Abstract
The functional role of nitric oxide (NO) was investigated in the systemic and pulmonary circulations of the South American rattlesnake, Crotalus durissus terrificus. Bolus, intra-arterial injections of the NO donor, sodium nitroprusside (SNP) caused a significant systemic vasodilatation resulting in a reduction in systemic resistance (Rsys). This response was accompanied by a significant decrease in systemic pressure and a rise in systemic blood flow. Pulmonary resistance (Rpul) remained constant while pulmonary pressure (Ppul) and pulmonary blood flow (Qpul) decreased. Injection of L-Arginine (L-Arg) produced a similar response to SNP in the systemic circulation, inducing an immediate systemic vasodilatation, while Rpul was unaffected. Blockade of NO synthesis via the nitric oxide synthase inhibitor, L-NAME, did not affect haemodynamic variables in the systemic circulation, indicating a small contribution of NO to the basal regulation of systemic vascular resistance. Similarly, Rpul and Qpul remained unchanged, although there was a significant rise in Ppul. Via injection of SNP, this study clearly demonstrates that NO causes a systemic vasodilatation in the rattlesnake, indicating that NO may contribute in the regulation of systemic vascular resistance. In contrast, the pulmonary vasculature seems far less responsive to NO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitric oxide (NO) is an important endogenously produced signalling molecule that exerts various physiological effects, including local regulation of blood flow, neurotransmission, and inflammatory responses (e.g. Mungrue et al. 2003). NO is synthesised from L-Arginine (L-Arg) by nitric oxide synthase (NOS) and three isoforms of NOS have been characterised in mammals. Endothelial NOS (eNOS), mainly produced in endothelial cells, is the most common isoform, and plays an important role in the regulation of vascular tone, tissue perfusion and blood pressure (Moncada 1992; Li et al. 2002; Wang et al. 2000). Neuronal NOS (nNOS) is normally expressed in the brain and the enteric nervous system (Gyurko et al. 2002; Toda and Okamura 2003), whilst inducible NOS (iNOS) is expressed predominantly in epithelial and inflammatory cells (Nathan 1997; Aktan 2004). The distributions of NOS and the functional roles of NO have been studied extensively in mammals, but similar information about reptiles is scarce. Immunohistochemical studies, particularly on the brain, have identified nNOS and eNOS in some species of reptiles (Luebke et al. 1992; Bruning et al. 1994; Jiang and Terashima 1996; Smeets et al. 1997; Zamuner et al. 2001; Moon et al. 2002, 2004), suggesting that these isoforms may have similar functions to that of mammals.
Few studies have characterised the roles of NO in the cardiovascular system of reptiles. In the garter snake, Thamnophis sirtalis parietalis, application of ACh and SNP onto the dorsal aorta causes a dose-dependent relaxation (Knight and Burnstock 1993). Similarly, SNP induces vasodilatation in the systemic circulation of the saltwater crocodile, Crocodylus porosus, and the freshwater turtle, Trachemys scripta (Stephens et al. 1983; Altimiras et al. 1998; Crossley et al. 2000). Topical application of ACh onto the brain increases cerebral blood flow (CBF) velocity in turtles, which can be abolished by N(1) nitro-L-Arginine methyl ester (L-NAME) (Hylland et al. 1996; Söderstrom et al. 1997). In all of these species, blockade of NO synthesis via L-NAME increases systemic vascular tone, indicating a tonic release of NO that contributes to basal regulation of the systemic vasculature (Söderstrom et al. 1997; Crossley et al. 2000; Knight and Burnstock 1993; Axelsson et al. 2001).
Most studies regarding the role of NO in the cardiovascular system of reptiles have concentrated on the systemic circulation, while only two studies report on the pulmonary circulation. In turtles (T. scripta) pulmonary vascular resistance is unaffected by SNP, whilst SNP causes a dilatation of the pulmonary vasculature in the python, Python regius (Crossley et al. 2000; Skovgaard et al. 2005). In both species, L-NAME had little effect on the pulmonary circulation, indicating a low basal regulation by NO. Similarly, in mammals, NO seems to contribute little to the basal tone of the pulmonary vasculature (reviewed by Hampl and Herget 2000). It is not clear why the structurally simple unicameral lung of pythons (see Perry 1998) is more responsive to NO than the more complex multicameral lung of turtles (Perry 1978), but pythons are unique amongst reptiles in having a functionally divided heart (Wang et al. 2003), and the larger responses may therefore be related to low pulmonary blood pressures.
The South American rattlesnake, Crotalus durissus, has a simple unicameral lung (Luchtel and Kardong 1981), but the ventricle is not functionally divided and, in comparison to the python, it has high pulmonary blood pressures (Galli et al. 2005). Crotalus has the typical vagal innervation of the pulmonary artery found in numerous reptiles (e.g. Berger 1972; Burggren 1977; Hicks 1994; E.W. Taylor, A.S. Abe and T. Wang, unpublished observation), but the role of local factors affecting pulmonary vascular resistance are not well known. In the present study we seek to identify the role of NO in the regulation of the systemic and pulmonary vasculature in Crotalus durissus. This study is part of a larger overall study on the cardiorespiratory physiology of this species (e.g. Wang et al. 1998, 2001; Andrade et al. 2004; Galli et al. 2005), but more specifically, the rattlesnake was also chosen because it represents a reptile with high pulmonary blood pressure and unicameral lungs.
Materials and methods
Experimental animals
Studies were undertaken on a total of 12 rattlesnakes, C. durissus terrificus, obtained from the Butantan Institute in São Paulo and transported to UNESP, Rio Claro, SP, Brazil. The snakes were housed in 0.5×0.5 m vivariums, maintained at 28±5°C on a natural light regime, and food was withheld 1 week prior to experimentation. All animals appeared healthy, with body masses ranging between 350 g and 950 g (490±10 g). The experiments were performed under anaesthesia, and the animals were killed at the end of the experiments by an overdose of pentobarbital.
Surgery and instrumentation for analysis of blood flow distribution in the systemic circulation
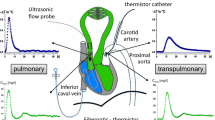
To access total systemic blood flow (Qsys) from measurements of blood flow in the left aorta (QLAo), blood flows in all major systemic arteries were measured in four snakes. These snakes were anaesthetised by injection of 30 mg kg−1 pentobarbital (Mebumal, Sygehusapotekerne, Denmark) into the tail muscle. All reflexes disappeared within 20 min and the animals were then placed in a prone position, so they could be traecheotomised for artificial ventilation, using a mechanical ventilator (Harvard Apparatus), at 4 breaths min−1 and a tidal volume of 25 ml kg−1. A 5 cm ventral incision was made cranial to the heart, and a PE50 catheter containing heparinised saline, was advanced towards the right aortic arch through the occlusively cannulated vertebral artery. Blood flow in the vertebral artery constitutes an insignificant fraction of the total systemic flow (Tobias Wang and Gina Galli, unpublished observation), and brain blood flow is mainly derived from the carotid artery, which remained intact. For measurements of blood flow, 3S or 3R transit-time ultrasonic blood flows probes (Transonic System, Inc., NY, USA) were placed around the left and right aortic arches (LAo and RAo, respectively) and the carotid artery (car). Acoustical gel was infused around the blood flow probes to enhance the signal. The flow probes were connected to two dual-channel blood flow meters (Transonic T206) and signals were recorded with a data acquisition system (Biopac MP100, Biopac Systems, Inc., Goleta, CA, USA) at 50 Hz.
Experimental protocol and calculations for blood flow distribution
Haemodynamic variables were allowed to stabilise for 45 min after instrumentation. Systemic blood flow distributions were then measured at rest and following injection of different pharmacological agents: adrenaline (0.01 mM; 2 μg kg−1), sodium nitroprusside (SNP, 0.008 mM; 2.5 μg kg−1), bradykinin (BK) (0.3 nmol kg−1) and propranolol (6 mM; 2 mg kg−1). These agents were chosen to manipulate vascular resistances with a variety of different vasoactive substances. All drugs were administered as bolus injections through the vertebral artery.
Surgery and instrumentation to assess NO tone
Eight snakes were anaesthetised and artificially ventilated as described above. The vertebral artery and a small branch of the pulmonary artery supplying the lower lung were occlusively cannulated with PE50 containing heparinised saline. Blood-flow probes were placed around the LAo and pulmonary artery. The catheters were connected to disposable pressure transducers (Baxter Edward model PX600, Irvine, CA, USA) and the signals were amplified using an in-house built preamplifier. The pressure transducers were positioned at the level of the heart of the snake and were calibrated daily against a static water column. Signals from the pressure transducers and the blood flow meter were recorded with a data acquisition system (Biopac MP100, Biopac Systems, Inc., Goleta, CA, USA) at 50 Hz.
Experimental protocol and calculations to assess NO tone
The animals received a sham injection of saline that was combined with rattlesnake serum (0.5 ml l−1 saline). Subsequently, an injection of 0.01 mM adrenaline (2 μg kg−1) was given, and once pressures and flows had returned to baseline values, the NO donor SNP was injected (0.008 mM; 2.5 μg kg−1). Following this, the substrate for endogenous production of NO, L-Arg, was injected (287 mM; 50 mg kg−1). Finally, NO synthesis was blocked by injection of the NOS inhibitor L-NAME (556 mM; 150 mg kg−1) to estimate the constitutive NO tone. To investigate whether NOS was inhibited by L-NAME, L-Arg was injected again, and a second infusion of SNP was administered to verify that the response to NO was intact. All drugs were administered through the catheter in the vertebral artery. All drugs were given in 0.2–1.0 ml kg−1 aliquots followed by 0.5 ml kg−1 of saline to flush the catheter, so that volume, rather than concentration, of the injected agent was constant relative to body mass. All drugs were purchased from Sigma (Denmark).
Data analysis and statistics
Recordings were analysed using data analysis software (AcqKnowledge, version 3.2.3). Mean blood pressures and flows were taken over a 2 min period prior to, and at the maximum effect of each agent.
Table 1 lists the distributions of blood flows in the systemic arteries following each pharmacological treatment. The ratio between QLAo and Qsys was then calculated from the data. A two-way ANOVA was performed to test whether the ratio differed significantly among treatments, and no significant differences were found. Therefore, as the relationship between QLAo and Qsys persists during all treatments, it is seems adequate to estimate Qsys as 3.3 times QLAo. Hence, this ratio was applied when estimating Qsys in the subsequent experimental protocol. Because there is only one pulmonary artery in the rattlesnake, measurements of blood flow in the pulmonary artery represent Qpul. Since the vertebral artery was occlusively cannulated, Qsys was estimated as 3.3 times QLAo. Total cardiac output (Qtot) was calculated as Qsys + Qpul. Heart rate (fH) was derived from the instantaneous blood flow trace from the left aortic arch, and total stroke volume (Vstot; pulmonary + systemic) was calculated as Qtot/f H . Rpul and Rsys were calculated from mean blood pressure and mean blood flow (Rpul=Ppul/Qpul and Rsys=Psys/Qsys), assuming that central venous blood pressures were negligible.
A paired t test was used to test for significant effects of the agents compared with the previous control values. This test was also employed to compare the effects of L-Arg and SNP before and after L-NAME. All data are presented as mean ± SEM and apparent differences are considered significant at the 95% level of confidence (P value<0.05).
Results
Figure 1 shows all haemodynamic variables recorded following the second protocol, before and after infusion of the various pharmacological agents, in one animal. Mean values of haemodynamic variables are shown in Figs. 2 and 3. Injection of SNP elicited a significant systemic vasodilatation causing Rsys to fall from 0.06±0.02 kPa ml−1 min kg to 0.03±0.00 kPa ml−1 min kg. This was associated with a significant decrease in Psys, and a small, but significant, rise in Qsys, (Fig. 2a–c). Rpul remained unchanged following injection of SNP, while Ppul and Qpul significantly decreased (Fig. 2d–f). Heart rate was significantly elevated after injection of SNP (45.4±3.7 to 48.3±3.5 min−1), but Qtot, and Vstot were not affected (Fig. 3a, c, d). A net R-L shunt, as indicated by Qpul/Qsys being less than one, was observed in resting animals (Fig. 3b). This ratio did not significantly change following injection of SNP.
Effects of injections of SNP, L-Arg and L-NAME on Psys, systemic pressure (a); Qsys, systemic blood flow (b); Rsys, systemic resistance (c); Ppul, pulmonary pressure (d); Qpul, pulmonary blood flow (e) and Rpul, pulmonary resistance (f). Open bars indicate pre-injection values and the closed bars indicate post-injection values. All data are presented mean ± SEM. (n=6–7). Values that are significantly different from the untreated control condition are marked with an asterisk (P<0.05)
Effects of injections of SNP, L-Arg and L-NAME on Vstot, total stroke volume (a), Qpul/Qsys (b), f H , heart rate (c), and Qtot total blood flow (d). Open bars indicate pre-injection value, closed bars indicate post-injection value. Values are mean with SEM. In the systemic circulation n=8. Values are mean with SEM. n=7, except for fH where n=8. Asterisk indicates significant difference of mean from control value (P≤0.05)
The effects of L-Arg were similar to those of SNP and induced an immediate systemic vasodilatation with a reduction in Rsys from 0.08±0.03 kPa ml−1 min kg to 0.03±0.01 kPa ml−1 min kg. This was manifested by a significant reduction in Psys and a significant increase in Qsys (Fig. 2a–c). There were no significant effects of L-Arg in the pulmonary circulation (Fig. 2d–f). L-Arg caused significant increases in Vstot, f H , and Qtot (Fig. 3a, c, d). No significant change in Qpul/Qsys was observed following injection of L-Arg (Fig. 3b).
No significant effects on systemic haemodynamic variables were observed following injection of L-NAME (Fig. 2a–c). There was a small, but significant rise in Ppul following L-NAME, while both Qpul and Rpul remained virtually unchanged (Fig. 2d–f). Heart rate was significantly increased, but there were no significant changes in Qtot, Vstot or Qpul/Qsys (Fig 3).
The reduction in Rsys following injection of L-Arg was significantly attenuated following treatment with L-NAME. Consequently, changes in Psys and Qsys were significantly less after injection of L-NAME compared to injection of L-Arg alone (Fig. 4f–h).
Maximum changes in physiological variables seen after injection of L-Arg before and after L-NAME. Ppul, pulmonary pressure (a); Qpul pulmonary blood flow (b), Rpul pulmonary resistance (c), Vstot total stroke volume (d), Qtot total blood flow (e), Psys systemic pressure (f), Qsys systemic blood flow (g), Rsys systemic resistance (h); f H , heart rate (I) and Qpul/Qsys (j). In the systemic circulation n=8. In the pulmonary circulation Ppul, n=7; Qpul, n=7; and Rpul, n=6. Vstot, Qpul/Qsys and Qtot n=7, while fH, n=8. Asterisk indicates significant difference of mean value following injection of L-NAME (P≤0.05)
Discussion
Critique of methods
Our study was performed on anaesthetised animals. Anesthesia leads to a higher fH and Qpul compared to fully recovered Crotalus, which appears to be caused by a withdrawal of vagal tone and elevated sympathetic activity (T. Wang, E.W. Taylor and A.S. Abe, unpublished observation). Furthermore, autonomic responses, such as barostatic regulation, are virtually abolished. The depressed autonomic function is advantageous for studies on local regulation of the cardiovascular system because reflex responses to altered blood pressure are lacking. It is, nevertheless, possible that barbiturates affect the tone of NO, so that it differs from fully recovered animals. However, because we characterised effects of both NO application (SNP) and inhibition of synthesis (L-NAME), the differences between the systemic and pulmonary circuits can be established conclusively. Nevertheless, future studies should address the role of NO in fully recovered and non-anaesthetised reptiles.
The role of NO in the systemic circulation
In mammals, NO is known to be a potent systemic vasodilator that is involved in a host of physiological mechanisms, including the regulation of blood flow and blood pressure in the cardiovascular system, as well as neurotransmission and inflammatory responses (for a review see Mungrue et al. 2003). Using the NADPH diaphorase reaction, nNOS has been demonstrated in the brain and gut of several reptiles (Bruning et al. 1994; Luebke et al. 1992; Smeets et al. 1997; Haverkamp and Eldred 1998; Lamanna et al. 1999; Olsson and Gibbins 1999). Furthermore, eNOS immunoreactivity has been identified in the trigiminal ganglia of the crotaline snake, Trimeresurus flavoviridis, which suggests a role in neurotransmission (Moon et al. 2004).
The NO-donor SNP, led to a large systemic vasodilatation in the rattlesnake, indicating a substantial potential for NO in regulating systemic vascular resistance. This finding is consistent with various other species of reptiles, where SNP induces a reduction in systemic resistance (Crossley et al. 2000; Skovgaard et al. 2005) or a marked reduction in Psys (Stephens et al. 1983; Altimiras et al. 1998). Furthermore, aortic strips of the garter snake and caiman vasodilate in response to SNP, and the vasodilatory responses to ACh can be blocked by L-NAME (Miller and Vanhoutte 1986; Knight and Burnstock 1993). Injection of L-Arg, the substrate for endogenous production of NO, caused similar effects to SNP in rattlesnakes. Following L-NAME infusion, the effects of L-Arg were significantly attenuated indicating that L-NAME had blocked NO production.
Topical application of ACh and SNP induces an increase in CBF in turtles, T. scripta (Hylland et al. 1996). The effects of ACh can be totally blocked by L-NAME, indicating that this effect is mediated through NO production. In the same species, blood flow fell to zero following L-NAME application, while systemic blood pressure persistently increased, indicating a profound basal regulation of NO in the cerebral blood vessels or the arteries supplying the brain (Söderstrom et al. 1997). In contrast, injection of another NOS inhibitor, nitro-L-Arginine (L-NA) in the estuarine crocodile, C. porosus, had no effect on CBF velocity or blood pressure (Söderstrom et al. 1999). However, this study was performed under hypoxic conditions, which may have affected the impact that L-NA had on cardiovascular variables.
Although SNP and L-Arg caused a clear vasodilatation in the rattlesnake, injection of L-NAME did not significantly alter systemic resistance. This is in contrast to turtles and pythons for which L-NAME increases Rsys (Crossley et al. 2000; Skovgaard et al. 2005). Thus, in the rattlesnake, it seems that NO contributes little to the basal regulation of the systemic vasculature.
The pulmonary circulation
In contrast to the clear regulatory role of NO in the systemic circulation of rattlesnakes, the pulmonary vasculature seems far less affected. Pulmonary resistance was unaffected by either SNP or L-Arg. Alongside this, L-NAME had no effect on Rpul, indicating a lack of contribution of NO to the maintenance of basal vascular tone in the pulmonary vasculature of Crotalus.
The extent to which NO contributes to the basal regulation of pulmonary resistance in mammals is controversial. Some studies have reported a significant vasoconstriction following NOS inhibition (Meyer et al. 1993; Lorente et al. 1993; Cremona et al. 1994, 1997; Celemajer et al. 1994), but most species of mammals studied do not exhibit a response (Asano et al. 1997; Crawley et al. 1990; Lindeborg et al. 1995; Nishiwaki et al. 1992; reviewed by Hampl and Herget 2000). Nevertheless, additional studies in mammals have shown that when the pulmonary vasculature is pre-constricted, for example during hypoxic pulmonary vasoconstriction or pulmonary hypertension, NO synthesis is increased (reviewed in Hampl and Herget 2000). Under these circumstances NOS inhibitors cause a potentiation of the pulmonary vasoconstriction already seen in hypoxic or chronically hypertensive animals.
Studies into the resting NO tone in the pulmonary circulation of reptiles are scarce. Our study is in agreement with data obtained from turtles where injection of SNP and L-NAME had little effect on the vascular tone of the pulmonary circulation (Crossley et al. 2000). Pulmonary flows and pressures were similar to recovered animals, so it was concluded that NO does not contribute to the vascular tone of the pulmonary circulation in turtles. In contrast, SNP caused a significant vasodilatation of the pulmonary vasculature of the python (P. regius). As in turtles and rattlesnakes, L-NAME, however, did not increase pulmonary vascular tone, indicating that NO contributes little to pulmonary resistance (Skovgaard et al. 2005).
The observation that SNP does not dilate the pulmonary vasculature of rattlesnakes and turtles (Crossley et al. 2000), but does reduce Rpul in python (Skovgaard et al. 2005), may suggest that reptiles with a divided circulation, and low pulmonary blood pressure are more dependant on NO regulation in the pulmonary circulation. The pulmonary artery of reptiles with an undivided circulation is densely innervated by the vagus nerve, which once stimulated, causes a constriction of the vessel, thereby acting to regulate pulmonary resistance (Berger 1972; Burggren 1977). This neural regulation of the pulmonary artery is the prime determinant of cardiac shunt patterns (Hicks 1994). However, in reptiles with functionally divided hearts, the vagal innervation is presumably less effective in regulating pulmonary blood flow, and other factors within the pulmonary vasculature, such as NO, may become more important.
Conclusion
In all species of reptiles studied to date, SNP causes a systemic vasodilatation, indicating an important role for NO in regulation of systemic vascular tone. Unlike other reptiles, however, the rattlesnake does not seem to rely on NO for maintenance of basal systemic vascular tone. In contrast to the potential vasodilatory role of NO in the systemic circulation of the rattlesnake, the pulmonary vasculature is relatively unresponsive to this form of local regulation. It seems that species with an undivided circulation, such as the rattlesnake and the turtle, may be less reliant on local regulation of the pulmonary vasculature via the production of NO, than those with divided hearts, such as the python.
References
Aktan F (2004) iNOS-mediated nitric oxide production and its regulation. Life sciences 75:639–653
Altimiras J, Franklin CE, Axelsson M (1998) Relationships between blood pressure and heart rate in the saltwater crocodile Crocodylus porosus. J Exp Biol 201:2235–2242
Andrade DV, Tattersall GJ, Brito SP, Soncini R, Branco LG, Glass ML, Abe AS, Milsom WK (2004) The ventilatory response to environmental hypercarbia in the South American rattlesnake, Crotalus durissus. J Comp Physiol B 174:281–291
Asano K, Yanagidaira Y, Yoshimura K, Sakai A (1997) The cGMP pathway is not responsible for the blunted hypoxic vasoconstriction in rat lungs alter altitude exposure. Acta Physiol Scand 160:393–400
Axelsson M, Olsen C, Gibbins I, Holmgren S, Franklin CE (2001) Nitric oxide, a potent vasodilator of the aortic anastomosis in the estuarine crocodile, Crocodylus porosus. Gen Comp Endocrinol 122:198–204
Berger PJ (1972) The vagal and sympathetic innervation of the isolated pulmonary artery of a lizard and a tortoise. Comp Gen Pharmacol 3:113–124
Bruning G, Wiese S, Mayer B (1994) Nitric oxide synthase in the brain of the turtle Pseudemys scripta elegans. J Comp Neurol 348:183–206
Burggren WW (1977) The pulmonary circulation of the chelonian reptile: morphology, haemodynamics and pharmacology. J Comp Physiol 116:303–323
Celemajer DS, Dollery C, Burch M, Deanfield JE (1994) Role of endothelium in the maintenance of low pulmonary vascular tone in normal children. Circulation 89:2041–2044
Crawley DE, Liu SF, Evans TW, Barnes PJ (1990) Inhibitory role of endothilum-derived relaxing factor in rat and human pulmonary arteries. Br J Pharmocol 101:166–170
Cremona G, Wood AM, Hall LW, Bower EA, Higenbottam T (1994) Effect of inhibitors of nitric oxide release and action on vascular tone in isolated lungs of pig, sheep, dog and man. J Physiol (Lond) 481:185–195
Cremona G, Higenbottam T, Takao M, Bower EA, Hall LW (1997) Nature and site of action of endogenous nitric oxide in vasculature of isolated pig lungs. J Appl Physiol 82:23–31
Crossley D, Wang T, Altimiras J (2000) Role of nitric oxide in the systematic and pulmonary circulation of anaesthetized turtles (Trachemys scripta). J Exp Biol 286:683–689
Galli GLJ, Skovgaard N, Abe A, Conlon M, Wang T, Taylor EW (2005) Cardiovascular actions of rattlesnake bradykinin ([Val1 Thr6]bradykinin) in the South American rattlesnake Crotalus durissus terrificus. Am J Physiol 288:R456-R465
Gyurko R, Leupen S, Huang PL (2002) Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology 143:2767–2774
Hampl V, Herget J (2000) Role of nitric oxide in the parthogenesis of chronic pulmonary hypertension. Physiol Rev 80:1337–1372
Haverkamp S, Eldred WD (1998) Localization of nNOS in photoreceptor, bipolar and horizontal cells in turtle and rat retinas. Neuroreport 9:2231–2235
Hicks JW (1994) Adrenergic and cholinergic regulation of intracardiac shunting. Physiol Zool 67:1325–1346
Hylland P, Nilsson GE, Lutz P (1996) Role of nitric oxide in the elevation of cerebral blood flow induced by acetylcholine and anoxia in the turtle. J Cereb Blood Flow Metab 16:290–295
Jiang PJ, Terashima S (1996) Distribution of NADPH-diaphorase in the central nervous system of an infrared-sensitive snake, Trimeresurus flavoviridis. Brain Res 713:168–177
KnightG, Burnstock G (1993) Acetylcholine induces relaxation via the release of nitric oxide from the endothelial cells of the garter snake (Thamnophis sirtalis parientalis). Comp Biochem Physiol 106C:383–388
Lamanna C, Costagliola A, Vittoria A, Mayer B, Assisi L, Botte V, Cecio A (1999) NADPH-diaphorase and NOS enzymatic activities in some neurons of reptilian gut and their relationships with two neuropeptides. Anat Embryol (Berl) 199:397–405
Li H, Wallerath T, Forstermann U (2002) Physiological mechanisms regulating the expression of endothelial-type NO synthase. Nitric Oxide 7:132–147
Lindeborg DM, Kavanagh BP, Van Meurs K, Pearl RG (1995) Inhaled nitric oxide does not alter the longitudinal distribution of pulmonary vascular resistance. J Appl Physiol 78:341–348
Lorente JA, Landin L, Renes E, De Pablo R, Jorge P, Rodena E, Liste D (1993) The Role of nitric oxide in the haemodyanmic changes of sepsis. Crit Care Med 21:759–767
Luchtel DL, Kardong KV (1981) Ultrastructure of the lung of the rattlesnake, Crotalus viridis oreganus. J Morphol 169: 29–47
Luebke JI, Weider JM, McCarley RW, Greene RW (1992) Distribution of NADPH-diaphorase positive somata in the brainstem of the monitor lizard Varanus exanthematicus. Neurosci Lett. 148:129–132
Meyer J, Lentz CW, Herendon DN, Nelson S, Traber LD, Traber DL (1993) Effects of halothane anaesthesia on vasoconstrictor response to NG-nitro-L-Arginine methyl ester, an inhibitor of the nitric oxide synthesis, in sheep. Anesth Analg 77:1221
Miller VM, Vanhoutte PM. (1986) Endothelium-dependent responses in isolated blood vessels of lower vertebrates. Blood Vessels 23:225–35
Moncada S (1992). Nitric oxide gas: mediator, modulator, and pathophysiologic entity. J Lab Clin Med 120:187–191
Moon C, Terashima S, Ahn M, Kang J, Shin T (2002). Immunohistochemical analysis of neuronal nitric oxide synthase in the trigeminal ganglia of the crotaline snake Trimeresurus flavoviridis. Neurosci Lett. 319:21–24
Moon C, Terashima S, Shin T (2004) Immunohistochemical study of endothelial nitric oxide synthase in the trigeminal ganglia of a crotaline snake Trimeresurus flavoviridis. J Vet Med Sci 66:1007–1009
Mungrue IN, Bredt DS, Stewart DJ, Husain M. (2003). From molecules to mammals: what’s NOS got to do with it?. Acta Physiol Scand 179:123–135
Nathan C (1997) Inducible nitric oxide synthase: what difference does it make?. J Clin Invest 100:2417–2423
Nishiwaki K, Kyhan DP, Rock P, Desai PM, Peterson WP, Pribble CG, Murray PA (1992) N-nitro-L-Arginine and pulmonary vascular pressure-flow relationship in consioud dogs. Am J Physiol 262:H1331–H1337
Olsson C, Gibbins I (1999) Nitric oxide synthase in the gastrointestinal tract of the estuarine crocodile, Crocodylus porosus. Cell Tissue Res 296:433–437
Perry SF (1978) Quantitative anatomy of the lungs of the red-eared turtle, Pseudemys scripta elegans. Respir Physiol 35:245–262
Perry SF (1998) Lungs: comparative anatomy, functional morphology and evolution. In: Gans C, Gaunt AS (eds) Biology of Reptilia, vol 19. Morphology G: visceral organs. SSAR Press, pp 1–92
Skovgaard N, Galli GLJ, Taylor EW, Conlon M, Wang T (2005) Hemodynamic effects of python neuropeptide γ in the anesthetized python, Python regius. Reg Pept (in press)
Smeets WJ, Alonso JR, Gonzalez A (1997) Distribution of NADPH-diaphorase and nitric oxide synthase in relation to catecholaminergic neuronal structures in the brain of the lizard Gekko gecko. J Comp Neurol 6(377):121–141
Söderstrom V, Nilsson G, Lutz P (1997) Effects of inhibition of nitric oxide synthesis and of hypercapnia on blood pressure and brain blood flow in the turtle. J Exp Biol 200:815–820
Söderstrom V, Nilsson GE, Renshaw GMC, Franklin CE (1999) Hypoxia stimulates cerebral blood flow in the estuarine crocodile, Crocodylus porosus. Neurosci Lett 267:1–4
Stephens GA, Shirer HW, Trank JW, Goetz KL (1983) Arterial baroreceptor reflex control of heart rate in two species of turtle. Am J Physiol 244:R544–R552
Toda N, Okamura T (2003) The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev 55:271–324
Wang T, Abe AS, Glass ML (1998). Effects of temperature on lung and blood gases in the South American rattlesnake, Crotalus durissus terrificus. Comp Biochem Physiol 121A:7–11
Wang T, Axelsson M, Jensen J, Conlon M (2000) Cardiovascular actions of python bradykinin and substance P in the anesthetized python, Python regius. Am J Physiol 279:R531–R538
Wang T, Warburton S, Abe A, Taylor T (2001) Vagal control of heart rate and cardiac shunts in reptiles: relation to metabolic state. Exp Physiol 86:777–784
Wang T, Altimiras J, Klein W, Axelsson M (2003) Ventricular haemodynamics in Python molurus: separation of pulmonary and systemic pressures. J Exp Biol 206:4241–4245
Zamuner SR, Gutierrez JM, Muscara MN, Teixeira SA, Teixeira CF (2001) Bothrops asper and Bothrops jararaca snake venoms trigger microbicidal functions of peritoneal leukocytes in vivo. Toxicon 39:1505–1513
Acknowledgements
This study was supported by the Danish Research Council, the NOVO foundation and FAPESP. Gina Galli is in receipt of a special studentship from the BBSRC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier
Rights and permissions
About this article
Cite this article
Galli, G.L., Skovgaard, N., Abe, A.S. et al. The role of nitric oxide in the regulation of the systemic and pulmonary vasculature of the rattlesnake, Crotalus durissus terrificus. J Comp Physiol B 175, 201–208 (2005). https://doi.org/10.1007/s00360-005-0476-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0476-2