Abstract
Neuromodulation by peptides and amines is a primary source of plasticity in the nervous system as it adapts the animal to an ever-changing environment. The crustacean stomatogastric nervous system is one of the premier systems to study neuromodulation and its effects on motor pattern generation at the cellular level. It contains the extensively modulated central pattern generators that drive the gastric mill (chewing) and pyloric (food filtering) rhythms. Neuromodulators affect all stages of neuronal processing in this system, from membrane currents and synaptic transmission in network neurons to the properties of the effector muscles. The ease with which distinct neurons are identified and their activity is recorded in this system has provided considerable insight into the mechanisms by which neuromodulators affect their target cells and modulatory neuron function. Recent evidence suggests that neuromodulators are involved in homeostatic processes and that the modulatory system itself is under modulatory control, a fascinating topic whose surface has been barely scratched. Future challenges include exploring the behavioral conditions under which these systems are activated and how their effects are regulated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Generating numerous behaviors that suit all facets of life is the most fascinating ability of the nervous system. Intriguingly, even small brains with a limited number of neurons perform this task. Because appropriate behavioral responses to the challenges of everyday life are vital to animal survival, there is a strong evolutionary pressure toward mechanisms that allow flexibility in the nervous system. The work on many model systems demonstrates that one common principle underlying nervous system flexibility is that most neural networks produce many different activity patterns and not a single stereotyped output only (Marder and Calabrese 1996). Network activity depends on the membrane and synaptic properties of the network neurons as well as on extrinsic input from other neural structures and sense organs. All of these properties are altered by substances, termed neuromodulators that are available in the bloodstream or released locally by neurons.
Neuromodulation is a pervasive influence throughout the nervous system of animals from nematodes to humans (Kupfermann 1979; Kaczmarek and Levitan 1987; Lopez and Brown 1992; Katz and Frost 1996; Birmingham and Tauck 2003; Dickinson 2006; Krichmar 2008). Neuromodulatory substances, such as amines, neuropeptides, gases and small-molecule neurotransmitters are important regulators of cellular and network activity (Cantrell and Catterall 2001; Gu 2002; Nusbaum and Beenhakker 2002; Dickinson 2006; Krichmar 2008), and their dysfunction is involved in numerous pathologies, such as Parkinson disease (Montague et al. 2004), personality disorders (Dagher and Robbins 2009) and schizophrenia (Knable and Weinberger 1997). Their paracrine effects are often mediated by the activation of metabotropic receptors and associated signaling pathways. They can therefore affect all stages of neuronal information processing and cause both immediate and long-term effects (Branchereau et al. 2002; Fénelon et al. 2002).
One of the premier systems for studying neuromodulator actions and their underlying mechanisms is the stomatogastric nervous system (STNS) of decapod crustaceans, including clawed and spiny lobsters, crayfish and crabs (Fig. 1a). The STNS provides an almost unique opportunity to record and manipulate identified modulatory systems and determine their influence on identified motor circuit neurons (Selverston and Moulins 1987; Harris-Warrick et al. 1992; Nusbaum and Beenhakker 2002; Marder and Bucher 2007). It controls various aspects of feeding and contains the well-defined gastric mill and pyloric central pattern generators (CPGs), plus several descending modulatory projections that control these circuits. Several peripheral systems which modulate gastric mill and pyloric activity, largely via activation of specific modulatory projection neurons, are also identified. For the purpose of this review, I will use the term “neuromodulator” for neurotransmitters released from modulatory neurons that alter the intrinsic and/or synaptic properties of their target cells, generally via a metabotropic action.
The stomatogastric nervous system of the crab. a Dorsal view of the crab Cancer pagurus with parts of its dorsal carapace removed. The heart, the STNS and the brain are visible. Hemolymph from the heart reaches the STNS via the ophthalmic artery. Courtesy of U. Hedrich (Ulm University). b Flat projection of the STNS as seen in the Petri dish. Blue cell bodies of neurons in the stomatogastric, esophageal and commissural ganglia. CoG commissural ganglion, OG esophageal ganglion, CoC circumesophageal commissure, ivn inferior ventricular nerve, ion inferior esophageal nerve, son superior esophageal nerve, stn stomatogastric nerve, mvn median ventricular nerve, dgn dorsal gastric nerve, dvn dorsal ventricular nerve, lvn lateral ventricular nerve, lgn lateral gastric nerve, pdn pyloric dilator nerve. c Antero-lateral view of the foregut, including esophagus, cardiac sac and gastric mill, as well as pylorus. The STNS is located dorsally on the foregut and innervates all of its compartments
The CPGs in the STNS are able to generate repetitive, rhythmic activity even in the absence of extrinsic timing cues such as sensory feedback. Similar pattern generating networks control a variety of rhythmic motor behaviors, including respiration, locomotion and the generation of the heartbeat in species with neurogenic hearts (Bässler and Büschges 1998; Cymbalyuk et al. 2002; Büschges et al. 2004; Kristan et al. 2005; Ramirez and Viemari 2005). Although the specifics of pattern generation differ to some extent among species, CPGs share a set of general organizing principles. One such principle is that CPGs are multifunctional networks, due to the influence of neuromodulators which enable them to produce the behavioral flexibility required for their proper function.
In the STNS, neuromodulators shape motor activity into many different forms (Nusbaum and Beenhakker 2002). Here, I will review the modulatory system of the STNS and some of the consequences of extrinsic neuromodulation (deriving from outside of the affected circuit) on pattern generation. I will also present ideas on the regulation of the modulatory system and on metamodulation, i.e. modulation of the modulatory system.
The crustacean stomatogastric nervous system
The STNS (Fig. 1a) is an extension of the CNS and consists of four interconnected ganglia (Fig. 1b): the paired commissural ganglia (CoGs; about 500 neurons each), the unpaired esophageal ganglion (14 neurons) and the stomatogastric ganglion (STG; 26–30 neurons depending on species). It contains distinct, but interacting CPGs that generate the motor patterns underlying rhythmic movements of the esophagus (swallowing), cardiac sac (food storage), gastric mill (chewing) and pylorus (filtering of chewed food). While the esophageal and cardiac sac pattern generators are located in the CoGs and the esophageal ganglion, the gastric mill and pyloric rhythms are generated by CPGs in the STG.
The striated muscles that are innervated by the STG motor neurons drive the movements of the gastric mill and the pyloric filter apparatus (for morphology of the foregut see Fig. 1c; Maynard and Dando 1974; Selverston and Moulins 1987). Food that enters the foregut via the esophagus is ground between three teeth located dorsally within the gastric mill before it passes into the pylorus. Here, a complicated filtering press separates solid food particles from fluids. The latter are taken up by the midgut gland, the functional equivalent of the liver and pancreas, while the solid particles move to the hindgut where they are excreted.
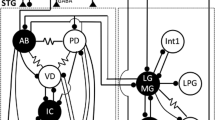
The circuits underlying the pyloric and gastric mill patterns have been extensively studied (Hartline and Maynard 1975; Harris-Warrick et al. 1992; Nusbaum and Beenhakker 2002; Hooper 2003; Hooper and DiCaprio 2004; Marder and Bucher 2007). Their circuit neurons are identified in several crustacean species and the cellular properties and synaptic interactions of these neurons (Fig. 2a) are well known. The synaptic connections, and in particular those between the gastric mill and pyloric circuits, vary substantially between species, which facilitated the comparative study of pattern generating mechanisms. The pyloric rhythm is triphasic (Fig. 2b) and has a cycle frequency between 0.5 and 2 Hz. It is driven by a set of electrically coupled pacemaker neurons and usually spontaneously active, even in the isolated nervous system. It results from the synaptic interactions and intrinsic properties of the circuit neurons. Most neurons in the pyloric circuit have dual function: they participate in pattern generation and they act as motor neurons. For example, the pyloric dilator (PD) neurons built the pyloric pacemaker ensemble together with the anterior burster (AB) and lateral posterior gastric neurons (Fig. 2a). Yet, they also drive the muscles that dilate the pyloric chamber during the first phase of a pyloric cycle. Simultaneously with the PDs, the ventricular dilator neuron VD becomes active and the cardiopyloric valve opens. In the second phase, the anterior region of the pyloric chamber constricts and the cardiopyloric valve closes due to the activities of the lateral pyloric (LP) and inferior cardiac neurons. During the third phase of the pyloric pattern the posterior region of pylorus constricts due to the activity of the pyloric constrictor neurons (for summary see Claiborne and Selverston 1987).
The pyloric and gastric mill networks a in Cancer borealis and Cancer pagurus consist of 20–22 neurons (11–13 pyloric and 9 gastric mill neurons) that interact via inhibitory and electrical synapses (gap junctions). The colors indicate the participation in a particular motor pattern. AB anterior burster, PD pyloric dilator, LPG lateral posterior gastric, LP lateral pyloric, IC inferior cardiac, LG lateral gastric, MG medial gastric, GM gastric mill, PY pyloric constrictor, VD ventricular dilator, Int1 interneuron 1, AM anterior median, DG dorsal gastric. The pyloric rhythm is a triphasic pattern and usually spontaneously active (b). It is driven by a pacemaker ensemble of neurons built by the electrically coupled AB, PD and LPG neurons. Shown are intracellular recordings of the PD and LP neurons plus extracellular recordings from the pyloric nerves pdn, lvn and mvn (see also Fig. 1b). Scale bar LP 13 mV, PD 20 mV. c Intracellular recording of a GM neuron along with extracellular recordings of the lateral and dorsal gastric nerves (lgn and dgn). Scale bar GM 20 mV
In contrast to the pyloric rhythm, the gastric mill rhythm is episodic and active in only about half of the isolated nervous systems (Stein et al. 2005). This rhythm depends on the release of neuromodulatory substances from descending modulatory projection neurons in most species. No classical pacemaker neuron can be found in the gastric mill circuit. Rather, pairs of neurons with reciprocal inhibitory connections (Fig. 2a) shape a two-phase rhythm (Fig. 2c) with cycle frequencies between 0.05 and 0.2 Hz. The gastric mill pattern appears to be more flexible than the pyloric rhythm (Hartline and Maynard 1975; Heinzel et al. 1993; Stein et al. 2006), but its basic mode consists of a closing of the lateral teeth, driven by the medial and lateral gastric neurons and a forward and downward movement of the medial tooth due to the activity of the gastric mill (GM) motor neurons. After that all three teeth retract (mediated by the lateral posterior gastric neurons and the dorsal gastric neuron, DG; for summary see Claiborne and Selverston 1987).
Sources of neuromodulators in the STNS
In the STNS, neuromodulators can reach the STG motor circuits either via release from the terminals of modulatory projection neurons and sensory neurons or via the bloodstream as circulating hormones (Marder and Thirumalai 2002; Nusbaum 2002; Nusbaum and Beenhakker 2002; Dickinson 2006). Neurohormones are released by neurohemal organs and neurohemal release zones such as the x-organ/sinus gland complex in the eyestalks, the postcommissural organ and the pericardial organs, the major neurosecretory structures in crustaceans (Christie et al. 1995; Pulver and Marder 2002; Li et al. 2003; Skiebe 2003; Chen et al. 2009). The STG is ideally located to be targeted by neurohormones, because it is situated within the ophthalmic artery, which connects the heart and the supraesophageal ganglion (the brain).
The same neuromodulatory substances that are released from the pericardial organs are often also found in the terminals of modulatory neurons that innervate the STG (Nusbaum et al. 2001; Skiebe 2001; Marder and Thirumalai 2002; Nusbaum and Beenhakker 2002). It is unknown why the same substances are used both as circulating neurohormones and as local neuromodulators. It is possible that local release targets specific regions of the nervous system while circulating hormones can coordinate multiple target areas, including tissues outside the nervous system. The complement of neuromodulatory substances available in modulatory neurons varies during development (Fenelon et al. 1999; Kilman et al. 1999; Cape et al. 2008) and between equivalent neurons in different species, as does the synaptic connectivity of these neurons and the response of the motor circuits (Rehm et al. 2008). A summary of identified modulatory neurons and their actions in different species is given in supplemental 1. More details on the variety of available modulators can be found in Skiebe (2001), Marder et al. (2002) and Billimoria et al. (2005) and Marder and Bucher (2007).
Modulatory projection neurons have distinct actions on their target circuits
In the crab, Cancer borealis, extensive investigations have characterized the connectivity of modulatory projection neurons and their effects on the STG circuits. Altogether, about 40 axonal projections from the CoGs reach the STG (Coleman et al. 1992) via the unilateral stomatogastric nerve (Fig. 3). A few of these projection neurons are identified, i.e. soma location, axonal projection and synaptic actions on the STG circuits have been described (Fig. 3, supplemental 2). These neurons, which include the modulatory commissural neurons (MCN) 1, 5 and 7 and commissural projection neuron 2 (CPN2), are identified by their physiological properties in each animal (Coleman and Nusbaum 1994; Norris et al. 1994, 1996; Blitz et al. 1999; Nusbaum and Beenhakker 2002). A functionally equivalent pair of projection neurons in the esophageal ganglion is also identified, called the modulatory proctolin neurons (MPNs; Nusbaum and Marder 1989a; Blitz and Nusbaum 1997). Many different neuromodulatory substances are contained within these projection neurons, including amino acids, amines and neuropeptides (Skiebe 2001). Traditionally, the transmitters used by neurons have been determined with immunocytochemical methods. More recently, mass spectrometry and related techniques such as matrix-assisted laser desorption/ionization time of flight mass spectrometry have been used to identify many amine and peptide transmitters in single neurons and small pieces of neural tissue (Li et al. 2002; Skiebe et al. 2002; Li et al. 2003; Christie et al. 2006; Stemmler et al. 2007). Most of these neuromodulators occur in only a small number of projection neurons. For example, the peptide CabTRP Ia (Cancer borealis tachykinin-related peptide Ia; Christie et al. 1997) is exclusively found in MCN1 (Fig. 3, supplemental 2; Coleman et al. 1995). In addition to CabTRP Ia, MCN1 releases γ-aminobutyric acid (GABA) and the peptide proctolin (Blitz et al. 1999). In general, neuromodulators are co-localized with other transmitters, with a common motif being one classical small-molecule transmitter and at least one peptide transmitter (Nusbaum and Beenhakker 2002).
The term ‘modulatory projection neuron’ is typically used for descending pathways that have rather slow, metabotropic actions on their target neurons, often as a result of using their peptide or amine co-transmitters. These same neurons commonly also have fast, ionotropic actions on at least some target neurons. In the STNS, projection neurons regulate the activities of the STG motor circuits (Nusbaum and Beenhakker 2002; Marder et al. 2005; Marder and Bucher 2007), but in contrast to classical ‘command neurons’, which are required to be both necessary and sufficient to elicit a behavior (Kupfermann and Weiss 1978; Edwards et al. 1999), multiple projection neurons appear to determine the activity of the STG circuits (Combes et al. 1999; Blitz et al. 2004; Hedrich et al. 2009). This may well be because CPGs generate continuous rhythmic activity and thus seem particularly unsuitable for being controlled by a single command neuron. For example, MPN accelerates the pyloric rhythm, and can elicit a rhythm if none was present before, but it is not necessary to generate the rhythm (Nusbaum and Marder 1989a, b). Yet, one can argue that each projection neuron causes distinct modifications of the motor pattern (Nusbaum and Beenhakker 2002) and that they act as command neurons that elicit a particular version of a motor pattern.
In principle, the actions of an extrinsic neuromodulatory neuron could largely be mimicked by other modulatory neurons that release the same neuromodulator if the modulator acts diffusely and is removed slowly. However, the proctolinergic neurons MPN, MCN1 and MCN7 elicit distinct types of rhythms (Nusbaum et al. 2001; Wood and Nusbaum 2002), even in conditions where differences in their co-transmitter complement are experimentally reduced. This results in part from a difference in the strength of the proctolin actions of these neurons onto the same pyloric neurons and is partly due to a differential regulation of proctolin by extracellular peptidase activity in the STG neuropil (Nusbaum 2002; Wood and Nusbaum 2002). This differential regulation of neuromodulator actions released from different projection neurons contradicts the hypothesis that bath application of neuromodulators is equivalent to activating the relevant modulatory neuron. Besides the fact that modulatory neurons typically contain more than one neurotransmitter, the response of the target neurons may desensitize during bath applications (von Bohlen und Halbach and Dermietzel 2006). In addition, the activity of some descending projection neurons is shaped by synaptic connections from the CPGs, resulting in transient transmitter release. MCN1, for example, can either be tonically active or its activity is gated by ascending feedback from the pyloric circuit (Fig. 4b; Wood et al. 2004; Blitz and Nusbaum 2008). Both MCN1 activity patterns excite the gastric mill CPG (example shown Fig. 4a), but the resulting motor activity differs when tonic and rhythmic MCN1 are compared (Wood et al. 2004). In addition, MCN1 transmitter release in the STG is locally regulated by a gastric mill CPG neuron LG (lateral gastric neuron), via presynaptic inhibition and an electrical synapse, leading to a complex pattern of activity in the MCN1 STG terminals (Fig. 4c; Coleman et al. 1995) and a correspondingly complex release of peptide modulators from MCN1. Furthermore, MCN1 co-transmitter release appears to be diminished by GABAergic crosstalk at the axon terminal from its contralateral counterpart (Stein et al. 2007). Despite the fact that neuromodulator actions are not always effectively mimicked by bath application, application of individual neuromodulators has provided helpful insights into the actions of hormonal release of neuromodulators and their synaptic and cellular actions. It was these experiments that introduced the concept of multifunctional pattern generating circuits.
Interactions of MCN1 and STG circuits. a Tonic MCN1 stimulation elicits a gastric mill rhythm and excites the pyloric rhythm (adapted from Stein et al. 2007). b MCN1 spike activity can be gated by feedback from the pyloric pacemaker ensemble (arrows; W. Stein, unpublished). c The MCN1 terminal is electrically coupled to LG and it receives presynaptic inhibition from it (arrow; W. Stein, unpublished). d Most gastric mill neurons respond to application of more of the MCN1 co-transmitters than to their release from MCN1. Left distribution of receptors for CabTRP Ia, GABA and proctolin in the gastric mill network and the MCN1 terminal. Right response of gastric mill neurons and the MCN1 terminal to release of CabTRP Ia, GABA and proctolin from MCN1. Gray sections no response to the indicated transmitter, color positive response to the indicated transmitter. Adapted from Stein et al. (2007)
Recent investigations on the gastric mill circuit indicate that not necessarily all neurons in a network will respond to the release of the adequate transmitter, despite possessing receptors for that signaling molecule. The DG neuron, for example, has receptors for both MCN1 peptide co-transmitters (CabTRP Ia, proctolin), and it is activated by MCN1 stimulation. However, the DG excitation is not proctolin-dependent as it is exclusively mediated by CabTRP Ia (Fig. 4d; Stein et al. 2007). Similarly, the gastric mill CPG neuron LG possesses receptors for the MCN1 co-transmitters GABA and CabTRP Ia, and consistently responds to focal GABA application of both (Swensen et al. 2000; Stein et al. 2007). Yet, MCN1 excites LG only via CabTRP Ia (Wood et al. 2000; Stein et al. 2007). This unmatched response to GABA may be caused by a spatial segregation of the GABA receptors, or by presynaptic segregation of GABA release sites of MCN1.
It is not clear why MCN1 uses only the peptide CabTRP Ia to influence the LG neuron, while its peptidergic excitation of most pyloric neurons involves both proctolin and CabTRP Ia (Swensen and Marder 2000; Wood et al. 2000; Wood and Nusbaum 2002; Stein et al. 2007). This divergence may relate to the response of the gastric mill circuit to different projection neurons. For example, MPN and MCN1 share the co-transmitters GABA and proctolin (Nusbaum and Marder 1989a; Blitz et al. 1999) and both excite the pyloric rhythm (Blitz et al. 1999; Wood and Nusbaum 2002). Yet, while MCN1 stimulation elicits a gastric mill rhythm, MPN stimulation suppresses it (Blitz and Nusbaum 1997), a fact mainly attributed to its GABAergic inhibition of CoG projection neurons (Blitz and Nusbaum 1999). If proctolin release from MCN1 excited LG, then MPN would likely have a similar influence on LG and would therefore activate the gastric mill rhythm rather than suppress it. This partly results from the fact that neurally released peptides often diffuse over relatively long distances (Jan and Jan 1982; Burnstock 2004; Seal and Edwards 2006), and that these two projection neurons share proctolinergic actions on many STG neurons (Nusbaum and Marder 1989b; Blitz et al. 1999; Wood et al. 2000; Wood and Nusbaum 2002). Clearly, there is no single description for the mechanisms used by different neuromodulators to affect the same neurons.
Sensory neurons are both sources and targets of neuromodulation
Several local mechanosensory and proprioreceptive pathways such as muscle tendon organs and stretch receptors have been demonstrated to affect STG neuron activities via the release of neuromodulators. One set of identified primary sensory neurons in this system is the gastropyloric receptors (GPR1 and 2; Katz et al. 1989; Katz and Harris-Warrick 1989, 1990; Katz 1998; Birmingham 2001; Birmingham et al. 2003; Blitz et al. 2004; Beenhakker et al. 2005; Beenhakker et al. 2007). The GPRs are bilaterally symmetric muscle stretch-sensitive neurons that project centrally and arborize in the STG and the CoGs (Fig. 5a; Katz et al. 1989; Katz and Harris-Warrick 1989, 1990). GPR2 participates in a positive feedback reflex with the gastric mill retractor motor neuron DG. Specifically, it excites DG whose activity causes the muscle to contract that is the functional antagonist of the GPR2-innervated muscle. This contraction stretches the muscle innervated by GPR2 and thus activates GPR2. GPR2 then feeds back to the STG where, among other actions, it elicits a burst of spike activity in DG. In C. borealis, the GPRs contain acetylcholine, allatostatin and serotonin (Katz and Harris-Warrick 1989; Skiebe and Schneider 1994). In fact, the GPR cells are the only neuronal source of serotonin in the STG. Their modulatory actions are manifold: (1) the responses of the pyloric network to GPR depends on the activation of pharmacologically distinct serotonin receptors (Zhang and Harris-Warrick 1994; Clark et al. 2004; Spitzer et al. 2008a; Spitzer et al. 2008b) and includes a long-lasting excitation of the pyloric pacemakers and changes in the pyloric cycle frequency and phase relationships (Katz and Harris-Warrick 1989; Katz 1998). (2) GPR2 excites the gastric mill neuron DG via fast nicotinic EPSPs and serotonergic modulation. The serotonin action alters the DG intrinsic properties, enabling DG to generate a plateau potential (Fig. 5b) that is triggered by the cholinergic depolarization (Katz and Harris-Warrick 1989; Kiehn and Harris-Warrick 1992a, b; Zhang and Harris-Warrick 1995; Zhang et al. 1995). (3) GPR2 also influences modulatory projection neurons in the CoGs that innervate the STG (Fig. 5c, d). Most prominently, it causes a long-lasting activation of MCN1 (Fig. 5c) and CPN2 (Fig. 5d), which, in turn, cause a prolonged alteration of the STG motor patterns (Fig. 5e; Blitz et al. 2004).
Sensory neurons in the STNS. a Location and axonal projection of the muscle stretch receptor GPR2, the muscle tendon organ anterior gastric receptor (AGR) and of the mechanosensitive ventral cardiac neurons (VCN) in Cancer crabs. The GPR2 soma is located in the gastro-pyloric nerve (gpn) and that of AGR in the STG, posterior to the motor neurons. The ventral cardiac neurons are activated by pressure application to the cardiac gutter (Beenhakker et al. 2004). Schematics of ventral cardiac neurons adapted from Beenhakker et al. (2004). AGR and GPR pictures: W. Stein, unpublished. Scale bars AGR 90 μm, GPR 100 μm, ventral cardiac neurons: 20 μm. cv3 posterior inferior cardiac muscle. b GPR exerts modulatory actions on DG. It enables it to generate plateau potentials. Injection of short current pulses does not elicit plateauing (arrows) unless GPR was active beforehand. Adapted from Katz (1998). c GPR elicits long-lasting activity in the projection neuron MCN1. d. GPR causes strong firing in CPN2 that outlasts the stimulus. c, d Adapted from Blitz et al. (2004). e When stimulated rhythmically, GPR elicits a gastric mill rhythm and modulates the pyloric rhythm, partly due to its actions on MCN1. Extracellular recordings of pyloric rhythm on lvn and gastric mill rhythm on lgn and dgn. In addition, MCN1 and GM are shown. Courtesy of N. Daur (Ulm University). f AGR possesses a spike initiation zone in the central section of its axon. A combination of original recording and plot of instantaneous firing frequency is shown. Below when AGR performs sensory functions (left, saline), action potentials are generated in the periphery and are thus first seen on the dgn. When octopamine is applied to the axon, spikes are generated centrally and propagate both ortho- and antidromically (adapted from Daur et al. 2009)
One early idea for the function of the GPR co-transmitters serotonin and acetylcholine was that GPR also released them onto the muscles, which would give GPR a motor function in addition to its sensory function. However, no such effect appears to be present (Katz et al. 1989). Yet, a release at the afferent terminals may affect sensory coding in GPR, because the responsiveness of GPR to muscle stretch is modulated by the co-transmitters it contains (Birmingham et al. 2003). The GPR neurons demonstrate that modulatory actions may not only arise from the CNS, but also from primary sensory receptors in the periphery. Additionally, these can evoke even long-lasting responses from motor circuits via their modulatory excitation of other modulatory inputs to those circuits. At first glance it appears counter-intuitive for a sensory neuron providing cycle-by-cycle feedback to a rhythmic motor circuit to also exert neuromodulatory effects. Yet, sensory receptors may be multifunctional. For example, they commonly provide timing cues and adapt the motor pattern accordingly with their fast ionotropic actions. Additionally, they may contribute to the initiation and maintenance of rhythmic motor patterns and change the state of the motor system such that it responds differently to other inputs (Daur et al. 2009).
Sensory neurons not only have modulatory actions, they are also targeted by neuromodulators. For example, the mode of activity of the anterior gastric receptor, a muscle tendon organ (Fig. 5a; Combes et al. 1997; Smarandache and Stein 2007), can be switched from encoding cycle-by-cycle muscle movements to detecting average levels of muscle tension by neuromodulator application (Birmingham et al. 1999). Modulation thus enables sensory neurons to encode different sorts of stimuli and to alter the relationship between sensory stimulus and the resulting spike activity. This leads to an ambiguity in the information content of a particular spike train such that two different sensory stimuli could result in very similar sensory activities if encoded in different neuromodulatory states. Moreover, the spike activity of sensory neurons may be altered on its way to the target neurons. The anterior gastric receptor possesses at least one additional spike initiation zone in a central part of its axon that is spatially distant from the innervated muscle (Smarandache and Stein 2007). This spike initiation zone cannot contribute to the sensory function, yet it is responsible for generating the conspicuous tonic activity present in this proprioreceptor. In fact, the central spike initiation zone is under neuromodulatory control (Fig. 5f; Daur et al. 2009): octopamine increases the firing rate of this zone and elicits action potentials that travel both orthodromically to the postsynaptic neurons and antidromically toward the periphery (Fig. 5f). Consequently, they may interfere with the spike activity generated in the periphery or even alter the sensory sensitivity. Furthermore, neuromodulators may affect the temporal precision of sensory spiking, i.e. change the jitter in spike timing, as shown for GPR and allatostatin (Billimoria et al. 2006).
Undoubtedly, ambiguity in spike activity is present in many, if not all, sensory neurons. It will be interesting to see how this issue is handled by the CNS and how it will be addressed by future studies.
Neuromodulators have diverse actions on the motor circuits
The actions of neuromodulators on a CPG network are highly distributed throughout the network. One conclusion from the studies on neuromodulation is that the activity phenotype of neuronal networks is state-dependent and a population response of all of the involved neurons and synapses. The critical network sites for modulation, however, are often unknown, but this fundamental question can be addressed in the stomatogastric system (e.g., Harris-Warrick et al. 1998). Many different types of neuromodulators, including small-molecule, gaseous and peptide neurotransmitters affect the STG circuits (Marder and Bucher 2007). Each neuromodulator and each neuromodulatory neuron can evoke characteristic and distinct types of rhythms and cause changes in rhythm frequency, the activity of each network neuron and its phase relationship (Marder and Weimann 1992). The STG provided evidence that all modulatory actions that affect synaptic strengths and excitability in the network are important for a given motor output and critically determine motor activity (Harris-Warrick et al. 1998; Nusbaum and Beenhakker 2002; Marder and Bucher 2007).
The presence of so many neuromodulators in this system has also provided insight into several principles of neuromodulator actions: (A) most neuromodulators act through second messenger cascades to alter ionic conductances available in their target cells (Hempel et al. 1996). As a consequence, cells may lose or gain autogenic capabilities such as postinhibitory rebound or plateau potentials and respond differently to synaptic input (Marder and Calabrese 1996; Marder and Thirumalai 2002). In fact, they may express additional, ectopic spike initiation zones that generate action potentials independent of the synaptic actions occurring in the ganglia (Fig. 6a; Meyrand et al. 1992; Bucher et al. 2003; Goaillard et al. 2004; Le et al. 2006; Ballo and Bucher 2009; Daur et al. 2009). (B) Multiple modulatory substances can target the same neuron (Fig. 6b; Swensen and Marder 2001; Nusbaum and Beenhakker 2002; Marder et al. 2005; Stein et al. 2007), and (C) different neurons within the same network can respond differently to the same neuromodulator (Fig. 6c; Zhang and Harris-Warrick 1994; Harris-Warrick et al. 1998; Katz 1998; Swensen et al. 2000; Stein et al. 2007). The latter can result from distinct second messenger pathways activated by modulatory substances (Spitzer et al. 2008a). Such pathways may be associated with signal amplification in a particular cell, but not in others. Similarly, the same second messenger molecule may be linked to other signaling pathways and thus have divergent effects on several conductances. On the other hand, different receptors may activate the same second messenger pathway such that the actions of different modulatory substances converge onto the same signaling pathway (Swensen and Marder 2000, 2001).
Modulator actions on STG circuit neurons. a Dopamine elicits action potentials at an extrasomatic spike initiation zone in the axon of PD (left). These action potentials (arrows) travel to the soma where they can interfere with the burst activity of PD. Right in dopamine, PD spikes are elicited in the lvn. Adapted from Bucher et al. (2003). b The same neuron can respond to several modulators. Red LP response to the hormone crustacean cardioactive peptide (CCAP). Green response to proctolin. Adapted from Swensen and Marder (2000). c Different neurons of the same network can respond differently to the same modulator. Top LG response to GABA application. Bottom response of Interneuron 1. Adapted from Stein et al. (2007). d Several peptide modulators converge onto the same ionic current (I MIC), shown for crustacean cardioactive peptide and proctolin (left). Middle and right distribution of proctolin and crustacean cardioactive peptide receptors, respectively, in pyloric neurons. Adapted from Swensen and Marder (2000)
The stomatogastric system has provided the opportunity to study the converging and diverging actions of neuromodulators. For example, serotonin released from the GPR neurons has distinct effects on different stomatogastric neurons owing to its differential actions on multiple ion currents and multiple types of receptors (summarized in Katz 1998). In contrast, several neuropeptides and muscarinic agonists including proctolin, crustacean cardioactive peptide, pilocarpine and FLRFamide-related peptides converge onto a single voltage-gated inward current (“neuromodulator-induced current” (I MIC); Fig. 6d; Swensen and Marder 2000), which acts as a pacemaker current. Yet, each pyloric neuron is directly targeted by several of these modulators and, when isolated from the network, responds similarly when they are bath applied separately and isolated from the network. Thus, the actions of several modulators converge on the cellular and current levels. Despite this convergence, these modulators elicit different motor patterns in the intact network. This is due to the differential distribution of peptide receptors on each network neuron (Fig. 6d). The same is true for the biogenic amines dopamine, serotonin and octopamine (Harris-Warrick et al. 1992; Harris-Warrick et al. 1998). Postsynaptic receptor distribution is thus one of the factors that determine which neurons participate in a motor pattern when neuromodulators are released. This was elegantly confirmed by Swensen and Marder (2001), who transformed the pyloric rhythm elicited by crustacean cardioactive peptide into the proctolin-configured pyloric rhythm during continued bath application of crustacean cardioactive peptide. This was done by using the dynamic clamp method to selectively inject the peptide-activated current into those pyloric neurons that respond to proctolin, but not to crustacean cardioactive peptide.
The rich modulation in the STG appears to pose a profound design problem, at least from the observer’s point of view: how can the pyloric circuit generate a stable rhythm, given the many possibilities to alter circuit parameters and the numerous modulatory substances present at the same time, and how can circuit activity be regulated? These are difficult questions to answer, but they have been addressed in the STNS (see also Marder and Bucher 2005). The actions of the peptide proctolin have shed light on some of the mechanisms used by the nervous system to counteract the effects of multiple modulators. The inward current activated by proctolin (I MIC; Fig. 6d) is also activated by several other modulators (Swensen and Marder 2000). One of the consequences of this convergence, at the current level, is that a modulator can saturate the neuronal response and occlude the actions of others. This ‘ceiling effect’ is one of the cellular mechanisms of ‘state-dependent neuromodulation’ during which the effects of a neuromodulator depend on the initial state of the system when the modulator is applied. In the case of proctolin, a ceiling effect does occur for the pyloric cycle frequency, which does not exceed a maximum of about 1 Hz with increasing proctolin concentrations (Nusbaum and Marder 1989b). Adding a second modulator, for example CabTRP Ia, does not further increase the rhythm frequency (Wood et al. 2000; Wood and Nusbaum 2002), although in control conditions the rhythm can be driven to up to twice this frequency. It is unclear, however, if and to what extend multiple modulators counteract each other’s synaptic or excitability effects.
Another mechanism that can limit the actions of coactive modulators is inherent in the voltage-dependence of the I MIC. Specifically, I MIC amplitude is small near the resting potential and increases with depolarization, but it peaks at membrane potentials that are more hyperpolarized than those reached during action potential generation (see Fig. 6d; Swensen and Marder 2000). At more depolarized potentials, the current amplitude decreases again. This inverted bell-like shaped voltage-dependence is beneficial for pacemaker neurons because, in contrast to the actions of conventional excitatory neurotransmitters, the amplitude of the slow wave in an oscillatory neuron will increase without the oscillatory pattern being lost to tonic firing. Hence, the voltage-dependence of IMIC keeps the membrane potential in a dynamic range and thus allows a control of oscillation frequency and amplitude without exhausting the response of the cell. This also prevents instabilities in the oscillations of the pyloric pacemakers that would otherwise occur due to tonically depolarized membrane potentials.
Moreover, an excessive modulation of the network may be prevented by the gating of sensory information that activates modulatory neurons. For example, the stretch receptor GPR and the mechanoreceptive ventral cardiac neurons both activate the projection neuron MCN1 when stimulated separately (Blitz et al. 2004). Yet, when both are activated simultaneously, the GPR excitation of MCN1 is absent (Beenhakker et al. 2007). This gating effect occurs within the CoGs and is not a result of a ceiling effect on firing frequency. While the underlying cellular and synaptic mechanisms remain to be determined, the gating effect limits the excitation that MCN1 receives when several sensory pathways are active simultaneously. It thus prevents an excessive modulation of the motor circuits.
Neuromodulators affect electrical and chemical synapses and change synaptic gain and dynamics
Neuromodulators not only affect the cellular properties of the circuit neurons, they also directly affect electrical and chemical synaptic transmission (Marder et al. 1997; Ayali et al. 1998; Harris-Warrick et al. 1998; Ayali and Harris-Warrick 1999; Thirumalai et al. 2006). This emphasizes the need to examine the whole circuit, in addition to the parts, and remind us that a circuit diagram, as the one shown in Fig. 2a, is like a roadmap: it shows the streets, but not the traffic. In the lobster Panulirus interruptus, all six electrical synapses in the pyloric network are affected by dopamine, serotonin and octopamine. Johnson et al. (1993, 1994) showed that each synapse is specifically altered by these modulators and that each modulator causes different effects. For example, the anterior burster neuron AB is coupled via a non-rectifying electrical synapse to the pyloric dilator neuron PD, and together they build the pacemaker ensemble that drives the pyloric rhythm (Fig. 2a). Dopamine enhances the coupling strength of this electrical synapse, but only in the PD to AB direction. In the opposite direction, it weakens the synapse, essentially creating a rectifying electrical synapse. Serotonin, on the other hand, enhances the synapse in PD to AB direction, but has no effect on the opposite direction.
Chemical synapses are also targeted by neuromodulators (Dickinson et al. 1990; Harris-Warrick et al. 1998; Thirumalai et al. 2006), and the actions of dopamine have been studied in particular detail. Every synapse in the pyloric network of the spiny lobster has been shown to be affected by dopamine, but in different directions and to different extents (summary: Harris-Warrick et al. 1998). While transmission at some synapses is strongly reduced, which effectively inactivates these synapses, transmission at others is enhanced or only functional in the presence of dopamine. An example for the latter is the synaptic connection between the pyloric constrictor neurons and the LP neuron, which requires dopamine (or other modulators) to be functional. In contrast, the PD output synapses fall silent when dopamine is present. In the pyloric network, neurons interact by both, spike-mediated and graded synaptic transmission (Graubard et al. 1980, 1983; Hartline and Graubard 1992). In the latter, transmitter release can even occur at the resting potential and typically depends on the membrane potential. Interestingly, dopamine can even have different effects on spike-mediated and graded transmission at the same synapse. It strengthens the LP to PD graded transmission, but at the same time diminishes spike-evoked transmission (Ayali et al. 1998). The molecular mechanisms underlying this differential modulation are unknown, but it has been shown that both presynaptic transmitter release and postsynaptic response can be modulated by dopamine (Harris-Warrick et al. 1998).
It is usually assumed that changes in synaptic strength that are caused by the effects of neuromodulators, for example, will have important functional consequences for the circuit. However, Thirumalai et al. (2006) demonstrate that this may not necessarily be true in all cases: LP is the only circuit neuron in the pyloric network that provides feedback to the pacemakers (Fig. 2a). This inhibitory synapse is potentiated by the peptide red pigment concentrating hormone (Fig. 7a; Thirumalai et al. 2006). Surprisingly, this synaptic enhancement does not alter the pyloric rhythm in the way predicted from the circuit diagram (Fig. 2a). Increased feedback inhibition to the pyloric pacemaker ensemble was anticipated to decrease cycle frequency. Instead, overall cycle frequency and the phase relationship of the neurons were relatively unchanged (Thirumalai et al. 2006). Apparently, the timing and duration of the synaptic input from LP was such that it failed to phase-advance or phase-delay the next burst of the pacemakers, a fact obvious from their phase-response curves (Prinz et al. 2003b). What, then, was the effect of the synaptic potentiation? One explanation might be that rather than altering the pyloric rhythm, the strengthened LP synapse stabilized the rhythm (Mamiya and Nadim 2004). If the synaptic inhibition occurred earlier or later in the cycle, then it would likely have advanced or delayed the start of the next cycle and thereby altered the cycle frequency. Thus, when the rhythm frequency changes, it would have advanced or delayed the start of the next cycle accordingly and thereby moved cycle frequency back toward its original frequency. The neuromodulatory control of synaptic strength may therefore, in this example, tend to stabilize ongoing network performance.
Neuromodulators affect synapses and muscles. a Synapses. Red pigment concentrating hormone (RPCH) enhances the LP to PD synapse. In saline (top) there are no obvious IPSP visible in PD. In contrast, in red pigment concentrating hormone (below), distinct IPSPs can be seen. This effect is mediated by an increase in synaptic efficacy (top right; voltage-clamp recording of LP). Bottom the pyloric pattern is not strongly affected by the synaptic potentiation. Adapted from Thirumalai et al. (2006). b Muscles. Modulators can either enhance or diminish muscle contractions. The example shows this for the gastric gm4 muscle. c Neuromuscular junction. In gm4 muscles, serotonin has little effect on the amplitude of the excitatory junction potential at motoneuronal firing frequencies of 5 Hz, but has a much more pronounced effect at 10 Hz. In contrast, the junction potentials in gm6 increase at 5 Hz, but show no marked difference at 10 Hz. b, c Adapted from Jorge-Rivera et al. (1998)
Neuromodulation alters muscle characteristics and response
In the crab, the STG motor circuits innervate 36 pairs of bilateral muscles in the gastric mill and pyloric regions of the foregut (Maynard and Dando 1974). Thirty of these muscles are considered intrinsic because they are confined to the stomach wall and six are extrinsic muscles because they attach the stomach to the carapace. The response of many of these muscles to neuronal input is altered by neuromodulators, for example from the FLRFamide family (Meyrand and Marder 1991; Jorge-Rivera and Marder 1996; Jorge-Rivera and Marder 1997; Jorge-Rivera et al. 1998). In fact, an extensive study by Jorge-Rivera et al. (1998) demonstrated that the gastric mill gm4 muscle activity is modulated by as many different substances as are likely to be released into the hemolymph (Fig. 7b; Christie et al. 1995; Skiebe 2001), including several peptides and amines that are found in pericardial organs and other neurosecretory structures. Since most motor neurons of the adult STG do not contain any modulatory substances other than the neurotransmitters acetylcholine or glutamate (Lingle 1980; Hooper et al. 1986; Skiebe 2001; with the possible exception of the lateral posterior gastric neurons; D. Bucher, personal communication) and the muscles show a sensitivity to rather small modulator concentrations, it appears that the muscles only detect hormonally released modulators.
The muscle responses depend on modulator concentration (Meyrand and Marder 1991), and both the amplitude of muscle force and its temporal dynamics are affected. For example, TNRNFLRFamide and serotonin both increase the force amplitude of the gm4 muscle, but have different effects on the gm4 dependence on motoneuronal firing frequencies. Specifically, while TNRNFLRFamide more effectively potentiates gm4 contractions elicited at 10 Hz than at 40 Hz, serotonin is more effective at 40 Hz than at 10 Hz (Jorge-Rivera et al. 1998). Thus, the effects of modulators not only depend on modulator concentration, but also on the prevailing motor activity. Given that most modulators are found in the hemolymph and in input fibers to the STG (such as the CoG projection neurons), the modulation of motor circuits and muscles must be regulated conjointly. The modulation of muscle properties also illustrates that the modulatory system can change the gain between a motor circuit and its effector system.
It is worth noting that some of the modulatory effects on the muscles are mediated via a modulation of the neuromuscular junction, i.e. the modulation of a synapse rather than intrinsic muscle properties (Dudel and Kuffler 1961; Atwood 1976; Atwood and Wojtowicz 1986; Katz et al. 1993; Jorge-Rivera and Marder 1996; Sen et al. 1996; Jorge-Rivera and Marder 1997; Qian and Delaney 1997; Worden et al. 1997; Jorge-Rivera et al. 1998; Msghina et al. 1998; Morris and Hooper 2001; Stein et al. 2006). For example, in the case of the gm4 and gm6 muscles, serotonin increases the synaptic strength by almost 50%, most likely due to presynaptic effects (Fig. 7c; Jorge-Rivera et al. 1998).
Interestingly, serotonin decreases facilitation at both synapses, leading to a steeper onset but weaker increase of the electrical response (Jorge-Rivera et al. 1998). This has consequences for muscle force production. With low initial transmitter release, the muscle force reached during a burst of motoneuronal activity will mainly depend on facilitation and thus on motor neuron firing frequency and duration. In contrast, in serotonin the initial synaptic strength is high and the effects of facilitation are small, but summation will have a considerable effect. The generated force should thus appear earlier and depend less on the duration of the motor activity. This illustrates that the modulatory system can bias the timing of muscle force production for particular motor patterns. The effects of neuromodulators on the muscles and on the motor circuits may even operate coordinately. For example, hormonally released crustacean cardioactive peptide affects both, the pyloric LP neuron and LP-innervated muscles (Weimann et al. 1997). The changes in the motor pattern caused by the excitatory effects of crustacean cardioactive peptide on LP produce significant changes in LP-innervated muscle movement. These movements are additionally potentiated by the effects of crustacean cardioactive peptide on the neuromuscular junction. Thus, motor neuron firing and the gain control of the neuromuscular junctions can operate in conjunction in response to hormonally released neuromodulators.
Homeostasis of neuronal activity and the potential influence of the neuromodulatory system
Rhythmic motor patterns drive behaviors that serve basic and frequently vital functions of the body, such as respiration, locomotion, heartbeat and digestion (Bässler and Büschges 1998; Cymbalyuk et al. 2002; Büschges et al. 2004; Ramirez and Viemari 2005; Marder and Bucher 2007). While a certain flexibility of neural activity is required to adapt to changing conditions, instability or disruption of rhythmic activity is potentially a threat to the survival of the animal.
In the STG, the maximum conductance levels of ionic currents in pyloric CPG cells are highly variable (Liu et al. 1998; Golowasch et al. 1999a; Golowasch et al. 1999b; Schulz et al. 2006), despite the fact that stable activity patterns are generated over long durations and changing conditions (Bucher et al. 2005). Modeling studies suggest that the high conductance variability in identified neurons can allow them to have different solutions for creating stable activity patterns (Prinz et al. 2003a, 2004; Taylor et al. 2009). Furthermore, the pyloric CPG can restore rhythmic activity after disruptive perturbations and injury (Fig. 8a; Thoby-Brisson and Simmers 1998; Luther et al. 2003; Saghatelyan et al. 2005; Davis 2006), an event attributed to a re-arrangement of intrinsic and synaptic properties (Thoby-Brisson and Simmers 2002; Faumont et al. 2005; Gansert et al. 2007).
Homeostasis in the STNS and modulation of the modulatory system. a Schematics showing the effects of proctolin on ionic currents during homeostatic processes. With intact neuromodulatory input from the CoG projection neurons (left), the pyloric CPG generates a rapid triphasic pattern (pyl.). In the PD neurons, the maximum conductance levels of I HTK and I A and I HTK and I h are co-regulated when measured across many animals (below). When the stomatogastric nerve (stn) is transected (decentralization; second from left), the pyloric rhythm stops and most pyloric neurons generate tonic activity. The co-regulation of I HTK and I A and I HTK and I h is lost. After 4 days (third from left), the pyloric rhythm has recovered, but no current co-regulation is present. In contrast, when decentralization occurs and proctolin is applied to substitute the absent neuromodulator input (right), current co-regulation is maintained even when circuit activity is completely suppressed by Tetrodotoxin. Adapted from Khorkova and Golowasch (2007). b Schematic representation of brain pathways that may regulate the modulatory system in the CoGs. The brain is connected to the CoGs via the circumesophageal commissures (CoCs) and the inferior ventricular nerve (ivn). Furthermore, projection neuron activity can be altered when the axons pass the junction of the superior esophageal and stomatogastric nerves (arrow)
To maintain functional activity patterns, regulatory mechanisms must be in place to keep cellular and network parameters within boundaries suitable to support the appropriate output. These mechanisms can be activity-dependent and triggered by external factors or by internal cellular changes of the cells, as occurs for example during development. Such mechanisms exist at the synaptic (Turrigiano 1999; Turrigiano and Nelson 2004), neuronal (Hong and Lnenicka 1995; Galante et al. 2001; Davis 2006) and network levels (Thoby-Brisson and Simmers 1998; Golowasch et al. 1999b; Gonzalez-Islas and Wenner 2006). For example, the maximum conductance levels of several ionic currents can be simultaneously regulated as a consequence of neuronal activity changes (Linsdell and Moody 1994; Turrigiano et al. 1995; Desai et al. 1999; Tobin and Calabrese 2005; Haedo and Golowasch 2006).
In addition, activity-independent homeostatic mechanisms act to maintain network output. MacLean et al. (2003) showed, for instance, that the expression of certain ionic conductances is co-regulated independently of network activity. Specifically, overexpression of the outward A-type K+ current (I A) by mRNA injection, which by itself should disrupt rhythmic activity, resulted in a compensatory increase of the hyperpolarization-activated inward current (I h) which led to the preservation of network activity (MacLean et al. 2003).
Neuromodulators are important regulators of cellular and network activity and can influence the regulation of ion channel activation and expression (DeLorme et al. 1988; Turrigiano et al. 1994; Desai et al. 1999; Haedo and Golowasch 2006). It is thus not surprising that they are also involved in homeostatic processes. A first indication comes from Khorkova and Golowasch (2007), who disabled the neuromodulatory input from the projection neurons to the STG by cutting the stomatogastric nerve (Fig. 8a; decentralization). After nerve transection, rhythmic activity in the STG ceased, but re-appeared in short activity bouts within the following 24 h until a new stable activity level was reached that was comparable to that before decentralization. In parallel, three voltage-gated ionic currents [I A, I h and I HTK (high-threshold potassium current); Khorkova and Golowasch 2007] that were analyzed in the pyloric PD neurons showed a high degree of correlation in their current density levels. Twenty-four hours after decentralization, I h and I A were still tightly correlated, but the I h/I HTK and I A/I HTK pairs were no longer correlated (Fig. 8a). The loss of the ion current co-dependence, as well as the changes in absolute conductance density levels, however, was prevented by a bath applying the neuromodulator proctolin after decentralization. Interestingly, these effects were not activity-dependent, because adding the Na+ channel blocker Tetrodotoxin had no effects on ion current level co-regulation (Khorkova and Golowasch 2007).
Maintaining a coordinated relationship between conductances could effectively reduce global conductance variance and diminish the potential for activity disruption during changes in single conductances. The coordinating mechanisms of current co-regulation, however, are not known. In fact, in most systems, it is even unclear to what extent coordinated expression of ion channels occurs. Yet, the experiments of Khorkova and Golowasch (2007) indicate that besides their actions on the neuronal excitability, neuromodulators have access to the intrinsic regulatory mechanisms that keep cellular parameters within the appropriate boundaries to support a stable motor output. Since the coordination between currents occurs at the transcript level (Schulz et al. 2006), neuromodulators may act on these coordinating mechanisms via the second messenger pathways they activate. The contribution of neuromodulators to homeostatic processes will certainly increase the degrees of freedom that will need to be studied, but can potentially shed further light on the mechanisms involved in the regulation of these processes.
Regulation and modulation of the modulatory system
The neuromodulatory system is a powerful agent for manipulating neural activity, via many different mechanisms. Further, in a rich modulatory environment, many modulatory substances interact. Naturally, the question arises, what regulates and controls the neuromodulatory system? While the connectivity of some projection neurons to their postsynaptic targets in the STG has been studied thoroughly, only few studies have addressed their synaptic input, with the exception of local sensory neurons. The STNS is connected to the brain via the paired circumesophageal commissures and the single inferior ventricular nerve (Figs. 3, 8b). Although the details are yet to be elucidated, these connections enable higher neural centers to control the STG circuits (Powers 1973; Spirito 1975; Fleischer 1981), and this control most likely involves the projection neurons in the CoGs (Kirby and Nusbaum 2007). Each CoG protrudes from one of the paired circumesophageal commissures (Fig. 1b), which are large fiber tracts that connect the brain with the thoracic ganglion. The CoG projection neurons are thus well-positioned to be influenced by neurons projecting from the brain. Indeed, more than 75 neurons project from various locations in the brain to the CoGs, at least some of which are likely to influence the CoG projection neurons (Kirby and Nusbaum 2007). The identity of these neurons has not been determined, but stimulating axons within the circumesophageal commissures can elicit different motor outputs, including tail movements, swimming, walking (Atwood and Wiersma 1967; Bowerman and Larimer 1974a; Bowerman and Larimer 1974b; Reed and Page 1977) and gastric mill rhythms (Blitz et al. 2008).
Several studies have documented the ability of the inferior ventricular neurons, which project from the brain to the CoGs and the STG, to regulate the pyloric and gastric mill rhythms (Dando and Selverston 1972; Russell and Hartline 1981; Sigvardt and Mulloney 1982a, b; Claiborne and Selverston 1984a, b; Cazalets et al. 1987, 1990a, b; Meyrand et al. 1991, 1994; Mulloney and Hall 1991; Tierney et al. 1997; Christie et al. 2004; Faumont et al. 2005; Hedrich and Stein 2008) and their actions involve the CoG projection neurons. In fact, these studies also demonstrate that the modulatory CoG projection neurons may be under modulatory control themselves. For example, the inferior ventricular neurons release an FLRFamide-related peptide and this peptide triggers prolonged bursting in at least some of the CoG projection neurons (Weimann et al. 1993; Christie et al. 2004; Stein, unpublished). In the lobster H. gammarus, the homologs of the inferior ventricular neurons, called the pyloric suppressor neurons, dismantle and reconfigure the STG and CoG circuits (Meyrand et al. 1991, 1994): typically, the gastric mill, pyloric and esophageal circuits generate distinct motor rhythms. Activation of the pyloric suppressor neurons eliminates these rhythms and replaces them with a single, conjoint motor pattern. The elicited motor pattern comprises the activities of subsets of neurons from each of the three circuits. The pyloric suppressor neurons evoke several different, target-specific effects (Faumont et al. 2005): they elicit a long-lasting, voltage-dependent excitation of two gastric mill neurons, a transient hyperpolarization of those gastric mill neurons that do not participate in the conjoint rhythm and a long-lasting gastro-pyloric inhibition of the pyloric pattern generator neurons. The modulatory actions of the pyloric suppressor neurons thus dismantle the gastric mill and pyloric circuits, with some components participating in the conjoint rhythm and other components falling silent.
The activity of modulatory neurons may even be modulated in the axon. Goaillard et al. (2004) show that the projection neuron MCN5 possesses a second spike initiation zone near the junction of the superior esophageal and stomatogastric nerves (Fig. 8b), a location known to contain synapses and neurophil-like varicosities (Marder et al. 1986, 1987; Skiebe and Wollenschlager 2002; Goaillard et al. 2004). The axonal spike initiation zone becomes functional when the biogenic amine octopamine is present, demonstrating that the modulatory system is affected by other modulatory structures. The functional relevance of this metamodulation is still speculative, but could relate to the fact that the octopamine modulation occurs at a location at which both MCN5 axons are affected. While it is generally assumed that bilaterally symmetric neurons such as MCN5 show similar activity, synchronization and simultaneous modulation of their activity is difficult to achieve in the spatially separated commissural ganglia.
In many systems, the activity of descending neurons is also influenced by ascending feedback from the motor system (Gillette et al. 1978; Nusbaum 1986; Arshavsky et al. 1988; Dubuc and Grillner 1989; Cazalets et al. 1990a; Frost and Katz 1996; Ezure and Tanaka 1997; Wood et al. 2004; Stein et al. 2005; Blitz and Nusbaum 2008; Buchanan and Einum 2008). For example, CPGs can provide feedback that imposes rhythmic activity patterns on the projection neurons. While the function of such CPG feedback remains to be determined in most systems, Wood et al. (2004) showed in the STNS the ability of the pyloric circuit to regulate the gastric mill rhythm via such ascending feedback. As a consequence, the presence of rhythmic CPG feedback elicits a distinct gastric mill rhythm that differs from that when CPG feedback is missing. Furthermore, Blitz and Nusbaum (2008) showed that the feedback from the pyloric pacemaker neuron AB to MCN1 is subject to a presynaptic state-dependent regulation, enabling the same descending pathway to elicit distinct motor patterns. Whether this state-dependent regulation of feedback also alters the impact of other incoming signals to projection neurons, for example deriving from higher order or sensory neurons, remains to be determined.
We have barely touched the universe of mechanisms that contribute to the electrical activity of the projection neurons. The possibility that these neurons themselves may be under modulatory control and hence may express a similar flexibility as do the STG circuit neurons provides yet another set of mechanisms contributing to the functional flexibility of the nervous system. We now need to explore the behavioral conditions that activate the modulatory systems. Future research is thus likely to involve experiments in which the STNS remains connected with the rest of the CNS, for example, in intact but tethered animals. These studies should, in turn, lead to a better understanding of the principles underlying the control and regulation of the modulatory system and, in the long term, provide insight into how comparable neural circuits operate in the numerically larger and less accessible vertebrate CNS.
Abbreviations
- AB:
-
Anterior burster
- CoG:
-
Commissural ganglion
- CPG:
-
Central pattern generator
- CPN2:
-
Commissural projection neuron 2
- GABA:
-
γ-Aminobutyric acid
- GM:
-
Gastric mill neurons
- GPR:
-
Gastro-pyloric receptor
- I A :
-
A-type current
- Ih :
-
h-type current
- I HTK :
-
High-threshold potassium current
- I MIC :
-
Neuromodulator-induced current
- LG:
-
Lateral gastric neuron
- LP:
-
Lateral pyloric neuron
- MCN:
-
Modulatory commissural neuron
- MPN:
-
Modulatory proctolin neuron
- PD:
-
Pyloric dilator neuron
- STG:
-
Stomatogastric ganglion
- STNS:
-
Stomatogastric nervous system
References
Arshavsky YI, Orlovsky GN, Perret C (1988) Activity of rubrospinal neurons during locomotion and scratching in the cat. Behav Brain Res 28:193–199
Atwood HL (1976) Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol 7:291–391
Atwood HL, Wiersma CA (1967) Command interneurons in the crayfish central nervous system. J Exp Biol 46:249–261
Atwood HL, Wojtowicz JM (1986) Short-term and long-term plasticity and physiological differentiation of crustacean motor synapses. Int Rev Neurobiol 28:275–362
Ayali A, Harris-Warrick RM (1999) Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J Neurosci 19:6712–6722
Ayali A, Johnson BR, Harris-Warrick RM (1998) Dopamine modulates graded and spike-evoked synaptic inhibition independently at single synapses in pyloric network of lobster. J Neurophysiol 79:2063–2069
Ballo AW, Bucher D (2009) Complex intrinsic membrane properties and dopamine shape spiking activity in a motor axon. J Neurosci 29:5062–5074
Bässler U, Büschges A (1998) Pattern generation for stick insect walking movements—multisensory control of a locomotor program. Brain Res Rev 27:65–88
Beenhakker MP, Blitz DM, Nusbaum MP (2004) Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol 91:78–91
Beenhakker MP, DeLong ND, Saideman SR, Nadim F, Nusbaum MP (2005) Proprioceptor regulation of motor circuit activity by presynaptic inhibition of a modulatory projection neuron. J Neurosci 25:8794–8806
Beenhakker MP, Kirby MS, Nusbaum MP (2007) Mechanosensory gating of proprioceptor input to modulatory projection neurons. J Neurosci 27:14308–14316
Billimoria CP, Li L, Marder E (2005) Profiling of neuropeptides released at the stomatogastric ganglion of the crab, Cancer borealis with mass spectrometry. J Neurochem 95:191–199
Billimoria CP, DiCaprio RA, Birmingham JT, Abbott LF, Marder E (2006) Neuromodulation of spike-timing precision in sensory neurons. J Neurosci 26:5910–5919
Birmingham JT (2001) Increasing sensor flexibility through neuromodulation. Biol Bull 200:206–210
Birmingham JT, Tauck DL (2003) Neuromodulation in invertebrate sensory systems: from biophysics to behavior. J Exp Biol 206:3541–3546
Birmingham JT, Szuts ZB, Abbott LF, Marder E (1999) Encoding of muscle movement on two time scales by a sensory neuron that switches between spiking and bursting modes. J Neurophysiol 82:2786–2797
Birmingham JT, Billimoria CP, DeKlotz TR, Stewart RA, Marder E (2003) Differential and history-dependent modulation of a stretch receptor in the stomatogastric system of the crab, Cancer borealis. J Neurophysiol 90:3608–3616
Blitz DM, Nusbaum MP (1997) Motor pattern selection via inhibition of parallel pathways. J Neurosci 17:4965–4975
Blitz DM, Nusbaum MP (1999) Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci 19:6774–6783
Blitz DM, Nusbaum MP (2008) State-dependent presynaptic inhibition regulates central pattern generator feedback to descending inputs. J Neurosci 28:9564–9574
Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP (1999) Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci 19:5449–5463
Blitz DM, Beenhakker MP, Nusbaum MP (2004) Different sensory systems share projection neurons but elicit distinct motor patterns. J Neurosci 24:11381–11390
Blitz DM, White RS, Saideman SR, Cook A, Christie AE, Nadim F, Nusbaum MP (2008) A newly identified extrinsic input triggers a distinct gastric mill rhythm via activation of modulatory projection neurons. J Exp Biol 211:1000–1011
Bowerman RF, Larimer JL (1974a) Command fibres in the circumoesophogeal connectives of crayfish. I. Tonic fibres. J Exp Biol 60:95–117
Bowerman RF, Larimer JL (1974b) Command fibres in the circumoesophogeal connectives of crayfish. II. Phasic fibres. J Exp Biol 60:119–134
Branchereau P, Chapron J, Meyrand P (2002) Descending 5-hydroxytryptamine raphe inputs repress the expression of serotonergic neurons and slow the maturation of inhibitory systems in mouse embryonic spinal cord. J Neurosci 22:2598–2606
Buchanan JT, Einum JF (2008) The spinobulbar system in lamprey. Brain Res Rev 57:37–45
Bucher D, Thirumalai V, Marder E (2003) Axonal dopamine receptors activate peripheral spike initiation in a stomatogastric motor neuron. J Neurosci 23:6866–6875
Bucher D, Prinz AA, Marder E (2005) Animal-to-animal variability in motor pattern production in adults and during growth. J Neurosci 25:1611–1619
Burnstock G (2004) Cotransmission. Curr Opin Pharmacol 4:47–52
Büschges A, Ludwar BC, Bucher D, Schmidt J, DiCaprio RA (2004) Synaptic drive contributing to rhythmic activation of motoneurons in the deafferented stick insect walking system. Eur J Neurosci 19:1856–1862
Cantrell AR, Catterall WA (2001) Neuromodulation of Na+ channels: an unexpected form of cellular plasticity. Nat Rev Neurosci 2:397–407
Cape SS, Rehm KJ, Ma M, Marder E, Li L (2008) Mass spectral comparison of the neuropeptide complement of the stomatogastric ganglion and brain in the adult and embryonic lobster, Homarus americanus. J Neurochem 105:690–702
Cazalets JR, Nagy F, Moulins M (1987) Suppressive control of a rhythmic central pattern generator by an identified modulatory neuron in crustacea. Neurosci Lett 81:267–272
Cazalets JR, Nagy F, Moulins M (1990a) Suppressive control of the crustacean pyloric network by a pair of identified interneurons. I. Modulation of the motor pattern. J Neurosci 10:448–457
Cazalets JR, Nagy F, Moulins M (1990b) Suppressive control of the crustacean pyloric network by a pair of identified interneurons. II. Modulation of neuronal properties. J Neurosci 10:458–468
Chen R, Ma M, Hui L, Zhang J, Li L (2009) Measurement of neuropeptides in crustacean hemolymph via MALDI mass spectrometry. J Am Soc Mass Spectrom 20:708–718
Christie AE, Skiebe P, Marder E (1995) Matrix of neuromodulators in neurosecretory structures of the crab Cancer borealis. J Exp Biol 198:2431–2439
Christie AE, Lundquist CT, Nassel DR, Nusbaum MP (1997) Two novel tachykinin-related peptides from the nervous system of the crab Cancer borealis. J Exp Biol 200:2279–2294
Christie AE, Stein W, Quinlan JE, Beenhakker MP, Marder E, Nusbaum MP (2004) Actions of a histaminergic/peptidergic projection neuron on rhythmic motor patterns in the stomatogastric nervous system of the crab Cancer borealis. J Comp Neurol 469:153–169
Christie AE, Stemmler EA, Peguero B, Messinger DI, Provencher HL, Scheerlinck P, Hsu YW, Guiney ME, de la Iglesia HO, Dickinson PS (2006) Identification, physiological actions, and distribution of VYRKPPFNGSIFamide (Val1)-SIFamide) in the stomatogastric nervous system of the American lobster Homarus americanus. J Comp Neurol 496:406–421
Claiborne BJ, Selverston AI (1984a) Histamine as a neurotransmitter in the stomatogastric nervous system of the spiny lobster. J Neurosci 4:708–721
Claiborne BJ, Selverston AI (1984b) Localization of stomatogastric IV neuron cell bodies in lobster brain. J Comp Physiol A 154:27–32
Claiborne BJ, Selverston AI (1987) Functional anatomy and behavior. The crustacean stomatogastric system—a model for the study of central nervous systems. Springer, Berlin, pp 9–27
Clark MC, Dever TE, Dever JJ, Xu P, Rehder V, Sosa MA, Baro DJ (2004) Arthropod 5-HT2 receptors: a neurohormonal receptor in decapod crustaceans that displays agonist independent activity resulting from an evolutionary alteration to the DRY motif. J Neurosci 24:3421–3435
Coleman MJ, Nusbaum MP (1994) Functional consequences of compartmentalization of synaptic input. J Neurosci 14:6544–6552
Coleman MJ, Nusbaum MP, Cournil I, Claiborne BJ (1992) Distribution of modulatory inputs to the stomatogastric ganglion of the crab, Cancer borealis. J Comp Neurol 325:581–594
Coleman MJ, Meyrand P, Nusbaum MP (1995) A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378:502–505
Combes D, Simmers J, Moulins M (1997) Conditional dendritic oscillators in a lobster mechanoreceptor neurone. J Physiol 499(Pt 1):161–177
Combes D, Meyrand P, Simmers J (1999) Motor pattern specification by dual descending pathways to a lobster rhythm-generating network. J Neurosci 19:3610–3619
Cymbalyuk GS, Quentin G, Masino MA, Calabrese RL (2002) Bursting in leech heart interneurons: cell-autonomous and network-based mechanisms. J Neurosci 22:10580–10592
Dagher A, Robbins TW (2009) Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron 61:502–510
Dando MR, Selverston AI (1972) Command fibres from the supra-oesophageal ganglion to the stomatogastric ganglion in Panulirus argus. J Comp Physiol A 78:138–175
Daur N, Nadim F, Stein W (2009) Regulation of motor patterns by the central spike-initiation zone of a sensory neuron. Eur J Neurosci 30:808–822
Davis GW (2006) Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci 29:307–323
DeLorme EM, Rabe CS, McGee R Jr (1988) Regulation of the number of functional voltage-sensitive Ca++ channels on PC12 cells by chronic changes in membrane potential. J Pharmacol Exp Ther 244:838–843
Desai NS, Rutherford LC, Turrigiano GG (1999) Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2:515–520
Dickinson PS (2006) Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol 16:604–614
Dickinson PS, Mecsas C, Marder E (1990) Neuropeptide fusion of two motor-pattern generator circuits. Nature 344:155–158
Dubuc R, Grillner S (1989) The role of spinal cord inputs in modulating the activity of reticulospinal neurons during fictive locomotion in the lamprey. Brain Res 483:196–200
Dudel J, Kuffler SW (1961) Mechanism of facilitation at the crayfish neuromuscular junction. J Physiol 155:530–542
Edwards DH, Heitler WJ, Krasne FB (1999) Fifty years of a command neuron: the neurobiology of escape behavior in the crayfish. Trends Neurosci 22:153–161
Ezure K, Tanaka I (1997) Convergence of central respiratory and locomotor rhythms onto single neurons of the lateral reticular nucleus. Exp Brain Res 113:230–242
Faumont S, Combes D, Meyrand P, Simmers J (2005) Reconfiguration of multiple motor networks by short- and long-term actions of an identified modulatory neuron. Eur J Neurosci 22:2489–2502
Fenelon VS, Kilman V, Meyrand P, Marder E (1999) Sequential developmental acquisition of neuromodulatory inputs to a central pattern-generating network. J Comp Neurol 408:335–351
Fénelon VS, Le Feuvre Y, Meyrand P (2002) Role of modulatory inputs in the ontogeny of neural networks. Springer-Verlag, New York, LLC
Fleischer AG (1981) The effect of eyestalk hormones on the gastric mill in the intact lobster, Panulirus interruptus. J Comp Physiol A 141:363–368
Frost WN, Katz PS (1996) Single neuron control over a complex motor program. Proc Natl Acad Sci USA 93:422–426
Galante M, Avossa D, Rosato-Siri M, Ballerini L (2001) Homeostatic plasticity induced by chronic block of AMPA/kainate receptors modulates the generation of rhythmic bursting in rat spinal cord organotypic cultures. Eur J Neurosci 14:903–917
Gansert J, Golowasch J, Nadim F (2007) Sustained rhythmic activity in gap-junctionally coupled networks of model neurons depends on the diameter of coupled dendrites. J Neurophysiol 98:3450–3460
Gillette R, Kovac MP, Davis WJ (1978) Command neurons in Pleurobranchaea receive synaptic feedback from the motor network they excite. Science 199:798–801
Goaillard JM, Schulz DJ, Kilman VL, Marder E (2004) Octopamine modulates the axons of modulatory projection neurons. J Neurosci 24:7063–7073
Golowasch J, Abbott LF, Marder E (1999a) Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab Cancer borealis. J Neurosci 19:1–5
Golowasch J, Casey M, Abbott LF, Marder E (1999b) Network stability from activity-dependent regulation of neuronal conductances. Neural Comput 11:1079–1096
Gonzalez-Islas C, Wenner P (2006) Spontaneous network activity in the embryonic spinal cord regulates AMPAergic and GABAergic synaptic strength. Neuron 49:563–575
Graubard K, Raper JA, Hartline DK (1980) Graded synaptic transmission between spiking neurons. Proc Natl Acad Sci USA 77:3733–3735
Graubard K, Raper JA, Hartline DK (1983) Graded synaptic transmission between identified spiking neurons. J Neurophysiol 50:508–521
Gu Q (2002) Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111:815–835
Haedo RJ, Golowasch J (2006) Ionic mechanism underlying recovery of rhythmic activity in adult isolated neurons. J Neurophysiol 96:1860–1876
Harris-Warrick RM, Marder E, Selverston AI, Moulins M (1992) Dynamic biological networks: the stomatogastric nervous system. MIT Press, Cambridge
Harris-Warrick RM, Johnson BR, Peck JH, Kloppenburg P, Ayali A, Skarbinski J (1998) Distributed effects of dopamine modulation in the crustacean pyloric network. Ann N Y Acad Sci 860:155–167
Hartline DK, Graubard K (1992) Cellular and synaptic properties in the crustacean stomatogastric nervous system. Dynamic biological networks: the stomatogastric nervous system. MIT Press, Cambridge, pp 31–86
Hartline DK, Maynard DM (1975) Motor patterns in the stomatogastric ganglion of the lobster Panulirus argus. J Exp Biol 62:405–420
Hedrich UB, Stein W (2008) Characterization of a descending pathway: activation and effects on motor patterns in the brachyuran crustacean stomatogastric nervous system. J Exp Biol 211:2624–2637
Hedrich UB, Smarandache CR, Stein W (2009) Differential activation of projection neurons by two sensory pathways contributes to motor pattern selection. J Neurophysiol (epub ahead of print)
Heinzel HG, Weimann JM, Marder E (1993) The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J Neurosci 13:1793–1803
Hempel CM, Vincent P, Adams SR, Tsien RY, Selverston AI (1996) Spatio-temporal dynamics of cyclic AMP signals in an intact neural circuit. Nature 384:166–169
Hong SJ, Lnenicka GA (1995) Activity-dependent reduction in voltage-dependent calcium current in a crayfish motoneuron. J Neurosci 15:3539–3547
Hooper SL (2003) Crustacean stomatogastric system. In: Arbib MA (ed) The handbook of brain theory and neural networks. MIT Press, Cambridge, pp 304–308
Hooper SL, DiCaprio RA (2004) Crustacean motor pattern generator networks. NeuroSignals 13:50–69
Hooper SL, O’Neil MB, Wagner R, Ewer J, Golowasch J, Marder E (1986) The innervation of the pyloric region of the crab, Cancer borealis: homologous muscles in decapod species are differently innervated. J Comp Physiol A 159:227–240
Jan LY, Jan YN (1982) Peptidergic transmission in sympathetic ganglia of the frog. J Physiol 327:219–246
Johnson BR, Peck JH, Harris-Warrick RM (1993) Amine modulation of electrical coupling in the pyloric network of the lobster stomatogastric ganglion. J Comp Physiol A 172:715–732
Johnson BR, Peck JH, Harris-Warrick RM (1994) Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J Comp Physiol A 175:233–249
Jorge-Rivera JC, Marder E (1996) TNRNFLRFamide and SDRNFLRFamide modulate muscles of the stomatogastric system of the crab Cancer borealis. J Comp Physiol A 179:741–751
Jorge-Rivera J, Marder YE (1997) Allatostatin decreases stomatogastric neuromuscular transmission in the crab Cancer borealis. J Exp Biol 200:2937–2946
Jorge-Rivera JC, Sen K, Birmingham JT, Abbott LF, Marder E (1998) Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J Neurophysiol 80:2559–2570
Kaczmarek LK, Levitan IB (1987) Neuromodulation: the biochemical control of neuronal excitability. Oxford University Press, USA
Katz PS (1998) Comparison of extrinsic and intrinsic neuromodulation in two central pattern generator circuits in invertebrates. Exp Physiol 83:281–292
Katz PS, Frost WN (1996) Intrinsic neuromodulation: altering neuronal circuits from within. Trends Neurosci 19:54–61
Katz PS, Harris-Warrick RM (1989) Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. II. Rapid nicotinic and prolonged modulatory effects on neurons in the stomatogastric ganglion. J Neurophysiol 62:571–581
Katz PS, Harris-Warrick RM (1990) Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. J Neurosci 10:1495–1512
Katz PS, Eigg MH, Harris-Warrick RM (1989) Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol 62:558–570
Katz PS, Kirk MD, Govind CK (1993) Facilitation and depression at different branches of the same motor axon: evidence for presynaptic differences in release. J Neurosci 13:3075–3089
Khorkova O, Golowasch J (2007) Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci 27:8709–8718
Kiehn O, Harris-Warrick RM (1992a) 5-HT modulation of hyperpolarization-activated inward current and calcium-dependent outward current in a crustacean motor neuron. J Neurophysiol 68:496–508
Kiehn O, Harris-Warrick RM (1992b) Serotonergic stretch receptors induce plateau properties in a crustacean motor neuron by a dual-conductance mechanism. J Neurophysiol 68:485–495
Kilman V, Fenelon VS, Richards KS, Thirumalai V, Meyrand P, Marder E (1999) Sequential developmental acquisition of cotransmitters in identified sensory neurons of the stomatogastric nervous system of the lobsters, Homarus americanus and Homarus gammarus. J Comp Neurol 408:318–334
Kirby MS, Nusbaum MP (2007) Central nervous system projections to and from the commissural ganglion of the crab Cancer borealis. Cell Tissue Res 328:625–637
Knable MB, Weinberger DR (1997) Dopamine, the prefrontal cortex and schizophrenia. J Psychopharmacol 11:123–131
Krichmar JL (2008) The neuromodulatory system: a framework for survival and adaptive behavior in a challenging world. Adapt Behav 16:385–399
Kristan WB Jr, Calabrese RL, Friesen WO (2005) Neuronal control of leech behavior. Prog Neurobiol 76:279–327
Kupfermann I (1979) Modulatory actions of neurotransmitters. Annu Rev Neurosci 2:447–465
Kupfermann I, Weiss KR (1978) The command neuron concept. Behav Brain Sci 1:3–39
Le T, Verley DR, Goaillard JM, Messinger DI, Christie AE, Birmingham JT (2006) Bistable behavior originating in the axon of a crustacean motor neuron. J Neurophysiol 95:1356–1368
Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E (2002) Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J Comp Neurol 444:227–244
Li L, Kelley WP, Billimoria CP, Christie AE, Pulver SR, Sweedler JV, Marder E (2003) Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J Neurochem 87:642–656
Lingle C (1980) The sensitivity of decapod foregut muscles to acetylcholine and glutamate. J Comp Physiol A 138:187–199
Linsdell P, Moody WJ (1994) Na+ channel mis-expression accelerates K+ channel development in embryonic Xenopus laevis skeletal muscle. J Physiol 480(Pt 3):405–410
Liu Z, Golowasch J, Marder E, Abbott LF (1998) A model neuron with activity-dependent conductances regulated by multiple calcium sensors. J Neurosci 18:2309–2320
Lopez HS, Brown AM (1992) Neuromodulation. Curr Opin Neurobiol 2:317–322
Luther JA, Robie AA, Yarotsky J, Reina C, Marder E, Golowasch J (2003) Episodic bouts of activity accompany recovery of rhythmic output by a neuromodulator- and activity-deprived adult neural network. J Neurophysiol 90:2720–2730
MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM (2003) Activity-independent homeostasis in rhythmically active neurons. Neuron 37:109–120
Mamiya A, Nadim F (2004) Dynamic interaction of oscillatory neurons coupled with reciprocally inhibitory synapses acts to stabilize the rhythm period. J Neurosci 24:5140–5150
Marder E, Bucher D (2005) Robustness in neuronal systems: the balance between homeostasis, plasticity, and modulation. In: Jen E (ed) Robust design: a repertoire of biological, ecological and engineering case studies. Oxford University Press, Oxford
Marder E, Bucher D (2007) Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69:291–316
Marder E, Calabrese RL (1996) Principles of rhythmic motor pattern generation. Physiol Rev 76:687–717
Marder E, Thirumalai V (2002) Cellular, synaptic and network effects of neuromodulation. Neural Netw 15:479–493
Marder E, Weimann JM (1992) Modulatory control of multiple task processing in the stomatogastric nervous system. Pergamon Press, New York
Marder E, Hooper SL, Siwicki KK (1986) Modulatory action and distribution of the neuropeptide proctolin in the crustacean stomatogastric nervous system. J Comp Neurol 243:454–467
Marder E, Calabrese RL, Nusbaum MP, Trimmer B (1987) Distribution and partial characterization of FMRFamide-like peptides in the stomatogastric nervous systems of the rock crab, Cancer borealis, and the spiny lobster, Panulirus interruptus. J Comp Neurol 259:150–163
Marder E, Jorge-Rivera JC, Kilman V, Weimann JM (1997) Peptidergic modulation of synaptic transmission in a rhythmic motor system. Advances in organ biology. JAI Press, Greenwich, CT, pp 213–233
Marder E, Blitz DM, Christie AE, Nusbaum MP (2002) Convergence and divergence of cotransmitter systems in the crab stomatogastric nervous system. In: Wiese K (ed) The crustacean nervous system. Springer, Berlin
Marder E, Bucher D, Schulz DJ, Taylor AL (2005) Invertebrate central pattern generation moves along. Curr Biol 15:R685–R699
Maynard DM, Dando MR (1974) The structure of the stomatogastric neuromuscular system in Callinectes sapidus, Homarus americanus and Panulirus argus (Decapoda Crustacea). Philos Trans R Soc Lond B Biol Sci 268:161–220
Meyrand P, Marder E (1991) Matching neural and muscle oscillators: control by FMRFamide-like peptides. J Neurosci 11:1150–1161
Meyrand P, Simmers J, Moulins M (1991) Construction of a pattern-generating circuit with neurons of different networks. Nature 351:60–63
Meyrand P, Weimann JM, Marder E (1992) Multiple axonal spike initiation zones in a motor neuron: serotonin activation. J Neurosci 12:2803–2812
Meyrand P, Simmers J, Moulins M (1994) Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. J Neurosci 14:630–644
Montague PR, Hyman SE, Cohen JD (2004) Computational roles for dopamine in behavioural control. Nature 431:760–767
Morris LG, Hooper SL (2001) Mechanisms underlying stabilization of temporally summated muscle contractions in the lobster (Panulirus) pyloric system. J Neurophysiol 85:254–268
Msghina M, Govind CK, Atwood HL (1998) Synaptic structure and transmitter release in crustacean phasic and tonic motor neurons. J Neurosci 18:1374–1382
Mulloney B, Hall WM (1991) Neurons with histamine-like immunoreactivity in the segmental and stomatogastric nervous systems of the crayfish Pacifastacus leniusculus and the lobster Homarus americanus. Cell Tissue Res 266:197–207
Norris BJ, Coleman MJ, Nusbaum MP (1994) Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis. J Neurophysiol 72:1451–1463
Norris BJ, Coleman MJ, Nusbaum MP (1996) Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system. J Neurophysiol 75:97–108
Nusbaum MP (1986) Synaptic basis of swim initiation in the leech. III. Synaptic effects of serotonin-containing interneurones (cells 21 and 61) on swim CPG neurones (cells 18 and 208). J Exp Biol 122:303–321
Nusbaum MP (2002) Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol 60:378–387
Nusbaum MP, Beenhakker MP (2002) A small-systems approach to motor pattern generation. Nature 417:343–350
Nusbaum MP, Marder E (1989a) A modulatory proctolin-containing neuron (MPN). I. Identification and characterization. J Neurosci 9:1591–1599
Nusbaum MP, Marder E (1989b) A modulatory proctolin-containing neuron (MPN). II. State-dependent modulation of rhythmic motor activity. J Neurosci 9:1600–1607
Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E (2001) The roles of co-transmission in neural network modulation. Trends Neurosci 24:146–154
Powers LW (1973) Gastric mill rhythms in intact crabs. Comp Biochem Physiol 46:767–783
Prinz AA, Billimoria CP, Marder E (2003a) Alternative to hand-tuning conductance-based models: construction and analysis of databases of model neurons. J Neurophysiol 90:3998–4015
Prinz AA, Thirumalai V, Marder E (2003b) The functional consequences of changes in the strength and duration of synaptic inputs to oscillatory neurons. J Neurosci 23:943–954
Prinz AA, Bucher D, Marder E (2004) Similar network activity from disparate circuit parameters. Nat Neurosci 7:1345–1352
Pulver SR, Marder E (2002) Neuromodulatory complement of the pericardial organs in the embryonic lobster, Homarus americanus. J Comp Neurol 451:79–90
Qian SM, Delaney KR (1997) Neuromodulation of activity-dependent synaptic enhancement at crayfish neuromuscular junction. Brain Res 771:259–270
Ramirez JM, Viemari JC (2005) Determinants of inspiratory activity. Respir Physiol Neurobiol 147:145–157
Reed RA, Page CH (1977) Circumesophageal connective control of the common inhibitory motoneuron in the crab, Carcinus maenas. Comp Biochem Physiol 56:567–571
Rehm KJ, Deeg KE, Marder E (2008) Developmental regulation of neuromodulator function in the stomatogastric ganglion of the lobster, Homarus americanus. J Neurosci 28:9828–9839
Russell DF, Hartline DK (1981) A multiaction synapse evoking both EPSPs and enhancement of endogenous bursting. Brain Res 223:19–38
Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM (2005) Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron 46:103–116
Schulz DJ, Goaillard JM, Marder E (2006) Variable channel expression in identified single and electrically coupled neurons in different animals. Nat Neurosci 9:356–362
Seal RP, Edwards RH (2006) Functional implications of neurotransmitter co-release: glutamate and GABA share the load. Curr Opin Pharmacol 6:114–119
Selverston AI, Moulins M (1987) The crustacean stomatogastric system: a model for the study of central nervous systems. Springer-Verlag, Heidelberg
Sen K, Jorge-Rivera JC, Marder E, Abbott LF (1996) Decoding synapses. J Neurosci 16:6307–6318
Sigvardt KA, Mulloney B (1982a) Properties of synapses made by IVN command-interneurones in the stomatogastric ganglion of the spiny lobster Panulirus interruptus. J Exp Biol 97:153–168
Sigvardt KA, Mulloney B (1982b) Sensory alteration of motor patterns in the stomatogastric nervous system of the spiny lobster Panulirus interruptus. J Exp Biol 97:137–152
Skiebe P (2001) Neuropeptides are ubiquitous chemical mediators: using the stomatogastric nervous system as a model system. J Exp Biol 204:2035–2048
Skiebe P (2003) Neuropeptides in the crayfish stomatogastric nervous system. Microsc Res Tech 60:302–312
Skiebe P, Schneider H (1994) Allatostatin peptides in the crab stomatogastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. J Exp Biol 194:195–208
Skiebe P, Wollenschlager T (2002) Putative neurohemal release zones in the stomatogastric nervous system of decapod crustaceans. J Comp Neurol 453:280–291
Skiebe P, Dreger M, Meseke M, Evers JF, Hucho F (2002) Identification of orcokinins in single neurons in the stomatogastric nervous system of the crayfish, Cherax destructor. J Comp Neurol 444:245–259
Smarandache CR, Stein W (2007) Sensory-induced modification of two motor patterns in the crab, Cancer pagurus. J Exp Biol 210:2912–2922
Spirito CP (1975) The organization of the crayfish oesophageal nervous system. J Comp Physiol A 102:237–249
Spitzer N, Cymbalyuk G, Zhang H, Edwards DH, Baro DJ (2008a) Serotonin transduction cascades mediate variable changes in pyloric network cycle frequency in response to the same modulatory challenge. J Neurophysiol 99:2844–2863
Spitzer N, Edwards DH, Baro DJ (2008b) Conservation of structure, signaling and pharmacology between two serotonin receptor subtypes from decapod crustaceans, Panulirus interruptus and Procambarus clarkii. J Exp Biol 211:92–105
Stein W, Eberle CC, Hedrich UBS (2005) Motor pattern selection by nitric oxide in the stomatogastric nervous system of the crab. Eur J Neurosci 21:2767–2781
Stein W, Smarandache CR, Nickmann M, Hedrich UB (2006) Functional consequences of activity-dependent synaptic enhancement at a crustacean neuromuscular junction. J Exp Biol 209:1285–1300
Stein W, DeLong ND, Wood DE, Nusbaum MP (2007) Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci 26:1148–1165
Stemmler EA, Peguero B, Bruns EA, Dickinson PS, Christie AE (2007) Identification, physiological actions, and distribution of TPSGFLGMRamide: a novel tachykinin-related peptide from the midgut and stomatogastric nervous system of Cancer crabs. J Neurochem 101:1351–1366
Swensen AM, Marder E (2000) Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci 20:6752–6759
Swensen AM, Marder E (2001) Modulators with convergent cellular actions elicit distinct circuit outputs. J Neurosci 21:4050–4058
Swensen AM, Golowasch J, Christie AE, Coleman MJ, Nusbaum MP, Marder E (2000) GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol 203:2075–2092
Taylor AL, Goaillard JM, Marder E (2009) How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci 29:5573–5586
Thirumalai V, Prinz AA, Johnson CD, Marder E (2006) Red pigment concentrating hormone strongly enhances the strength of the feedback to the pyloric rhythm oscillator but has little effect on pyloric rhythm period. J Neurophysiol 95:1762–1770
Thoby-Brisson M, Simmers J (1998) Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J Neurosci 18:2212–2225
Thoby-Brisson M, Simmers J (2002) Long-term neuromodulatory regulation of a motor pattern-generating network: maintenance of synaptic efficacy and oscillatory properties. J Neurophysiol 88:2942–2953
Tierney AJ, Blanck J, Mercier J (1997) FMRFamide-like peptides in the crayfish (Procambarus clarkii) stomatogastric nervous system: distribution and effects on the pyloric motor pattern. J Exp Biol 200:3221–3233
Tobin AE, Calabrese RL (2005) Myomodulin increases Ih and inhibits the NA/K pump to modulate bursting in leech heart interneurons. J Neurophysiol 94:3938–3950
Turrigiano GG (1999) Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci 22:221–227
Turrigiano GG, Nelson SB (2004) Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5:97–107
Turrigiano G, Abbott LF, Marder E (1994) Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264:974–977
Turrigiano G, LeMasson G, Marder E (1995) Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J Neurosci 15:3640–3652
von Bohlen und Halbach O, Dermietzel R (2006) Neurotransmitters and neuromodulators: handbook of receptors and biological effects. Wiley, New York
Weimann JM, Marder E, Evans B, Calabrese RL (1993) The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol 181:1–26
Weimann JM, Skiebe P, Heinzel HG, Soto C, Kopell N, Jorge-Rivera JC, Marder E (1997) Modulation of oscillator interactions in the crab stomatogastric ganglion by crustacean cardioactive peptide. J Neurosci 17:1748–1760
Wood DE, Nusbaum MP (2002) Extracellular peptidase activity tunes motor pattern modulation. J Neurosci 22:4185–4195
Wood DE, Stein W, Nusbaum MP (2000) Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J Neurosci 20:8943–8953
Wood DE, Manor Y, Nadim F, Nusbaum MP (2004) Intercircuit control via rhythmic regulation of projection neuron activity. J Neurosci 24:7455–7463
Worden MK, Bykhovskaia M, Hackett JT (1997) Facilitation at the lobster neuromuscular junction: a stimulus-dependent mobilization model. J Neurophysiol 78:417–428
Zhang B, Harris-Warrick RM (1994) Multiple receptors mediate the modulatory effects of serotonergic neurons in a small neural network. J Exp Biol 190:55–77
Zhang B, Harris-Warrick RM (1995) Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. I. Calcium current and its modulation by serotonin. J Neurophysiol 74:1929–1937
Zhang B, Wootton JF, Harris-Warrick RM (1995) Calcium-dependent plateau potentials in a crab stomatogastric ganglion motor neuron. II. Calcium-activated slow inward current. J Neurophysiol 74:1938–1946
Acknowledgments
I thank M.P. Nusbaum, F. Nadim and H. Wolf for critically reading the manuscript and polishing the English. I would also like to thank N. Daur, U. Hedrich and J. Ausborn for helpful discussions. Research support in our laboratory is from German Research Foundation (DFG) grants.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stein, W. Modulation of stomatogastric rhythms. J Comp Physiol A 195, 989–1009 (2009). https://doi.org/10.1007/s00359-009-0483-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-009-0483-y