hicks JW, Thornburg KL (2010) Maturation of the angiotensin II cardiovascular response in the embryonic White Leghorn chicken (Gallus gallus). J Comp Physiol B 180:1057–1065
Eme J, Altimiras J, Hicks JW, Crossley DA II (2011a) Hypoxic alligator embryos: chronic hypoxia, catecholamine levels and autonomic responses of in ovo alligators. Comp Biochem Physiol A Mol Integr Physiol 160:412–420
Eme J, Crossley DA II, Hicks JW (2011b) Role of the left aortic arch and blood flows in embryonic American alligator (Alligator mississippiensis). J Comp Physiol B 181:391–401
Eme J, Hicks JW, Crossley DA II (2011c) Chronic hypoxic incubation blunts a cardiovascular reflex loop in embryonic American alligator (Alligator mississippiensis). J Comp Physiol B 181:981–990
Enok S, Simonsen LS, Pedersen SV, Wang T, Skovgaard N (2012) Humoral regulation of heart rate during digestion in pythons (Python molurus and Python regius). Am J Physiol Regul Integr Comp Physiol 302:R1176–R1183
Ferguson MWJ (1985) Reproductive biology and embryology of the crocodilians. In: Gans C, Billett F, Maderson PFA (eds) Biology of the Reptilia: development A. Wiley-Interscience, New York, pp 329–491
Galli G, Skovgaard N, Ab A, Taylor T, Wang T (2004) The role of nitric oxide in the regulation of the systemic and pulmonary vasculature of the rattlesnake, Crotalus durissus terrificus. Comp Biochem Phys B 139:146–156
Galli GL, Skovgaard N, Abe AS, Taylor EW, Conlon JM, Wang T (2005a) Cardiovascular actions of rattlesnake bradykinin ([Val1, Thr6]bradykinin) in the anesthetized South American rattlesnake Crotalus durissus terrificus. Am J Physiol Regul Integr Comp Physiol 288:R456–R465
Galli GLJ, Skovgaard N, Abe AS, Taylor EW, Wang T (2005b) The role of nitric oxide in the regulation of the systemic and pulmonary vasculature of the rattlesnake, Crotalus durissus terrificus. J Comp Physiol B Biochem Syst Environ Physiol 175:201–208
Genovese A, Spadaro G (1997) Highlights in cardiovascular effects of histamine and H1-receptor antagonists. Allergy 52:67–78
Granata AR, Reis DJ (1987) Hypotension and bradycardia elicited by histamine into the C1 area of the rostral ventrolateral medulla. Eur J Pharmacol 136:157–162
Hakanson R, Bottcher G, Sundler F, Vallgren S (1986) Activation and hyperplasia of gastrin and enterochromaffin-like cells in the stomach. Digestion 35(Suppl 1):23–41
Hicks JW, Wang T, Bennett AF (2000) Patterns of cardiovascular and ventilatory response to elevated metabolic states in the lizard Varanus exanthematicus. J Exp Biol 203:2437–2445
Jutel M, Akdis M, Akdis CA (2009) Histamine, histamine receptors and their role in immune pathology. Clin Exp Allergy 39:1786–1800
Levi R, Kuye JO (1974) Pharmacological characterization of cardiac histamine receptors: sensitivity to H1-receptor antagonists. Eur J Pharmacol 27:330–338
Li M, Hu J, Chen Z, Meng J, Wang H, Ma X, Luo X (2006) Evidence for histamine as a neurotransmitter in the cardiac sympathetic nervous system. Am J Physiol Heart Circ Physiol 291:H45–H51
Llenas J, Cardelus I, Heredia A, de Mora F, Gristwood RW (1999) Cardiotoxicity of histamine and the possible role of histamine in the arrhythmogenesis produced by certain antihistamines. Drug safety 21(Suppl 1):33–38 (discussion 81–37)
Mills TA, Taggart MJ, Greenwood SL, Baker PN, Wareing M (2007) Histamine-induced contraction and relaxation of placental chorionic plate arteries. Placenta 28:1158–1164
Morris LJ, Nilssen S (1994) Comparative physiology and evolustion of the autonomic nervous system. In: Nilsson S, Holmgren S (eds) Harwood Academic Publishers, Newark, pp 193–246
Reite OB (1970) The evolution of vascular smooth muscle responses to histamine and 5-hydroxytryptamine. 3. Manifestation of dual actions of either amine in reptiles. Acta Physiol Scand 78:213–231
Reite OB (1972) Comparative physiology of histamine. Physiol Rev 52:778–819
Schwartz JC, Arrang JM, Garbarg M, Pollard H, Ruat M (1991) Histaminergic transmission in the mammalian brain. Physiol Rev 71:1–51
Secor SM, Diamond J (1998) A vertebrate model of extreme physiological regulation. Nature 395:659–662
Secor SM, Hicks JW, Bennett AF (2000) Ventilatory and cardiovascular responses of a python (Python molurus) to exercise and digestion. J Exp Biol 203:2447–2454
Skovgaard N, Galli G, Abe A, Taylor EW, Wang T (2005a) The role of nitric oxide in regulation of the cardiovascular system in reptiles. Comp Biochem Phys A 142:205–214
Skovgaard N, Galli G, Taylor EW, Conlon JM, Wang TB (2005b) Hemodynamic effects of python neuropeptide gamma in the anesthetized python, Python regius. Regul Pept 128:15–26
Skovgaard N, Moller K, Gesser H, Wang T (2009) Histamine induces postprandial tachycardia through a direct effect on cardiac H2-receptors in pythons. Am J Physiol Regul Integr Comp Physiol 296:R774–R785
Tanaka H, Uesato N, Shigenobu K (1995) Chronotropic and inotropic effects of histamine in developing chick heart: differential mechanisms before and after hatching. Naunyn-Schmiedeberg’s Arch Pharmacol 351:391–397
Tate KB, Eme J, Swart J, Conlon JM, Crossley DA II (2012) Effects of dehydration on cardiovascular development in the embryonic American alligator (Alligator mississipiensis). Comp Biochem Physiol A Mol Integr Physiol 162:252–258
Taylor EW, Jordan D, Coote JH (1999) Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev 79:855–916
Tokita M, Kuratani S (2001) Normal embryonic stages of the Chinese softshelled turtle Pelodiscus sinensis (Trionychidae). Zool Sci 18:705–715
Wang T, Hicks JW (1996a) Cardiorespiratory synchrony in turtles. J Exp Biol 199:1791–1800
Wang T, Hicks JW (1996b) The interaction of pulmonary ventilation and the right-left shunt on arterial oxygen levels. J Exp Biol 199:2121–2129
Wang T, Krosniunas EH, Hicks JW (1997) The role of cardiac shunts in the regulation of arterial blood gases. Am Zool 37:12–22
Wang T, Taylor EW, Andrade D, Abe AS (2001a) Autonomic control of heart rate during forced activity and digestion in the snake Boa constrictor. J Exp Biol 204:3553–3560
Wang T, Warburton S, Abe A, Taylor T (2001b) Vagal control of heart rate and cardiac shunts in reptiles: relation to metabolic state. Exp Physiol 86:777–784
Yntema CL (1968) A series of stages in the embryonic development of Chelydra serpentina. J Morphol 125:219–251
Acknowledgments
This work was supported by a National Science Foundation Career award IBN IOS-0845741 to DAC and Sao Paulo research foundation (FAPESP) awards to ASA, MRS and EWT.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Rights and permissions
About this article
Cite this article
Crossley, D.A., Sartori, M.R., Abe, A.S. et al. A role for histamine in cardiovascular regulation in late stage embryos of the red-footed tortoise, Chelonoidis carbonaria Spix, 1824. J Comp Physiol B 183, 811–820 (2013). https://doi.org/10.1007/s00360-013-0746-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-013-0746-3