Abstract
The digestive system of the malacostracan crustaceans, namely the decapods, isopods, amphipods and mysids, is among the most complex organ systems of the animal kingdom serving multiple functions such as food processing, absorption and storage of nutrients, synthesis of digestive enzymes and blood proteins, detoxification of xenobiotics and osmoregulation. It is rather well investigated compared to other invertebrates because the Malacostraca include many ecological keystone species and food items for humans. The Decapoda and Peracarida share food processing with chewing and filtering structures of the stomach but differ with respect to morphology and ultrastructure of the digestive glands. In the Peracarida, the digestive glands are composed of few, relatively large lateral caeca, whereas in the Decapoda, hundreds to thousands of blindly ending tubules form a voluminous hepatopancreas. Morphogenesis and onset of functionality of the digestive system strongly depend on the mode of development. The digestive system is early developed in species with feeding planktonic larvae and appears late in species with direct lecithotrophic development. Some structures of the digestive system like the stomach ossicles are rather constant in higher taxa and are of taxonomic value, whereas others like the chewing structures are to some degree adapted to the feeding strategy. The nutrient absorbing and storing cells of the digestive glands show considerable ultrastructural variation during moult cycle, vitellogenesis and starvation. Some of the various functions of the digestive system are already assigned to specific sections of the digestive tract and cell types, but others still await precise localization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malacostraca (“higher crustaceans”) is the largest clade of the Crustacea comprising more than 40,000 species, including almost 15,000 described decapods (De Grave et al. 2009) and 25,000 peracarids (Ahyong et al. 2011). It contains familiar animals such as shrimps, lobsters, crayfish and crabs (Decapoda), krill (Euphausiacea), woodlice, amphipods and mysids (Peracarida) and some smaller and less known taxa. Malacostracans are mostly water-dwelling organisms, but many peracarids, which represent almost one-third of all crustaceans, have successfully invaded terrestrial environments, with woodlice being entirely terrestrial (Martin and Davis 2001). Regarding their food sources and supply, malacostracans can be carnivorous, omnivorous, herbivorous or detritivorous and act as predators, scavengers or ectoparasites (Schram 1986). Decapods are mostly predators, herbivores, suspension and deposit feeders, and peracarids feed on suspended particles, sediment, algae, wood and decaying organic material. Inhabiting marine habitats from the littoral to the deep sea including the pelagial, freshwater habitats from epigean to subterranean waters and terrestrial habitats from the sea shore to high mountains including deserts (Schram 1986), malacostracans exhibit a large diversity in feeding strategy and architecture of the digestive system (Icely and Nott 1992; Watling 2013).
Because of the economic and ecological importance of the Malacostraca, knowledge on the anatomy, morphogenesis and function of their digestive system is of utmost importance. Moreover, because of the high diversity of lifestyles and feeding strategies, the malacostracan digestive system is an interesting paradigm for the morphological and functional diversification of an organ system in a larger animal group. In the Malacostraca, the digestive system serves not only for the digestion and absorption of the food but also for a variety of other functions including detoxification of xenobiotics, synthesis of immune defence molecules and osmoregulation as will be detailed in this paper. The complex anatomy and physiology of the malacostracan digestive system have been extensively studied, and numerous data are available on the morphology and ultrastructure of the various parts of the alimentary system in adults, larvae and embryos (e.g. Wägele et al. 1981; Storch 1982; Icely and Nott 1984; Vogt 1985, 1993, 1994; Friesen et al. 1986; Storch and Štrus 1989, 2004; Hames and Hopkin 1989; Wägele 1992; Schmitz 1992; Coleman 1994; Biesiot and McDowell 1995, Štrus et al. 1995, 2008; Štrus and Blejec 2001; Storch et al. 2001, 2002; Tziouveli et al. 2011; McGaw and Curtis 2013; Trevisan et al. 2014; Sonakowska et al. 2015). Several papers focus on adaptations of the digestive system to different lifestyles and feeding strategies (Vogt et al. 1985; Ahearn 1987; Icely and Nott 1992; Watling 2013; Saborowski 2015; Spitzner et al. 2018). The microscopic anatomy, biochemistry and physiology of digestion in crustaceans as well as differentiation of the digestive tract were reviewed by Ceccaldi (2006) and Watling (2013).
It is noteworthy that complex digestive organs have already been found in early arthropods from the Cambrian period (Vannier et al. 2014) which appear functionally similar to some modern branchiuran and highly derived decapod crustaceans such as spiny lobsters. Macrophagy and carnivory and enzymatic breakdown of related food stuff were seemingly important for the evolutionary success and wide distribution of the ancient arthropods.
In this paper, we review structural and functional features of the digestive system of malacostracan crustaceans, focussing on decapods and peracarids. Particular emphasis is given to the elaboration of similarities and differences between these two taxa. Emphasis is also given to variations during development and between different life histories, lifestyles, feeding strategies and nutritional conditions. Since parts of the malacostracan digestive tract, particularly the triturating and filtering structures, are regularly renewed by moulting, we also address this crustacean-specific aspect.
Anatomy of the malacostracan digestive system
The malacostracan digestive system is composed of three regions, stomodeum or foregut with oesophagus and stomach, mesenteron or midgut comprising midgut canal and dorsal and lateral caeca and proctodeum with hindgut and rectum (Gruner 1993). In both decapods (Fig. 1a) and peracarids (Fig. 1b), the stomodeum and proctodeum derive from the ectoderm and are thus lined by a cuticle that is shed and replaced during growth by moulting. The stomodeum usually includes cuticular chewing and filtering structures for processing of the food.
a Schematic illustration of the digestive tract of the Decapoda on the example of freshwater crayfish (medio-lateral sagittal plane). The foregut (oesophagus, cardiac stomach and pyloric stomach) and hindgut are lined by a cuticle. A atrium of hepatopancreas, AC anterior dorsal caecum, CF cardiac filter, CP cardiopyloric filter channel, CV cardiopyloric valve, DC dorsal pyloric chamber, G gastrolith, HV hepatopancreatic-intestinal valve, LT lateral tooth, MC medial pyloric chamber, MT medial tooth, O oesophagus, PF pyloric filter, PV pyloro-intestinal valve (modified after Vogt 2002). b Digestive system of isopod Porcellio scaber. The ectodermal digestive tract is composed of the foregut (F) comprising oesophagus and stomach and the hindgut divided into anterior chamber (AC), papillate region (PR) and rectum (R). Four endodermal hepatopancreatic tubules (Hp) are connected to the digestive tract at the junction of the foregut and hindgut. Scale bar 1 mm
The mesenteron of Decapoda is characterized by extensively ramified lateral caeca, which together form the hepatopancreas. The midgut is long in some decapods like shrimps and lobsters and has small anterior and posterior dorsal caeca but is short in others like crayfish and crabs (Icely and Nott 1992). In crayfish, the posterior dorsal caecum is lacking. In gammaridean amphipods, the mesenteron is long (Schmitz 1992) and bears seven blindly ending tubes: a single anterior caecum, two pairs of lateral caeca forming the digestive gland (hepatopancreas) and a pair of posterior caeca involved in mineral translocation during the moult cycle (Graf and Michaut 1980). In isopods, the midgut tube is short in amphibious species of the family Ligiidae (Štrus and Drašlar 1988; Štrus et al. 2008) and completely absent in strictly terrestrial isopods. The endodermal part of the gut is then only represented by two or rarely three pairs of lateral midgut gland tubules connected to the atrium of the stomach at the foregut-hindgut junction. Dorsal caeca are lacking in isopods.
Comparison of the functional morphology of the digestive system in amphibious and terrestrial oniscideans by Štrus et al. (1995) revealed key adaptations to life on land. It was shown that the variety of cuticular structures, the compartmentalization of the stomach, the reduction of the endodermal midgut, the complexity of the foregut-hindgut junction, the presence of a typhlosole and the water-conserving properties of the posterior hindgut are specific traits of terrestrial species. The ultrastructure and dynamics of the epithelia of the digestive system in terrestrial isopods reflect both feeding strategies and moult cycle. During premoult and exuviation, food intake is reduced and the ectodermal epithelia are involved in formation of the new cuticle (Štrus et al. 1985; Štrus and Storch 1991).
Morphogenesis of the malacostracan digestive system
The development of the digestive tract is very variable in the Malacostraca, depending on life history. In species with pelagic larvae like many shrimps, the digestive tract is formed early and serves for feeding on plankton and suspended food particles (Anger 2001). Particularly, the stomach is considerably modified during the larval stages and acquires a complex gastric armature involved in the mastication and filtration of food. These changes are particularly obvious when the feeding habits change during the transition from pelagic larvae to benthic juveniles (e.g. Reedy 1935; Hinton and Corey 1979; Factor 1981, 1982; Nishida et al. 1990; Wolfe and Felgenhauer 1991; Suh et al. 1994; Lemmens and Knott 1994; Abrunhosa and Kittaka 1997a; Abrunhosa et al. 2006, 2011; Abrunhosa and Melo 2008; Queiroz et al. 2011; Tziouveli et al. 2011; Castejon et al. 2015a, b). In groups with direct development such as the Peracarida and freshwater crayfish, the digestive system develops and becomes functional rather late (Anger 2001).
Diversity of morphogenetic trajectories in the digestive tract of Decapoda
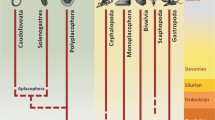
The developmental pattern in the Dendrobranchiata (penaeid shrimps), which are regarded as plesiomorphic within the Decapoda sensu strictu (Richter and Scholtz 2001), comprises embryonic stages that develop within the eggs, several pelagic larval stages (nauplius, zoea and mysis) and a transitional decapodid stage that settles on the ground (Anger 2001). However, in most Decapoda, this scheme is abbreviated starting with the zoea as first larva.
The foregut, midgut and hindgut primordia are separately formed in the pelagic nauplius stages of the penaeid shrimps (Penaeoidea) and the late embryos of the decapods with abbreviated development, which live on abundant yolk reserves (Hinton and Corey 1979). The most extreme case is the freshwater crayfish (Astacidea) with direct development, which completely lost the pelagic larval phase and hatch from the eggs as benthic adult-like juveniles (Gherardi et al. 2010). In cambarid crayfish, the stomach develops late and is only functional in stage-3 juveniles, the first feeding stage (Vogt 2008a). In these juveniles, the yolk is stored in the large lumina of the anterior dorsal coeca (Fig. 5a) and digested by their epithelial cells. In contrast, the external food is processed in the stomach-hepatopancreas system, and therefore, stage-3 juveniles can digest yolk and external food at the same time, facilitating slow adaptation to active feeding (Vogt 2008a).
The ectodermal foregut in decapod larvae consists of a simple tubular oesophagus (Ullrich and Storch 1993; Castejon et al. 2018) and a rather complex stomach, both lined with cuticle. The stomach in early pelagic larvae is generally divided into cardiac and pyloric chamber by a cardiopyloric valve and has a well-developed gland filter in the pyloric chamber. The gastric mill in the cardiac chamber that can triturate harder food stuff is usually formed in late larval stages during the transition to benthic juveniles. With the formation of the gastric mill, the mastication of food often partially shifts from the external mouthparts to the internal gastric mill. The teeth of the gastric mill are further modified and calcified in the subsequent juvenile stages (Hinton and Corey 1979; Factor 1981, 1982; Abrunhosa et al. 2006, 2011; Abrunhosa and Melo 2008; Castejon et al. 2015a, b).
The transitional stages that leave the pelagial and settle on the ground are non-feeding in some cases. Examples are the puerulus larvae of Palinuridae (Achelata, spiny lobsters) and the glaucothoe larvae of the king crabs Paralithodes sp. (Anomura). Both have reduced mouthparts, which are ineffective in mastication of the food, and at the same time still lack the gastric mill in the cardiac stomach, which is formed only later in the juveniles (Nishida et al. 1990; Wolfe and Felgenhauer 1991; Lemmens and Knott 1994; Abrunhosa and Kittaka 1997a). The puerulus larvae of the Palinuridae depend on the extensive lipid reserves stored in the fat bodies within the haemocoel (Takahashi et al. 1994; Nishida et al. 1995), while the glaucothoe larvae of Paralithodes utilize the lipids stored in the anterior midgut caecum and hepatopancreas (Abrunhosa and Kittaka 1997b).
In the Penaeoidea, the development of the foregut is slightly delayed compared to the derived groups of the Decapoda. The pelagic protozoea larvae have a simple foregut without gland filter, which is formed later in the mysis stage. The gastric mill is gradually established during postlarval development (Lovett and Felder 1989; Abrunhosa and Melo 2008; Diaz et al. 2008; Muhammad et al. 2012). In the caridean shrimps, the first pelagic larva, the zoea, already has a functional gland filter in the pyloric chamber, which is further elaborated during transition to the benthic lifestyle. In contrast to other decapods, a sophisticated gastric mill is not developed in the adults, so that the food is masticated solely by the mouthparts in all larval, postlarval and adult stages (Queiroz et al. 2011; Tziouveli et al. 2011).
The hindgut, another ectodermal part of the digestive tract, is present in decapod larvae as a simple cuticle lined tube. During larval development, the hindgut wall often folds into longitudinal ridges that run along the entire length of the hindgut (Hinton and Corey 1979; Factor 1981; Lovett and Felder 1989; Mikami et al. 1994; Tziouveli et al. 2011; Spitzner et al. 2018).
The formation of the endodermal midgut in the Penaeoidea begins in nauplius larvae when the abundant yolk cells in the body cavity assemble into a midgut anlage. The anterior midgut caeca and hepatopancreas emerge before the last nauplius stage (Talbot et al. 1972; Nakamura and Seki 1990; Abubakr and Jones 1992; Muhammad et al. 2012). In lobsters (Astacidea), which show prolonged embryonic development and abbreviated larval development, midgut formation starts around mid-embryonic development (Hinton and Corey 1979; Factor 1981). Late embryos have a midgut anlage with a distinct separation in anterior and posterior regions. The anterior region occupies the majority of the thoracic area and contains large amount of yolk surrounded by vitellophage epithelium (Hinton and Corey 1979).
The endodermal part of the digestive system in the postnaupliar protozoea stages of Penaeoidea and the zoea stages of the other Decapoda generally consists of the tubular midgut canal, the anterior midgut caeca and the hepatopancreas. The midgut canal is connected with the stomach anteriorly and with the hindgut posteriorly. The anterior midgut caeca and hepatopancreas originate from the midgut canal near its junction with the stomach. The anterior midgut caeca are usually paired structures and the hepatopancreas anlage consists of several paired lobes (Hinton and Corey 1979; Factor 1981; Lovett and Felder 1989; Nakamura and Seki 1990; Abubakr and Jones 1992; Abrunhosa and Kittaka 1997b; Muhammad et al. 2012; Spitzner et al. 2018).
The anterior midgut caeca reach the largest volume in the mysis stages of Penaeoidea and in the early larval stages of the other Decapoda and often contain yolk and storage lipids. During the subsequent development, the anterior midgut caeca are reduced in size and fuse into a single anterior midgut diverticulum while the yolk and storage lipids are utilized. The posterior midgut caecum at the junction between the midgut and hindgut develops in postlarval stages of Penaeoidea (Lovett and Felder 1989; Diaz et al. 2008; Muhammad et al. 2012) and in late larval stages of the majority of the other Decapoda (Hinton and Corey 1979; Factor 1981; Abrunhosa and Kittaka 1997b; Spitzner et al. 2018).
The hepatopancreas increases in volume during larval development and the initial hepatopancreatic lobes ramify into numerous tubules of smaller diameter, which multiply and elongate progressively (Factor 1981; Lovett and Felder 1989; Nakamura and Seki 1990; Abubakr and Jones 1992; Diaz et al. 2008; Tziouveli et al. 2011; Muhammad et al. 2012). All developmental stages include the same hepatopancreatic cell types as the adults (see below), but they show their typical ultrastructural features only in feeding stages. The epithelial cells in the hepatopancreas are subject to structural modifications associated with active feeding and the utilization of storage lipids in non-feeding stages. Particularly, the content of lipid droplets in the R cells of actively feeding larvae depends on their nutritional status and is depleted during shortage (Storch and Anger 1983; Anger et al. 1985; Mikami et al. 1994; Abrunhosa and Kittaka 1997b). Interestingly, in some non-feeding developmental stages like the puerulus larvae of Palinuridae or stage-1 juveniles of freshwater crayfish lipids are transferred via the haemolymph from external lipid stores to the R cells of the hepatopancreas where they are first stored in large lipid droplets and later metabolized (Takahashi et al. 1994; Nishida et al. 1995; Vogt 2008a).
Diversity of morphogenetic trajectories in the digestive tract of Peracarida
Peracarida generally develop directly in a brood chamber (marsupium) and following release the juveniles reach maturity after several moults. The development of digestive system starts with stomodeal and proctodeal invaginations that fuse with the embryonic yolk sac and progresses gradually with formation of the midgut and embryonic digestive glands. The morphogenesis of the digestive system is described for a few peracaridan species displaying diverse lifestyles. Most data are available from developmental studies in the marine amphipod and common laboratory model Parhyale hawaiensis (Browne et al. 2005) and the terrestrial isopod Porcellio scaber (Štrus et al. 2008; Wolff 2009; Milatovič et al. 2010; Mrak et al. 2015). Some data are also provided from the study of isopod Paragnathia formica, a fish ectoparasite with a unique brooding mechanism: the embryos remain within the ovaries until hatching to the free-swimming juveniles (Manship et al. 2011).
The common characteristic of morphogenesis of the peracarid digestive system is the development of the stomodeal invagination shortly before the proctodeal invagination. Both elongate towards each other and join with the midgut during further development (Nair 1956; Stromberg 1964, 1967; Browne et al. 2005; Wolff 2009; Milatovič et al. 2010; Manship et al. 2011). At the anterior lateral edges of the newly formed stomodeum, the oesophageal projections become visible, resulting in a Y-shaped stomodeal opening (Browne et al. 2005; Wolff, 2009). In the isopod Porcellio scaber, the stomodeum and proctodeum are formed during mid-embryogenesis (Fig. 2a) (Wolff 2009; Milatovič et al. 2010) and fuse just prior to hatching of the embryo from the chorion (Fig. 2b) (Milatovič et al. 2010). During early morphogenesis, the digestive tract is a simple tube with the gut epithelial cells secreting a thin apical matrix. In late embryogenesis, a finely elaborate cuticle lines the foregut and the cuticular structures in the filtering region of the stomach are already formed (Štrus et al. 2008). In the hindgut, the anatomical and ultrastructural distinction between anterior and posterior region becomes evident with typhlosole folds forming in the anterior region. The hindgut cells differentiate simultaneously (Fig. 2c). The first cuticle is represented by a thin layer of matrix covering the plasma membrane of the epithelial cells (Fig. 2d).
Morphogenesis of the digestive system in terrestrial isopod Porcellio scaber: differentiation of the ectodermal parts of the digestive tract during embryonic (a–d) and postembryonic (e–h) development. a Mid-stage embryo characterized by the onset of foregut and hindgut development. The proctodeal invagination (arrow) is visible in the dorsal posterior part, elongating towards the yolk sac (YS) in the middle of the embryo body. The midgut glands (MG) are filled with yolk. Scale bar 100 μm. b Cross section of foregut-hindgut junction in late embryo. The digestive tract is now continuous, with ventrally located stomach (S) connected to the hindgut (H) dorsally and midgut glands with yolk and lipids laterally. Richardson’s staining. Scale bar 50 μm. c Digestive tract surrounded by midgut gland diverticula. The epithelial cells (EC) of the hindgut are columnar in shape and the typhlosole (T) forms in the anterior hindgut. Richardson’s staining. Scale bar 20 μm. d Secretion of first cuticle (arrow) below the precuticular matrix (PM) covering the apical plasma membrane of the hindgut epithelial cells. TEM. Scale bar 0.5 μm. e Cross section of marsupial manca stage at stomach-hindgut junction. The stomach is separated into dorsal, lateral and ventral chambers, and the filter region (arrow) is discernible. In the hindgut, the typhlosole is present. Richardson’s staining. Scale bar 100 μm. f Formation of first cuticle (C) in hindgut with thin epicuticle (arrow) and procuticle (Pr). TEM. Scale bar 0.5 μm. g Cross section of postmarsupial manca. The gut tube is extensive and filled with diverse food particles (arrow). There are two pairs of laterally aligned midgut gland diverticula. Richardson’s staining. Scale bar 100 μm. h Elaborate cuticle in hindgut region composed of epicuticle (Ep) and procuticle with discernible pattern of chitin-protein fibers arrangement. TEM. Scale bar 0.5 μm
In postembryonic manca stages of Porcellio scaber, the filters, ridges and folds of the stomach and typhlosole in the anterior chamber are elaborated and functional (Fig. 2e). The hindgut cuticle consists of procuticle and epicuticle (Fig. 2f) with cuticular spines. The cuticle is replaced during release of the manca from the marsupium to the terrestrial environment (Mrak et al. 2015). After this moult, the gut lumen is usually filled with diverse food particles (Fig. 2g) and a complete functional gut with elaborated cuticle in the entire hindgut region is evident (Fig. 2h).
The functionality of the midgut and midgut glands in Peracarida begins already during embryonic and early postembryonic development with their involvement in the degradation and absorption of yolk. The midgut primordia are first visible as lateral disc-shaped structures early during embryogenesis, in the amphipod Parhyale hawaiensis even before the body appendages start to develop. Labelling with fluorescent dye revealed several layers of nuclei within the disc-shaped midgut in Parhyale hawaiensis during early morphogenesis (Browne et al. 2005). The midgut cells are later in development clearly recognized as endoderm and visceral mesoderm derivates as demonstrated with fluorescent labelling of blastomeres traced during development (Gerberding et al. 2002). In Parhyale hawaiensis, the yolk is first completely enveloped by the midgut forming a dorso-ventrally oriented wedge and then by the tubes of the digestive caeca that begin to project posteriorly from the midgut. The extension of the caecum anlagen is accompanied by peristaltic, muscular contractions and yolk transfer from the medial midgut to the bilateral digestive caeca, changing from coarse yolk granules to finer structured yolk (Browne et al. 2005).
In the isopod Porcellio scaber, there are only the paired midgut gland anlagen, which elongate towards the posterior region simultaneously with inclusion of the egg yolk. A pair of midgut gland primordia becomes prominent around mid-embryogenesis, when the buds of the body appendages are already apparent. Anteriorly, the two lobes of the midgut gland fuse and the yolk from the embryonic yolk sac gradually moves into the gland lumen displaying a finer texture (Fig. 3a, b) (Wolff 2009; Milatovič et al. 2010; Manship et al. 2011). In Porcellio scaber embryos, the gland epithelium encloses yolk and large lipid globules. Numerous lipid droplets are now present in the epithelial cells (Fig. 3c). In late embryos, the yolk-filled glands are directly connected via the hepatopancreatic duct to the stomach in the area of the foregut-hindgut junction (Fig. 3d) and the outgrowths of the second pair of digestive gland tubules from the previously-formed tubules are already observed (Wolff 2009; Milatovič et al. 2010). The typical B- and S cells of the digestive caeca are both flattened, but already discernible, based on the characteristic orange autoflorescence of the S cells under ultraviolet excitation. The main ultrastructural features of the B cells, such as microvillar apical surface and electron dense cytoplasm containing abundant lipid droplets and mitochondria, are already apparent (Štrus et al. 2008).
Morphogenesis of the digestive system in the terrestrial isopod Porcellio scaber: differentiation of the endodermal midgut glands during embryonic (a–d) and postembryonic (e–h) development. a Mid-stage embryo with egg envelopes (EE). Body segmentation and appendages are clearly visible (arrow) and prominent midgut gland primordia (MG) are filled with yolk which is also present in the embryonic yolk sac (YS). Scale bar 200 μm. b Sagital section of late embryo. The yolk with lipid globules is completely enclosed in the midgut glands. Richardson’s staining. Scale bar 100 μm. c Epithelial cells (EC) of midgut glands in direct apical contact with yolk (Y) and filled with lipid droplets (asterisks). Richardson’s staining. Scale bar 20 μm. d Cross section of late embryo in the area of the stomach (S)-hindgut (H) junction. The midgut glands join the digestive tract via the hepatopancreatic duct (arrow). Richardson’s staining. Scale bar 20 μm. e Two midgut gland diverticula in marsupial manca filled with yolk which is gradually reduced during the intramarsupial postembryonic development. Scale bar 200 μm. f Cross section of marsupial manca with two pairs of midgut gland diverticula around the hindgut. Richardson’s staining. Scale bar 100 μm. g Cross section of midgut gland diverticulum with two cell types, B cells (B) and S cells (S). Before release of the manca from the marsupium the B cells are large, protrude apically into the lumen and contain numerous lipid droplets. Richardson’s staining. Scale bar 20 μm. h Cross section of midgut gland diverticulum in postmarsupial manca. The midgut glands are devoid of yolk, and the epithelial cells are poor in lipid. The apical parts of the cells have prominent microvillar surface (arrow). Richardson’s staining. Scale bar 20 μm
In all three peracarid species described, the yolk in the digestive glands is consumed until the young are released from the female, although the developmental time of yolk consumption is different among species, most likely as the consequence of different lifestyles. The yolk stores in the digestive caeca of the amphipod Parhyale hawaiensis are significantly depleted towards the end of embryogenesis and the hatchlings become dependent on external food sources (Browne et al. 2005). In the ectoparasitic isopod Paragnathia formica, which develops within the ovaries, the yolk inclusions in the digestive glands are largest in chorion-free embryos inside the ovaries. The yolk is gradually diminished until motile embryos are released from the female (Manship et al. 2011).
In Porcellio scaber, the yolk in the gland lumen is gradually consumed during the intramarsupial postembryonic stages (Fig. 3e–g). In this developmental period, the midgut gland epithelial cells are larger than in the embryonic stages, begin to protrude apically into the lumen and contain numerous lipid droplets (Fig. 3f, g). The most significant changes in the morphology of the gland tubules and cells are evident in the stage just before the release of the manca from the marsupium. The cells protrude deeper into the gland lumen and acquire a characteristic shape as in the midgut glands of the adults (Fig. 3g). In postmarsupial manca stages, the gland lumen is devoid of yolk and the apical parts of the epithelial cells with abundant microvillar surface protrude into the lumen. The amount of lipid droplets in their cytoplasm is low, indicating that the nutrients from the yolk were already consumed (Fig. 3h). The postmarsupial mancae and juveniles are able to feed on different food sources available in the surroundings, as is evident by the abundance of diverse food particles in their gut.
Structure, function and dynamics of the ectodermal parts of the malacostracan digestive system
The ectodermal parts of the digestive system serve for mastication of the food, filtration of the chymus, compaction of undigestable food components into faecal pellets and regulation of water and ions.
Food processing in the decapod stomach and hindgut
In decapods, the food is usually taken with the pereopods and delivered to the mouthparts. The mouthparts typically consist of six pairs of appendages, the mandibles, maxillae 1 and 2 and maxillipeds 1–3 (Garm 2004). They are rich in cutting structures, pestles, mechano- and chemoreceptors (Fig. 4a) and tear the food into pieces. At the entrance of the oesophagus, the food is lubricated by mucus secreted from mucus glands (Fig. 4b). The oesophagus is a short extensible tube that channels the food into the stomach and transports large indigestible food components back to the exterior. The stomach is divided into two parts, the anterior cardiac stomach that serves for physical and chemical breakdown of the food and the posterior pyloric stomach that serves for filtration of the chymus and compaction of the solids (Fig. 1a) (Icely and Nott 1992). The stomach is armed by a system of calcified cuticular ossicles that support the chewing structures, participate in formation of the pyloric filter and provide attachment sites for the extensive musculature (Brösing 2010). The number and arrangement of these ossicles vary between the higher taxa of the Decapoda (e.g. 41 in brachyuran crabs) and can be used for taxonomic purposes.
Mouthparts, oesophagus and stomach of the Decapoda. a Cutting edges of mouthparts of crayfish Procambarus virginalis. Ma mandible; M1 maxilla 1. SEM. Scale bar 10 μm (from Vogt 2008a). b Oesophageal gland (OG) of shrimp Palaemon elegans including acidic mucopolysaccharides (blue). E epithelium of oesophagus. Alcian blue staining. Scale bar 2 μm. c View from oesophagus on gastric mill and cardiac filters (CF) of crayfish Faxonius limosus. The gastric mill is composed of the median tooth (MT) at the roof of the stomach, two lateral teeth (LT) and two accessory lateral teeth (AT). Scale bar 1 mm (from Vogt 2002). d Transversal resin section through pyloric stomach of postlarva of shrimp Penaeus monodon showing dorsal chamber (DC) with lateral channels (LC), medial chamber (MC) and ventral chambers (VC) with cuticular press plate (P) and cuticular pyloric filter channels (arrow). Arrowhead example of cuticular ossicle, asterisk fused filter channels leading to hepatopancreas, M musculature. Richardson’s staining. Scale bar 100 μm. e Lateral tooth of crayfish Astacus astacus. SEM. Scale bar 1 mm (from Vogt 2002). f Pyloric filter channels of Astacus astacus covered by filter setae. SEM. Scale bar 50 μm (from Vogt 2002). g Filter setae with lateral setules in Astacus astacus. SEM. Scale bar 2 μm (from Vogt 2002)
In the cardiac stomach, the food is mixed with the gastric fluid and is triturated by masticatory cuticular structures. In crayfish Astacus astacus, the gastric fluid has a volume of about 0.4 ml and contains 50 mg/ml protein. It is brown, slightly acidic (pH 5.6) and bitter tasting (Vogt et al. 1989a). The gastric fluid contains a variety of digestive enzymes and a fat emulsifier (Saborowski 2015) and is synthesized in the hepatopancreas. The chewing structures of the cardiac stomach vary considerably in the Decapoda. They are relatively simple in caridean shrimps (Storch et al. 2001) but elaborate in crayfish and crabs (Icely and Nott 1992; Vogt 2002). Thus, they can be used for taxonomic purposes. On the other hand, within a given higher taxon, they are modified according to different feeding strategies (Brösing and Türkay 2011).
Crayfish possess a gastric mill composed of a medial tooth located at the roof of the stomach, two lateral teeth and two smaller accessory lateral teeth (Vogt 2002), all consisting of thick calcified cuticle which is replaced by each moult (Fig. 4c, e). The calcium of the teeth and other cuticular parts of the body is withdrawn in pre-ecdysis, stored in the haemolymph and the digestive tract and used again for calcification of the new cuticle. In brachyuran crabs, the calcium is stored in large calcium phosphate granules of the hepatopancreatic R cells (Greenaway 1985) but in crayfish, it is stored in special organs in the inner wall of the cardiac stomach called gastroliths (Fig. 1a) (Shechter et al. 2008).
The gastric mill can perform a variety of cutting, grinding and squeezing movements (Böhm 1996). The chymus is filtered a first time through the cardiac filters at the floor of the cardiac stomach (Fig. 4c) (Storch et al. 2001). The filtrate is then transported via the cardiopyloric filter channels to the pyloric filters and the solids are transferred through the cardiopyloric valve into the medial chamber of the tri-storied pyloric stomach (Fig. 4d). Each of the two ventral pyloric chambers consists of a press plate and numerous filter channels covered by dense mats of setae (Fig. 4d, f, g) that retain particles larger than ~ 100 nm (Vogt 2002). These filters serve for further filtration of the first filtrate from the cardiac filters and of fluids that are pressed out from the solids in the medial chamber of the pyloric stomach. This secondary filtrate is then transported into the hepatopancreas tubules where the nutrients are absorbed. The medial chamber of the pyloric stomach serves for compaction of the solids. The dorsal chamber and the dorsolateral channels may serve for the transport of fluids and solids from the midgut to the cardiac stomach, e.g. for remastication.
The midgut and hindgut further solidify the faeces and transport them to the exterior. In the semi-transparent shrimp Palaemon elegans, we have repeatedly observed antiperistalsis of the gut and retrograde transport of the entire gut content into the cardiac stomach where it was again masticated (unpublished). This transport probably occurred through the dorsal chamber of the pyloric stomach. The histology and function of the midgut are described in detail in the “endodermal parts” section below. The hindgut is additionally involved in rapid uptake of water after ecdysis, seems to contribute to ion- and osmoregulation and can harbour bacterial symbionts (Icely and Nott 1992).
Cross sections of the hindgut of Astacus astacus typically showed six longitudinal folds which are lined by a single-layered epithelium (Fig. 5f) (Vogt 2002). This epithelium is covered by a cuticle and consists of a single columnar cell type without microvilli but with numerous apical membrane infoldings typical of osmoregulatory cells (Mykles 1979). Basally, the epithelium is covered by a thick layer of spongy connective tissue including longitudinal muscle fibres and a peripheral layer of circular musculature. These muscles produce peristaltic and antiperistaltic movements. Two nerves originating from the terminal ganglion run on either side of the hindgut (Fig. 5f) and regulate activity of this organ (To et al. 2004).
Midgut and hindgut of the Decapoda. a Transversal section of cephalothorax of stage 1 juvenile of Procambarus virginalis showing yolk (Y) in lumen of anterior midgut coecum, midgut (asterisk) and developing hepatopancreatic tubule system (H). Arrow ventral nerve cord, G gills. Azan staining. Scale bar 200 μm (from Vogt 2008a). b Midgut cells of Penaeus monodon with plenty of lipid globules (LG) and apical granules (arrow). BL basal lamina, N nucleus, L midgut lumen. TEM. Scale bar 5 μm. c Apex of midgut cell of Penaeus monodon showing apocrine secretion (arrow) of granular material for assembly of the peritrophic membrane (arrowheads). TEM. Scale bar 0.5 μm. d Golgi body of midgut cell of Penaeus monodon producing vesicles for secretion. TEM. Scale bar 0.3 μm. e Nerve (arrow) between basal cell membrane and basal lamina of midgut cell of Penaeus monodon. TEM. Scale bar 1 μm. f Transversal section of hindgut of Astacus astacus showing longitudinal folds lined by cuticle-lined epithelium. Arrow circular musculature, arrowhead longitudinal muscle, N nerve. HE staining. Scale bar 200 μm (from Vogt 2002)
The arrangement of the musculature is quite different in the various regions of the digestive tract. The oesophagus, midgut and hindgut have closed layers of circular musculature and some longitudinal muscles enabling swallowing of the food and peristalsis and antiperistalsis. The stomach is equipped with numerous individual muscles (Fig. 4d) that enable precise movement of the teeth and filtering structures (Icely and Nott 1992). The hepatopancreas tubules are enveloped by a muscle net consisting of longitudinal and circular muscles (see below), similarly to that shown for isopods in Fig. 13a. The alimentary tract except of the hepatopancreas is equipped with nerves (Fig. 5e, f) (Mykles 1979). The movements of the oesophagus, gastric mill and pyloric filters are controlled by the stomatogastric nervous system, which in crayfish consists of the oesophageal ganglion, the stomatogastric ganglion and two commissural ganglia of the tritocerebrum (Skiebe 2003).
Food processing in the peracarid stomach and hindgut
Food processing in peracarids starts in the mouth region with diversified mouthparts which manipulate the food and enable ingestion. In scavengers who ingest large quantities of food, the food is held with the maxillipeds and the mouthparts move antero-posteriorly or laterally to triturate it and direct it to the oral cavity (Ceccaldi 2006; Watling 2013). The muscular oesophagus is equipped with valve-like structures to prevent regurgitation and draws the food into the stomach. The structural features of the amphipod and isopod stomach were described by Icely and Nott (1984), Storch (1987) and Storch and Štrus (2004) and were revised by Schmitz (1992) and Wägele (1992). The available literature on gut ultrastructure and digestive processes in terrestrial isopods concerns Porcellio scaber (Hames and Hopkin 1989) and Ligia italica (Storch and Štrus 1989). The feeding strategies and data on physicochemical conditions in the gut of woodlice were reviewed by Zimmer (2002). Food choice and feeding strategies of woodlice with respect to evolutionary adaptations to terrestrial lifestyle were reviewed by Hornung (2011).
The mechanical and chemical degradation of food takes place in a complex stomach with elaborate masticating and filtering structures (Fig. 6a, b). The food is masticated and squeezed in the anterior part of the stomach (cardia) with prominent lateralia which are generally present in peracarid stomachs but their position and structure are variable. They are equipped with bristles, spines or denticles. Their armature is related to feeding strategy and represents a valuable trait for phylogenetic reconstructions (Coleman 1992). An extensive study of the mysid stomach from the phylogenetic viewpoint was presented by Kobusch (1998), and the armature of the lateralia and the funnel region were described as apomorphies of the Mysida. In amphibious woodlice, the lateralia are covered with long bristles and in the amphipod Arcitalitrus sylvaticus, they are equipped with additional spines which are involved in grinding larger food particles (Fig. 6c, d). Lateralia armoured with spines were also described in the stomach of the terrestrial isopod Porcellio scaber (Storch 1987).
Stomach of the Peracarida. a Sagital section of stomach of amphipod Arcitalitrus sylvaticus consisting of cardia (C) with lateralia (L), pylorus (P) with inferolateralia (Il) and funnel (F). Food is filtered through primary filter (PF) and secondary filter (SF). SEM. Scale bar 100 μm (from Štrus and Storch 2004). b Sagital section of stomach of isopod Ligia italica connected to midgut (M) and hindgut (H) with three pairs of midgut glands (MG) located ventrally. VL ventral lamella, DL dorsal lamella. SEM. Scale bar 120 μm. c Lateralia in the stomach of Ligia italica covered with long bristles. SEM. Scale bar 100 μm. d Lateralia in the stomach of Arcitalitrus sylvatica covered with setae and spines. SEM. Scale bar 50 μm (from Štrus and Storch 2004). e Primary filter (arrows) in terrestrial isopod Armadillidium vulgare showing filtering surfaces on both sides of the anteromedianum (Am) and dorsal lateralia. Azan staining. Scale bar 100 μm. f Secondary filter (arrows) in amphibious isopod Ligia italica on both sides of the inferomedianum (Im) and inferolateralia. The endodermal midgut is located dorsally and the midgut glands are located ventro-laterally. HE staining. Scale bar 50 μm
Partly degraded food is filtered through two sets of ventrally located filters (Fig. 6e, f). Paired primary and secondary filters are composed of Y-structured chitinous setae with different filtering capacities. Setae of the secondary filter form a fine sieve preventing larger particles to enter the midgut glands, where the filtrate is further processed and absorbed. In amphipods and mysids, partly degraded food is conveyed to the midgut/hindgut region through an extensive canal system of the stomach (Storch and Štrus 2004) that ends with a funnel (Fig. 6a) which voids food particles to the midgut. In isopods, the canals and funnel are not prominent as the midgut is reduced and coarse particles are directed to the hindgut through the dorsal part of the stomach (Fig. 6b).
The long hindgut in terrestrial isopods is divided into the anterior chamber, papillate region and rectum, which is separated from the papillate region by a muscular sphincter. The hindgut is generally in direct contact with the stomach (Vernon et al. 1974; Hassall and Jennings 1975; Holdich and Mayes 1975; Bettica et al. 1987; Storch and Štrus 1989; Wägele 1992; Rode and Drašlar 1998).
The anterior hindgut is a large storage chamber where food can be further digested or stored in species with limited food supply. A prominent typhlosole forms two dorsolateral channels (Fig. 7a, b) connected to the stomach anteriorly, while in amphibious species the typhlosole is a transient structure (Štrus et al. 1995). In the anterior hindgut, the food is processed and partially absorbed, as indicated by the structural characteristics of its epithelium (Hryniewiecka-Szyfter and Storch 1986). However, the digestion products are mainly transported along the typhlosole channels back to the stomach, where they are filtered into the hepatopancreas where nutrients are absorbed (Hames and Hopkin 1989).
Hindgut structures in terrestrial isopod Porcellio scaber. a Internal view of dorsal hindgut wall in anterior chamber. The dorsal hindgut wall is folded into a prominent typhlosole (T) and two dorsolateral channels (Ch). SEM. Scale bar 100 μm. b Cross section of hindgut in anterior chamber. Distended dorsal hindgut cells form a typhlosole and two dorsolateral channels. Lateral and ventral hindgut cells (HC) protrude into the hindgut lumen (L) with their dome-shaped apical parts. Richardson’s staining. Scale bar 100 μm. c External view of hindgut papillate region. Hindgut cells protrude with their basal parts (arrows) between the longitudinal and circular muscles surrounding the hindgut. SEM. Scale bar 200 μm. d Cross section of hindgut epithelium in papillate region. Dome-shaped basal parts (arrows) of hindgut cells bulge into the haemocoel (H). The hindgut lumen is filled with food. Richardson’s staining. Scale bar 100 μm. e Ultrastructure of hindgut cuticle in anterior chamber. The hindgut cuticle (C) consists of epicuticle (Ep) and procuticle (Pr) with discernible sublayers (arrows). TEM. Scale bar 1 μm. f Surface of hindgut cuticle bearing numerous posteriorly oriented spines. SEM. Scale bar 1 μm. g Apical plasma membrane of hindgut cells covered by cuticle and forming densely stacked apical infoldings (arrows) associated with numerous mitochondria (M). TEM. Scale bar 1 μm. h Basal plasma membrane of hindgut cells in papillate region folded into extensive basal labyrinth. Numerous basal infoldings (arrows) are in close contact with the abundant mitochondria (M). BL basal lamina. TEM. Scale bar 1 μm
The posterior hindgut of terrestrial species is also described as the papillate region due to prominent protuberances (papillae) on its outer surface (Fig. 7c). The papillae are the dome-shaped basal parts of the epithelial cells (Fig. 7d), protruding into the haemocoel between the longitudinal and circular muscles that envelop the hindgut (Hassal and Jennings 1975; Štrus et al. 1995; Rode and Drašlar 1998). The main functions of the papillate region, as indicated by its structural characteristics, are ion transport and water conservation (Vernon et al. 1974; Coruzzi et al. 1982; Palackal et al. 1984). The dry faecal pellets produced by terrestrial isopods are compacted in a short and extensively folded rectum and excreted through the anus (Hames and Hopkin 1989; Storch and Štrus 1989).
The entire hindgut is lined by a thin cuticle, which consists of an electron dense epicuticle and electron lucent procuticle (Fig. 7e). The surface of the epicuticle bears numerous posteriorly oriented spines (Fig. 7f), which direct the faeces towards the anus (Vernon et al. 1974; Palackal et al. 1984; Storch and Štrus 1989; Mrak et al. 2015). Bacteria are attached to the tips of these cuticular spines (Kostanjšek et al. 2006, 2007).
The characteristic ultrastructural features of the hindgut epithelial cells associated with their transport and barrier functions are extensive apical and basal plasma membrane infoldings associated with numerous mitochondria (Fig. 7g, h), abundant apico-basally oriented microtubules and extensive septate junctions on the interdigitated lateral plasma membranes (Vernon et al. 1974; Coruzzi et al. 1982; Palackal et al. 1984; Hryniewiecka-Szyfter and Storch 1986; Storch and Štrus 1989). The presence of Na+/K+-ATPase was demonstrated in the apical membranous invaginations of the epithelial cells in the hindgut of Armadillo officinalis (Warburg and Rosenberg 1989). In P. scaber, the ultrastructural differences between the anterior and posterior hindgut cells are consistent with the proposed absorption of digestion products in the anterior hindgut and the transepithelial transport of water and ions in the posterior hindgut (Bogataj et al. 2018).
Food consumption and food assimilation efficiency in decapods and peracarids
McGaw and Curtis (2013) compiled the duration of the entire digestive process in several Decapoda and found considerable variation, depending on species, meal and environmental conditions. The shortest retention time of food in the digestive tract was 1–2 h in penaeid shrimps fed on shrimps, and the longest reliably measured retention time was 72 h in crab Liocarcinus puber fed with brown algae, suggesting that animal prey is faster digested than plant material.
The variation in food consumption in different species of terrestrial isopods was described by Warburg (1987). In Porcellio scaber, the consumption is about 1.2 to 2.7% body mass/day, when fed on different leaf litters, and food assimilation efficiency is between 30 and 50% (Nair et al.1994). Food can remain in the gut for 4–17 h (Hartenstein 1964) and is digested and absorbed in a 24-h digestive cycle (Hames and Hopkin 1991). When the feeding rate is lower, food remains in the gut for a longer period of time. Porcellio scaber produce 10–35 faecal pellets per day with up to 7 pellets filling the entire gut (Kostanjšek et al. 2003).
Bacterial symbionts in the intestine of decapods and peracarids
Bacteria are important components of crustacean nutrition. They regularly enter the gut together with the food. Real intestinal endosymbionts were described in decapods from hydrothermal vents (Pakes et al. 2014; Zhang et al. 2017). The foregut of crustaceans is generally poorly inhabited by microorganisms, while in the hindgut, varied microbiota is often present.
The intestine of Decapoda was repeatedly shown to harbour bacterial communities. These communities were mostly analysed by DNA fingerprinting. However, it is still controversial whether these bacteria were beneficial symbionts resembling the microflora of humans or whether they were accidentally internalized with the food. For example, in the hindgut of Astacus astacus, Astacus leptodactylus and Pacifastacus leniusculus 350 bacterial strains were identified, mainly Pseudomonas, Aeromonas and Enterobacteriaceae (Mickėnienė 1999). Their concentration was 106–108 bacteria per gram gut content, and they produced 18 essential amino acids, which could principally contribute to crayfish nutrition. The digestive tract of Litopenaeus vannamei harboured diverse bacteria as well, which have the potential to degrade the dietary components (Tzuc et al. 2014). In the hepatopancreas of shrimps and crayfish, we have never seen bacteria except in individuals infected by pathogens (Vogt and Štrus 1998).
There are several reports on the presence of bacteria and fungi in the digestive system of terrestrial isopods, but their role in digestion still remains to be elucidated. Terrestrial isopods support microbial activity by mechanical degradation of the substrate and use the microorganisms in their guts as nutritional source. Molecular approaches applying 16S rRNA sequence analysis indicated the presence of resident bacteria in the gut of terrestrial isopods (Kostanjšek et al. 2002). Rod-like bacteria attached to the hindgut cuticle were described in the posterior hindgut of Porcellio scaber (Kostanjšek et al. 2003). Phylogenetic analysis based on 16S rRNA gene sequences grouped the bacteria within Mollicutes. In 16S rRNA clone libraries, mycoplasma-like bacteria (Firmicutes) were the most common bacteria present in sea slaters of the genus Ligia from the intertidal zone along the Eastern Pacific (Eberl 2012).
Whereas attached Mollicutes were recognized as isopod commensals highly adapted to their gut, intracellular Rhabdochlamydiae represent the first description of a chlamydial infection in arthropods and are currently recognized as human emerging pathogen. The isopod gut contains cellulolytic enzymes, which may be produced by microorganisms. However, functional cellulases of host origin have been extracted from the midgut caeca in Porcellio scaber (Kostanjšek et al. 2010) and production of endogenous cellulases was also reported for different decapod crustaceans (Linton et al. 2006), indicating that symbionts are not principally required for the digestion of cellulose. Several bacterial symbionts were described in the hepatopancreas of terrestrial isopods, and current knowledge on these microbiomes is reviewed by Bouchon et al. (2016).
Structure, function and dynamics of the endodermal parts of the malacostracan digestive tract
The endodermal parts of the digestive tract of malacostracans, mainly the midgut glands and hepatopancreas, serve for secretion of the digestive enzymes, absorption of nutrients, storage of energy and nutrient reserves, supply of the body with metabolites, storage of calcium during ecdysis, synthesis of blood proteins and detoxification of xenobiotics.
Midgut and hepatopancreas of the Decapoda
The endodermal part of the decapod digestive tract consists of the midgut canal, the anterior and posterior dorsal caeca and the numerous lateral caeca that form the hepatopancreas (Icely and Nott 1992).
The midgut is long in some decapod groups like penaeid shrimps and lobsters and very short in brachyuran crabs and crayfish (Icely and Nott 1992). The midgut cells of the shrimp Penaeus monodon have a well-developed microvillar border (Fig. 5b). Since they also include lipid droplets (Fig. 5b), they obviously can absorb, metabolize and store nutrients. However, most nutrients are absorbed and metabolized by the hepatopancreatic R cells as detailed below. The midgut cells of decapods further include plenty of rER and Golgi bodies (Fig. 5d) that are involved in synthesis of a peritrophic membrane (Fig. 5c) that surrounds the faeces. This structure creates a space between the microvillar border and the faeces that allows transport of fluids anterio-posteriorly and vice versa.
The anterior dorsal caecum of adults is composed of the same cell type as the midgut as revealed by us in the shrimps Penaeus monodon and Palaemon elegans and the crayfish Astacus astacus. It includes stem cells that regenerate the midgut epithelium from the anterior and is thus the origin proper of the midgut in which we have not seen mitotic stages. In developing stages, the anterior dorsal caecum absorbs the yolk as described above. The posterior dorsal midgut caecum also consists of the same cell type (Mykles 1979) and seems to regenerate the midgut from the posterior end. It is unknown whether the anterior and posterior caeca have additional functions in adults (Icely and Nott 1992).
The hepatopancreas of decapods is a bilobed organ composed of hundreds to thousands of blindly ending tubules (Fig. 8a). Each tubule is composed of a single layered epithelium (Fig. 8c) surrounded by haemolymph sinuses (Fig. 8d). The tubules include E cells (embryonic cells), R cells (resorptive cells), F cells (fibrillar cells), B cells (blister-like cells) and M cells (midget cells) (Fig. 8c) (Loizzi 1971; Al-Mohanna and Nott 1986, 1987, 1989; Vogt 1985, 1993, 1994).
Hepatopancreas of the Decapoda. a Bilobed hepatopancreas of crayfish Pacifastacus leniusculus consisting of hundreds of blindly ending tubules (arrow). Scale bar 10 mm (from Vogt 2002). b Oblique section through distal end of hepatopancreas tubule of Procambarus virginalis showing various mitotic stages (arrow). HS haemolymph space, L tubular lumen. Goldner’s staining. Scale bar 20 μm (from Vogt 2008b). c Transversal section of hepatopancreas tubule of Astacus astacus showing R cells (R), F cells (F), B cells (B) and M cells (arrow). PAS staining. Scale bar 50 μm (from Vogt 2002). d Transversal section of hepatopancreas of Astacus astacus showing tubules and interspersed haemolymph sinuses (yellow). Immunohistochemistry with haemocyanin antibodies. Scale bar 100 μm (from Vogt 2002). e Longitudinal section of hepatopancreas tubule of Penaeus monodon showing increase of copper deposits (arrow) from E cell zone (EZ) to B cell zone (BZ), i.e. along the age gradient of cells. Arrowhead tubular lumen, DZ differentiation zone. Rubeanic acid histochemistry for copper. Scale bar 30 μm (from Vogt and Quinitio 1994)
Four zones can be distinguished along each tubule: E cell zone, differentiation zone, B cell zone and proximal zone (Fig. 8e). The E cells are stem cells and located at the distal tubular ends (Fig. 8b) (Al-Mohanna et al. 1985a; Vogt 1994). They give rise to new R-, F- and B cells (Fig. 8b), which are pushed downstream by further mitotic pulses establishing a distinct age gradient along the tubule (Vogt 1993, 1994). The B cell zone is dominated by mature B cells. The proximal zone is devoid of B cells because these are discharged at the end of the B cell zone by holocrine secretion during a late phase of digestion (see below). R cells and F cells are discharged from the epithelium at the proximal end of the tubules. In crayfish, regeneration of the complete hepatopancreas seems to last less than 2 weeks as revealed with autoradiography (Davis and Burnett 1964).
Each hepatopancreas tubule is surrounded by a muscle net consisting of longitudinal and circular fibres and muscle cell bodies protruding into the haemolymph sinuses (Fig. 11f). These cell bodies include the nucleus, myofilaments, mitochondria, rER and free ribosomes. The muscle net serves for filling and emptying of the tubules.
Nerves have been found along neither the muscle net nor the hepatopancreas epithelium, suggesting that this organ is hormonally controlled.
Synthesis of digestive enzymes in F cells
The hepatopancreas synthesizes carbohydrases including cellulase, lipases and proteinases. An overview of the digestive enzymes of Decapoda and their properties is given in Saborowski (2015). The digestive enzymes are synthesized in the F cells as shown by immunocytochemistry (Fig. 9d, e) (Vogt et al. 1989a; Möhrlen et al. 2001) and in situ hybridization (Lehnert and Johnson 2002). The F cells show the typical ultrastructure of protein synthesizing and exporting cells. They are characterized by abundant rough endoplasmic reticulum (rER) and Golgi bodies (Fig. 9a). The Golgi bodies are considerably larger than those of the other hepatopancreatic cell types and produce large Golgi vesicles (Fig. 9c), which migrate to the microvillar border (Fig. 9b) to discharge their contents into the tubular lumen. Immunohistochemistry with antibodies raised against the proteinases astacin, trypsin and carboxypeptidase revealed intense staining of the Golgi bodies of the F cells and the lumina of the hepatopancreatic tubules (Fig. 9d, e) (Vogt et al. 1989a). The R cells and B cells were generally negative.
Hepatopancreatic F cell and cytological aspects of the synthesis of digestive enzymes in the Decapoda. a Longitudinal section of F cell of Penaeus monodon characterized by abundant rough endoplasmic reticulum (rER) and large Golgi bodies (arrow). Arrowhead basal lamina, B B cell, M mitochondrium, MB microvillar border, N cell nucleus, R R cell. TEM. Scale bar 3 μm (from Vogt 1985). b Apex of F cell of Penaeus monodon showing Golgi vesicles ready for secretion (arrow). TEM. Scale bar 1 μm. c Golgi body of F cell of Astacus astacus with large Golgi vesicles (arrow) at trans-side and small shuttling vesicles (arrowhead) at cis-side. TEM. Scale bar 1 μm (from Vogt et al. 1989a). d Oblique section through hepatopancreas epithelium of Astacus astacus showing intense staining of Golgi bodies in F cells for the digestive enzyme astacin (arrow). Immunohistochemistry with astacin antibodies. Scale bar 30 μm (from Vogt et al. 1989a). e Longitudinal section of two F cells in Astacus astacus showing astacin-related immunofluorescence in Golgi bodies (arrows) and tubular lumen (asterisk). Immunohistochemistry with astacin antibodies. Scale bar 20 μm (from Vogt 2002). f 3D-model of astacin. Arrow substrate binding cleft, Zn zinc in active centre of enzyme (from Bode et al. 1992)
The mode of synthesis and storage of the digestive enzymes in the Decapoda deviates considerably from the well-known scheme of the vertebrate pancreas. After synthesis in the rER and processing in the Golgi bodies, the digestive enzymes are directly transferred to the tubular lumen via Golgi vesicles. There is no extensive intracellular storage of the proenzymes in zymogen granules as is the case in the exocrine cells of the vertebrate pancreas. The proenzymes are activated on their way to the stomach or in the stomach as demonstrated for astacin (Möhrlen et al. 2001). Astacin (Fig. 9f) is a zinc-endopeptidase named after Astacus astacus (Bode et al. 1992). It consists of 200 amino acids, occurs in the gastric fluid of Astacus astacus in a concentration of ~ 1 mg per ml and cleaves native collagen without prior acidic denaturation.
In the cardiac stomach, the enzymes are stored in an active molecular (i.e. with the pro-part cut off) form to await the next meal as demonstrated for astacin, trypsin and carboxypeptidase (Vogt et al. 1989a; Vogt 2002). The enzymes are highly stable and well adapted to this extraordinary mode of storage (Saborowski 2015). In crayfish, the gastric fluid can be experimentally withdrawn through the oesophagus with a pipette, which stimulates enzyme synthesis in the hepatopancreas. Highest synthetic activity of the F cells was observed 1–2 h after stimulation. The stomach was refilled after about 4 h. When Astacus astacus was fed with a single portion of minced meat, the cardiac stomach was emptied in the following 3–4 h and refilled with new digestive fluid after about 5 h (Vogt et al. 1989a). A similar pattern of refilling of the stomach with digestive fluid was observed in crayfish Cherax quadricarinatus (Loya-Javellana et al. 1995).
Absorption of nutrients and storage of reserves in R cells
The nutrients are mainly absorbed, processed and stored by the R cells, the most abundant cell type of the hepatopancreas. The R cells have a polar architecture. Their apical part consists of a microvillar border, mitochondria and short sER cisternae of smooth endoplasmic reticulum (sER) (Fig. 10a, d). The basal part adjoining the haemolymph sinus includes a sER-like tubular system, rER and mitochondria (Figs. 10h and 11a, f). The medial cell area contains the nucleus, lipid globules, rER, mitochondria, Golgi bodies, peroxisomes, autophagosomes and electron dense lysosomes (Fig. 10a) (Loizzi 1971; Vogt 1985, 1994; Al-Mohanna and Nott 1987). The Golgi bodies produce vesicles with characteristic electron dense content, resembling stapled discs (Fig. 10e). These vesicles are not discharged into the tubular lumen suggesting that their content is used for intracellular purposes.
Hepatopancreatic R cell and ultrastructural aspects of the absorption, metabolization and storage of nutrients in Decapoda. a Oblique section through R cells of Penaeus monodon showing microvillar border (MB), cell nucleus (N), lipid globules (LG), rough endoplasmic reticulum (rER), mitochondria (M), autophagosome (A) and lysosome (Ly). TEM. Scale bar 3 μm. b R cell of Penaeus monodon after extended starvation. The cell height is drastically reduced and most cell organelles have been catabolized. Arrow microvillar border, arrowhead basal lamina, HS haemolymph space, L tubular lumen, Mu fibre of muscle net. TEM. Scale bar 1 μm (from Vogt et al. 1985). c Transversal section of hepatopancreas tubule of Astacus astacus showing unspecific esterase activity 5 h after feeding. Enzyme activity is particularly strong along the microvillar border (arrowhead) and in the bases of the R cells (arrow). Histochemistry for unspecific esterase. Scale bar 100 μm (from Gherardi et al. 2010). d Apex of R cell of Astacus astacus 2 h after feeding showing special arrangement of mitochondria and cisternae of smooth endoplasmic reticulum (arrow) underneath the microvillar border. TEM. Scale bar 0.5 μm (from Vogt 1994). e Golgi body of R cell of Astacus astacus showing Golgi vesicles with electron dense material at trans-face (arrow) and small shuttling vesicles at cis-face (arrowhead). TEM. Scale bar 0.2 μm (from Vogt 1994). f Glycogen field (GF) and lipid globules in R cell of Astacus astacus. TEM. Scale bar 1 μm (from Vogt 2002). g Catabolism of lipid globule in R cell of Penaeus monodon in close association with rough endoplasmic reticulum (rER) cisternae (arrow). TEM. Scale bar 0.4 μm. h Tubules of basal labyrinth in vitellogenic Penaeus monodon female including numerous, relatively large electron dense granules (arrow). TEM. Scale bar 0.2 μm (from Vogt et al. 1989b)
Ultrastructure of B cell (a–d) and further hepatopancreatic cell types (e–g) of the Decapoda. a Longitudinal section of young B cell of Penaeus monodon showing microvillar border (MB), absorptive apical complex (AC), zone with subapical vacuoles (S) and basal cell part with nucleus (N), rough endoplasmic reticulum (rER), Golgi bodies (G) and mitochondria (arrow). Arrowhead basal lamina, HS haemolymph space, R R cell. TEM. Scale bar 3 μm (from Vogt 1993). b Longitudinal section of mature B cell of Penaeus monodon showing microvillar border (arrow), apical complex, subapical vacuoles, large central vacuole (CV) and rather electron dense basal cytoplasm with nucleus. The cell is largely detached from the basal lamina (arrowhead). TEM. Scale bar 5 μm (from Vogt 1993). c Apical complex of B cell of Penaeus monodon showing endocytotic channels (arrow), endocytotic vesicles, darker Golgi vesicles (arrowhead) and subapical vacuoles. TEM. Scale bar 1 μm (from Vogt 1993). d Golgi body of B cell of Astacus astacus with small Golgi vesicles at trans-face (arrow) and shuttling vesicles at cis-face (arrowhead). TEM. Scale bar 0.2 μm. e M cell of Penaeus monodon with nucleus and large granular inclusion (GI). TEM. Scale bar 1 μm (from Vogt 1985). f Muscle net around hepatopancreas tubule showing cell body with nucleus and circular (CM) and longitudinal (LM) muscle fibrils. Arrow basal lamina, arrowhead basal tubule system of R cell. TEM. Scale bar 1 μm (from Vogt 1985). g Longitudinal resin section of hepatopancreas tubule of cavernicolous shrimp Troglocaris anophthalmus showing large terminal oleosphere (O) filled with lipid. Arrow lipid globule in R cell, arrowhead tubular lumen, B B cell. Richardson’s staining. Scale bar 50 μm (from Vogt and Štrus 1999)
In the first 2 h after feeding of Astacus astacus, there were marked ultrastructural alterations in the cell apices when compared to unfed specimens. Numerous mitochondria accumulated beneath the microvillar border and sER cisternae became vertically arranged between the mitochondria and the microvillar border (Fig. 10d). This special arrangement of cell organelles may reflect the uptake of fatty acids and their transport along the sER membranes to the mitochondria as observed in nutrient absorbing cells of mammals. This cell area also stained intensely for unspecific esterase (Fig. 10c), probably reflecting uptake and processing of the nutrients. In unfed specimens, only the microvillar border was stained for this enzyme. The nutrients pass the apical plasma membrane in molecular form, mostly via carriers for monosaccharides, amino acids and dipeptides, which have been identified and characterized in lobsters (Ahearn 1987; Saborowski 2015). At 5 h after feeding of Astacus astacus, unspecific esterase activity was mainly found in the basal part of the R cells (Fig. 10c), reflecting final processing of the nutrients for export into the haemolymph. The basal tubule system probably synthesizes the high density lipoproteins that are delivered to other organs via the haemolymph. Interestingly, the HDL of decapods includes phospholipids instead of triglycerides as a major lipid component (Abdu et al. 2000).
The R cells can store large amounts of lipids and glycogen (Fig. 10a, f) (Loizzi 1971; Vogt 1994). Lipid globules were found in all shrimps, crayfish and crabs investigated, but glycogen fields composed of α-glycogen particles were lacking in penaeid shrimps. These nutrient and energy reserves are mobilized during moulting (Al-Mohanna and Nott 1989), starvation (Vogt et al. 1985) and vitellogenesis (Vogt et al. 1989b). They are sufficient to meet the energy requirements during moulting and short-term starvation (Storch and Anger 1983; Vogt et al. 1985), but if starvation continues after their depletion, the cell organelles of all hepatopancreatic cells are catabolized (Fig. 10b) (Vogt et al. 1985). The breakdown of the lipid globules is often reflected by their close association with rER cisternae (Fig. 10g) and peroxisomes.
An unusual mode of extensive lipid storage aside of the lipid globules in the R cells was found in the cavernicolous shrimp Troglocaris anophthalmus. This shrimp has evolved large chambers at the distal ends of the hepatopancreas tubules that are completely filled with oil (Fig. 11g). These energy reserves enabled the shrimps to survive in the laboratory without feeding for more than 2 years (Vogt and Štrus 1999).
Vitellogenic females produce vitellogenins in the hepatopancreas that are delivered to the ovary to be transformed into vitellins. Vitellogenins are glycolipoproteins and have a higher molecular mass than the normally produced lipoproteins (Soroka et al. 2000). The vitellogenin synthesizing cell type is probably the R cell as may be deduced from striking ultrastructural changes in this life period. The R cells of vitellogenic Penaeus monodon and Palaemon elegans were characterized by large whorls of elongate and annulate rER cisternae, the above-mentioned signs of catabolism of the lipid stores and the presence of high amounts of electron dense particles in the basal tubule system (Fig. 10h) (Vogt et al. 1989b). These particles were much bigger than corresponding particles in non-vitellogenic females and probably represent the vitellogenins.
Ultrastructure and potential function of B cells
The B cells are the most enigmatic cell types of the hepatopancreas. Mature B cells are characterized by a large central vacuole. They are discharged from the epithelium at the end of the B cell zone by holocrine secretion in an unknown relationship to digestion. Earlier, B cells were considered as mature F cells and their central vacuole was interpreted as a large zymogen storing compartment (Al-Mohanna et al. 1985a; Al-Mohanna and Nott 1986). However, immunohistochemistry and in situ hybridization have clearly shown that B cells do not synthesize the proteolytic digestive enzymes (Fig. 9d). Moreover, B cells originate directly from E cells as shown for Penaeus monodon (Vogt 1993) and Astacus astacus (Vogt 1994). Young B cells and F cells look similar and can only be distinguished from each other by their characteristic Golgi bodies. These are small in B cells (Fig. 11d) but large in F cells (Fig. 9e) and produce different Golgi vesicles as shown by us for Penaeus monodon, Palaemon elegans and Astacus astacus.
Young B cells have contact to the tubular lumen and the haemolymph sinus (Fig. 11a) but detach from the basal lamina when they grow older (Fig. 11b) (Vogt 1993). B cells are characterized by an apical complex consisting of endocytotic channels and vesicles and Golgi vesicles that fuse with the endocytotic vesicles to form subapical vacuoles (Fig. 11c). These vacuoles then fuse with each other to form larger vacuoles and finally the central vacuole (Fig. 11a–c). The basal part of B cells includes the nucleus, rER, mitochondria and Golgi bodies (Fig. 11a, b) (Vogt 1993, 1994).
There is no doubt that B cells synthesize proteins on the one hand and absorb material from the tubular lumen on the other hand. One of the synthesized proteins is cathepsin L, a typical lysosomal enzyme that is also present in the digestive fluid of decapods (Hu and Leung 2007). It is not yet known what kind of material the B cells absorb. It is certainly not the low molecular weight products of digestion because these are absorbed by the R cells. Perhaps they absorb special nutrients like cholesterol, which is also absorbed in the vertebrate intestine by endocytosis. The relatively early detachment of the cells from the basal lamina does not fit to a cell type that absorbs nutrients for delivery into the haemolymph. The cell ultrastructure rather suggests that the absorbed material is degraded and a considerable proportion of the degradation product is accumulated in the central vacuole which may be interpreted as a large heterophagosome. The vacuolar content is discharged into the tubular lumen by holocrine secretion of the B cells and may be added to the gastric fluid either for further digestion or, more likely, as an essential component of the gastric fluid.
In Astacus astacus, the B cells were discharged from the epithelium about 6 h after feeding at a time, when the cardiac stomach was already refilled with digestive enzymes. The discharged B cells and their vacuoles disintegrated in the hepatopancreas tubules releasing the content of the central vacuole. Interestingly, Penaeus monodon fed for a longer period of time with an artificial lipid diet had plenty of B cells with particularly large central vacuoles, whereas specimens fed with pure protein and carbohydrate diets had only B cells with small central vacuoles. This may speak for the involvement of B cells in the digestion of lipids. Perhaps, they synthesize and even recycle the fat emulsifiers, which are acyltaurines and acylsarcosyltaurines in the Decapoda (Van den Oord 1966).
Ultrastructure and potential function of M cells
The M cells are the smallest and least frequent cells in the hepatopancreas epithelium (Fig. 8c) and were first described in Penaeus semiculcatus as cells with storage function by Al-Mohanna et al. (1985b). They are individually located on the basal lamina with close contact to the haemolymph sinuses and have no contact to the tubular lumen (Fig. 11e). They show the typical ultrastructure of peptide synthesizing endocrine cells. The form of their granular inclusions varies considerably between species. In Penaeus monodon, there was mainly one large inclusion (Fig. 11e), whereas in Astacus astacus, there were generally numerous small granules (Vogt 1994). M cells are probably not descendants of the hepatopancreatic E cells because they also occur in the midgut. They may be immigrants of a different cell lineage that colonize the hepatopancreas in an early phase of development and establish a self-perpetuating cell population.
The content of the M cells of the hepatopancreas is not yet identified, but they probably include the same hormones as their identically looking counterparts in the midgut (Christie et al. 2007). M cells are thought to regulate either the function and dynamics of neighbouring epithelial cells or the activity of the muscle net that lacks neuromotoric connections (Vogt 1994). The regulation of hepatopancreatic functions by hormones from extrinsic sources is better known. For example, injection of crustacean hyperglycemic hormone (CHH) from the x-organ-sinus gland into the haemolymph of crayfish Faxonius limosus elicited catabolism of the glycogen stores in the R cells (Kummer and Keller 1993). Release of glucose was detectable from 10 min after injection and attained a maximum after 2 h. CHH also seems to stimulate release of fatty acids and phospholipids from the hepatopancreatic lipid stores (Santos et al. 1997).
Synthesis of haemolymph proteins
The hepatopancreas synthesizes at least five macromolecules for secretion into the haemolymph, namely haemocyanin, lipoprotein, vitellogenin, pathogen recognizing and binding protein and clotting protein. The lipoproteins and vitellogenins are probably synthesized in the R cells as discussed above.
The dominant protein in the haemolymph is the oxygen carrier haemocyanin, which often exceeds 90% of the protein fraction. It is a polymer composed of several subunits (Stöcker et al. 1988) and is synthesized in the hepatopancreas as shown by Khayat et al. (1995). In situ hybridization of probes of the haemocyanin gene to hepatopancreas sections of Penaeus monodon suggests that the haemocyanin is synthesized in F cells (Lehnert and Johnson 2002; Wang 2007). We were not able to localize haemocyanin in the hepatopancreas cells of Astacus astacus by means of antibodies against the polymer although the intertubular haemolymph sinuses stained intensely (Fig. 8d). This feature may suggest that only the subunits are synthesized within the epithelial cells and the polymers are assembled outside in the haemolymph.
Bacterial and fungal pathogens release lipopolysaccharide (LPS) and β-1,3-glucan from their cell walls, respectively. In decapods, these molecules are recognized and bound by the LPS and β-1,3-glucan-binding protein (LGBP) (Cerenius et al. 1994). This complex then binds to receptors of the haemocytes and triggers the melanization and encapsulation reaction that eliminates the pathogens. In shrimp Litopenaeus vannamei, LGBP is synthesized in the hepatopancreas (Phupet et al. 2018). Its expression was upregulated after injection of Vibrio parahaemolyticus, white spot syndrome virus, lipopolysaccharide and ß-1,3-glucan into the haemolymph. The synthesizing cell type is probably the F cell.
Decapods possess an effective system of wound closure. The involved clotting protein is a homologue of the vitellogenins but occurs in both sexes (Hall et al. 1999). It is produced in the hepatopancreas, but the synthesizing cell type is unknown.
Detoxification of xenobiotics
The hepatopancreas of decapods is also a centre of the detoxification of xenobiotics. Environmental heavy metals are removed by storage excretion in lysosomes of the R cells and F cells. For instance, in the shrimp Penaeus monodon exposed to water-borne copper, the metal was deposited in the lysosomes of the R cells as an inert sulphite (Fig. 8e) (Vogt and Quinitio 1994), whereas in crayfish, water-borne iron was mainly accumulated in the F cells and only to a lower degree in the R cells (Roldan and Shivers 1987; Vogt 2002). The copper accumulating lysosomes in Penaeus monodon became larger along the tubules (i.e. with age of the cells) and were extruded from the epithelium at the proximal end of the tubules where the oldest cells are discharged. The metal granules were added to the faeces. Heavy metals can also be detoxified by binding to cytoplasmic metallothioneins as shown for cadmium in crayfish Procambarus clarkii (Del Ramo et al. 1989).
The hepatopancreas is also capable of detoxifying organic compounds with the help of cytochrome P450, which was repeatedly found in this organ (James and Boyle 1998). Cytochrome P450 is inducible as was shown in Pacifastacus leniusculus by the injection of TCDD (2,3,7,8-tetrachloro-dibenzo-p-dioxin) (Ashley et al. 1996).
Special structures and functions
Curious transformation of parts of the hepatopancreas are the lipid storing oleospheres of the cave-dwelling shrimp Troglocaris anophthalmus described above and the light-emitting organs of shrimps of the genus Sergestes. These bioluminescent organs are transformed hepatopancreas tubules consisting of cells with fluorescent paracrystalline platelets, which are the sources of the luminescence, and adjacent cells filled with lipid droplets which act as a diffuse reflector (Herring 1982). Further hepatopancreatic photophores with different architectures are known from other shrimp genera. Some caridean shrimps from the deep sea can eject a substance from the stomach that forms a luminous cloud upon contact with the water. This fluorescent material is thought to be synthesized in the hepatopancreas as well (Herring 1976).
Midgut and hepatopancreas of the Peracarida
The endodermal part of the digestive system differs markedly between the higher taxa of the peracarids. The amphipod midgut is a long weakly muscularized tube with variable numbers of caeca with different functions (Schmitz 1992). In isopods, the midgut portion of the alimentary canal is reduced or even completely absent in terrestrial woodlice (Holdich and Mayes 1975, Bettica et al. 1987; Storch and Štrus 1989). In amphibious woodlice of the family Ligiidae, a short ring of midgut cells is present in the area of the foregut-hindgut junction (Fig. 12a, b). These dome-shaped cells with microvilli, highly vacuolated cytoplasm and abundant Golgi fields (Fig. 12c, d) may secrete digestive juices.
Midgut of the amphibious isopod Ligia italica. a Cross section of digestive system in the area of the foregut-hindgut junction. The posterior part of the stomach (S) with dorsal and ventral lamellae (DL, VL) is dorsally surrounded by the hindgut (H), laterally by the midgut (M) and ventrally by the midgut glands (MG) which are connected to the atrium (A) of the stomach. HE staining. Scale bar 50 μm. b SEM micrograph of endodermal midgut separated from hindgut by circular junctional fold (plica circularis, PC), both covered by cuticle. Scale bar 100 μm. c Midgut cells with prominent microvillar surface. SEM. Scale bar 10 μm. d Section of midgut cells with large nuclei, numerous Golgi fields and vacuolated cytoplasm. TEM. Scale bar 5 μm
The cells of the midgut canal are very similar in all Peracarida. Cuboidal or columnar cells with prominent microvillar border have numerous mitochondria and smooth ER in their basal cytoplasm. They lie on a thick and densely folded basal lamina. In amphipods, they contain abundant vacuoles which are probably involved in the production of the peritrophic membrane surrounding the faecal pellets. Cell shape and ultrastructure change in relation to feeding and moulting. A detailed description of the midgut cells during the moult cycle of the amphipod Gammarus pulex is presented by Trevisan et al. (2014).
In terrestrial isopods, the midgut region is restricted to the paired blindly-ending tubules of the hepatopancreas, which open into the digestive tract at the foregut-hindgut junction. The hepatopancreas is composed of four or rarely six blindly-ending tubules, which extend into the body cavity along the hindgut (Hames and Hopkin 1989; Wägele 1992; Štrus et al. 1995). The hepatopancreas of Porcellio scaber consists of only four tubules. Each tubule is surrounded by a muscular network consisting of longitudinal and circular fibres running obliquely around the tubules. These fibres can change their length and diameter and enable peristaltic flux of fluids from and into the hepatopancreas (Fig. 13a). The hepatopancreas of isopods performs a broad range of functions such as absorption and storage of lipids, carbohydrates, proteins and metals, secretion of digestive enzymes, excretion of metals and probably osmoregulation (Storch 1984).
Histology and ultrastructure of the isopod hepatopancreas. a SEM micrograph of hepatopancreatic tubule in Ligia italica. Circular and longitudinal muscles extend between the epithelial cells. Scale bar 50 μm. b Cross section of hepatopancreatic tubule in Porcellio scaber. Large, dome-shaped B cells (B) alternate with small, wedge-shaped S cells (S). Richardson’s staining. Scale bar 100 μm. c Apical membranes of B and S cells forming regular microvilli (arrow). B cells harbour numerous lipid droplets (LD). TEM. Scale bar 10 μm. d SEM micrograph of B cells in Ligia italica secreting digestive juices. Scale bar 10 μm. e Ultrastructure of B cells. Abundant rough endoplasmic reticulum (ER) organized in stacks of parallel cisternae, numerous Golgi stacks (GS), lipid droplets and glycogen fields (arrows) are characteristic. TEM. Scale bar 2 μm. f Basal plasma membrane of both cell types enlarged by deep invaginations (arrows) associated with mitochondria (M). BL basal lamina. TEM. Scale bar 5 μm
In the single-layered hepatopancreatic epithelium, dome-shaped large cells named B cells alternate with wedge-shaped small cells named S cells (Fig. 13b). It has to be noted here that the B cells of isopods are not homologous and ultrastructurally and functionally comparable to the B cells of decapods and amphipods. They have just got the same name historically. The general ultrastructural characteristics of both hepatopancreatic cell types of isopods have been described by several authors (Storch 1984; Bettica et al. 1984; Wägele 1992; Žnidaršič et al. 2003).
The larger B cells are secretory and absorptive and contain energy reserves, mostly lipids and glycogen (Fig. 13c, d). The smaller S cells are the site of accumulation of metals, calcium and urates. The ultrastructure of the B cells is very dynamic and changes according to feeding cycles and environmental influences, resembling the R cells of decapods. Usually, B cells show dense cytoplasm and contain lipid droplets, glycogen fields, abundant rER and Golgi fields (Fig. 13e). The basal membrane area of both cell types is enlarged by deep invaginations associated with mitochondria (Fig. 13f). The morphology of the B cells was monitored during a 24 h digestive cycle (Lešer et al. 2008). It was shown that the daily cycle does markedly affect neither the epithelial thickness nor the abundance of lipid droplets. In contrast, the B cells undergo significant ultrastructural changes during starvation, moulting and when fed with metal contaminated food (Szyfter 1966; Štrus et al. 1985; Štrus 1987; Köhler et al. 1996; Žnidaršič et al. 2003; Odendaal and Reinecke 2003). These structural changes are reversible.
The ultrastructure of the S cells is characterized by abundant sER and electron dense granules. It remains rather constant irrespective of changes in feeding or moult cycle (Hames and Hopkin 1991). Terrestrial isopods can store outstanding amounts of copper in these cells (Wieser 1966; Hopkin and Martin 1982). Copper is accumulated in intracellular granules, which were reported to belong to the lysosomal system, but there is only a few data about the structure, chemical composition and function of these organelles (Wieser and Klima 1969; Prosi and Dallinger 1988).
In terrestrial isopods, the digestive juices are produced in the hepatopancreas tubules and in amphibious species possibly also in the short midgut canal (Štrus et al. 1995). The food consumed by woodlice contains large quantities of carbohydrates, especially lignins and cellulose. The enzymatic composition of the digestive juice was not yet thoroughly investigated but there are some reports on the presence of cellulolytic enzymes in woodlice (Hartenstein 1964; Hassall and Jennings 1975; Kozlovskaja and Striganova 1977). The authors argued that the enzymes might be of endogenous origin or produced by microbiota in the gut. The role of endosymbiotic bacteria in the production of cellulases was proposed by Zimmer and Topp (1998), but the presence of respective endosymbionts was not confirmed with the methods applied. On the other hand, Kostanjšek et al. (2010) report on the presence of endogenous cellulases in terrestrial isopods and gives strong support for the involvement of isopod endo-ß-1,4-glucanases in the degradation of cellulose.
Gammaridean amphipods possess a midgut canal, four paired digestive caeca, a single anterior caecum and a pair of posterior caeca (Schmitz 1992). The digestive caeca form together the hepatopancreas and include R, F and B cells resembling ultrastructurally the corresponding cell types in the Decapoda and Euphausiacea (Schultz 1976). Sequencing of the whole genome of the model amphipod Parhyale hawaiensis revealed the presence of an enzyme set for the digestion of lignocellulose (Kao et al. 2016). In Gammarus pulex, moult-related changes in the midgut and digestive glands were analysed and found to correspond to those described for decapods (Trevisan et al. 2014). The midgut canal remains filled with plant debris until late pre-ecdysis. The accumulation and consumption of lipid reserves in the R cells of the hepatopancreas are synchronized with the moult cycle. They accumulate during intermoult, are most abundant in late pre-ecdysis and are reduced during ecdysis and early postecdysis.
Conclusion and future directions
There is already a considerable amount of literature on the structural features of the malacostracan digestive tract covering different aspects from gross morphology to ultrastructure. However, the vast majority of articles come from relatively few, common and easily available species, species exploited in fisheries and aquaculture and laboratory models.
Most functions of the digestive tract were derived from structural and ultrastructural features and their dynamics in response to changes of the environmental and nutritional conditions. Some functions were observed directly in living specimens, for instance, in semi-transparent species and by endoscopy. Relatively few functions were assigned to particular cell types by in situ hybridization and immunocytochemistry.
Most parts of the malacostracan digestive system are subject to dynamic changes during lifetime. For example, the chewing structures of the foregut are progressively elaborated during larval development and the ultrastructure of the hepatopancreas cells, particularly the nutrient absorbing and storing cells, can reversibly change their appearance in all life stages, mainly in relation to moulting, starvation and vitellogenesis. They can thus be used to estimate the nutritional status of their carriers.
Some structures like the ossicles of the stomach are rather constant within higher taxa and can be used for systematics. Others like the chewing structures of the stomach are to some degree modified in adaptation to the feeding strategy. The most dramatic structural and functional adaptation is shown by terrestrial isopods which have significantly changed the midgut-hindgut system when compared to their aquatic relatives. Very few species are presently known to have evolved unique structures of the digestive system not shared by the other Malacostraca. Examples are the oleospheres in troglobitic shrimps and the light emitting organs in deep sea shrimps.
Future studies should include species with exceptional ecology, lifestyle and feeding strategy and apply modern morphological and cytological techniques. Examples of species from extreme environments are amphipods from the deep sea, isopods from deserts and high mountains, shrimps, amphipods and isopods from polar waters and various malacostracan taxa from subterranean environments. The application of techniques like microtomography, in situ hybridization, immunohistochemistry and the biochemical investigation of isolated cells is expected to provide new insights into the structural-functional relationships of the malacostracan digestive system. Last but not least, the present progress in genomics and transcriptomics, particularly in the amphipod and decapod laboratory models Parhyale hawaiensis and Procambarus virginalis, may greatly facilitate the identification of functions, particularly in the endodermal parts of the digestive tract.
References
Abdu U, Yehezkel G, Sagi A (2000) Oocyte development and polypeptide dynamics during ovarian maturation in the red-claw crayfish Cherax quadricarinatus. Invert Reprod Dev 37:75–83
Abrunhosa F, Kittaka J (1997a) Functional morphology of mouthparts and foregut of the last zoea, glaucothoe and first juvenile of the king crabs Paralithodes camtschaticus, P. brevipes and P. platypus. Fish Sci 63:923–930
Abrunhosa F, Kittaka J (1997b) Morphological changes in the midgut, midgut gland and hindgut during the larval and postlarval development of the red king crab Paralithodes camtschaticus. Fish Sci 63:746–754
Abrunhosa F, Melo M (2008) Development and functional morphology of the foreguts of larvae and postlarvae of three crustacean decapods. Braz J Biol 68:221–228
Abrunhosa F, Melo M, Lima JF, Abrunhosa J (2006) Developmental morphology of mouthparts and foregut of the larvae and postlarvae of Lepidophthalmus siriboia Felder & Rodrigues, 1993 (Decapoda: Callianassidae). Acta Amaz 36:335–342
Abrunhosa FA, Simith DJ, Monteiro JR, Souza Junior AN, Oliva PA (2011) Development and functional morphology of the larval foregut of two brachyuran species from Northern Brazil. An Acad Bras Cienc 83:1269–1278
Abubakr MA, Jones DA (1992) Functional morphology and ultrastructure of the anterior mid-gut diverticulae of larvae of Penaeus monodon Fabricius, 1798 (Decapoda, Natantia). Crustaceana 62:142–158
Ahearn GA (1987) Nutrient transport by the crustacean gastrointestinal tract: recent advances with vesicle techniques. Biol Rev 62:45–63
Ahyong ST, Lowry JK, Alonso M, Bamber RN, Boxshall GA, Castro P, Gerken S, Karaman GS, Goy JW, Jones DS, Meland K, Rogers DC, Svavarsson J (2011) Subphylum Crustacea Brünnich, 1772. Zootaxa 3148:165–191
Al-Mohanna SY, Nott JA (1986) B-cells and digestion in the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda). J Mar Biol Assoc UK 66:403–414
Al-Mohanna SY, Nott JA (1987) R-cells and the digestive cycle in Penaeus semisulcatus (Crustacea: Decapoda). Mar Biol 95:129–137
Al-Mohanna SY, Nott JA (1989) Functional cytology of the hepatopancreas of Penaeus semisulcatus (Crustacea: Decapoda) during the moult cycle. Mar Biol 101:535–544
Al-Mohanna SY, Nott JA, Lane DJW (1985a) Mitotic E- and secretory F-cells in the hepatopancreas of the shrimp Penaeus semisulcatus (Crustacea: Decapoda). J Mar Biol Assoc UK 65:901–910
Al-Mohanna SY, Nott JA, Lane DJW (1985b) M-‘midget’ cells in the hepatopancreas of the shrimp, Penaeus semisulcatus De Haan, 1844 (Decapoda, Natantia). Crustaceana 48:260–268
Anger K (2001) The biology of decapod crustacean larvae. Crustacean issues 14. A.A. Balkema, Rotterdam
Anger K, Storch V, Anger V, Capuzzo JM (1985) Effects of starvation on moult cycle and hepatopancreas of stage I lobster (Homarus americanus) larvae. Helgol Meeresunters 39:107–116
Ashley CM, Simpson MG, Holdich DM, Bell DR (1996) 2,3,7,8-Tetrachloro-dibenzo-p-dioxin is a potent toxin and induces cytochrome P 450 in the crayfish, Pacifastacus leniusculus. Aquat Toxicol 35:157–169
Bettica A, Shay MT, Vernon G, Witkus R (1984) An ultrastructural study of cell differentiation and associated acid phosphatase activity in the hepatopancreas of Porcellio scaber. Symp Zool Soc Lond 53:199–215
Bettica A, Witkus R, Vernon GM (1987) Ultrastructure of the foregut-hindgut junction in Porcellio scaber Latreille. J Crust Biol 7:619–623
Biesiot PM, McDowell JE (1995) Midgut-gland development during early life-history stages of the American lobster Homarus americanus. J Crust Biol 15:679–685
Bode W, Gomis-Rüth FX, Huber R, Zwilling R, Stöcker W (1992) Structure of astacin and implications for activation of astacins and zinc-ligation of collagenases. Nature 358:164–167
Bogataj U, Praznik M, Mrak P, Štrus J, Tušek-Žnidarič M, Žnidaršič N (2018) Comparative ultrastructure of cells and cuticle in the anterior chamber and papillate region of Porcellio scaber (Crustacea, Isopoda) hindgut. In: Hornung E, Taiti S, Szlavecz K (eds) Isopods in a changing world. ZooKeys, vol 801, pp 427–458
Böhm H (1996) Activity of the stomatogastric system in free-moving crayfish, Orconectes limosus Raf. Zoology 99:247–257
Bouchon D, Zimmer M, Dittmer J (2016) The terrestrial isopod microbiome: an all-in-one toolbox for animal microbe interactions of ecological relevance. Front Microbiol 7:1472
Brösing A (2010) Recent developments on the morphology of the brachyuran foregut ossicles and gastric teeth. Zootaxa 2510:1–44
Brösing A, Türkay M (2011) Gastric teeth of some thoracotreme crabs and their contribution to the brachyuran phylogeny. J Morphol 272:1109–1115
Browne WE, Price AL, Gerberding M, Patel NH (2005) Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 42:124–149
Castejon D, Ribes E, Dufort M, Rotlland G, Guerao G (2015a) Foregut morphology and ontogeny of the mud crab Dyspanopeus sayi (Smith, 1869) (Decapoda, Brachyura, Panopeidae). Arthropod Struct Dev 44:33–41
Castejon D, Rotlland G, Ribes E, Dufort M, Guerao G (2015b) Foregut morphology and ontogeny of the spider crab Maja brachydactyla (Brachyura, Majoidea, Majidae). J Morphol 276:1109–1122
Castejon D, Rotlland G, Ribes E, Dufort M, Guerao G (2018) Morphology and ultrastructure of the esophagus during the ontogeny of the spider crab Maja brachydactyla (Decapoda, Brachyura, Majidae). J Morphol 279:710–723
Ceccaldi HJ (2006) The digestive tract: anatomy, physiology, and biochemistry. In: Forest J, von Vaupel Klein JC (eds) Treatise on zoology – anatomy, taxonomy, biology. The Crustacea, vol 2. Brill, Leiden, pp 85–203
Cerenius L, Liang Z, Duvic B, Keyser P, Hellmann U, Palva ET, Iwanaga S, Söderhäll K (1994) Structure and biological activity of a 1,3-ß-D-glucan-binding protein in crustacean blood. J Biol Chem 269:29462–29467
Christie AE, Kutz-Naber KK, Stemmler EA, Klein A, Messinger DI, Goiney CC, Conterato AJ, Bruns EA, Hsu Y-WA, Li L, Dickinson PS (2007) Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus. J Exp Biol 210:699–714
Coleman CO (1992) Foregut morphology of Amphipoda (Crustacea). An example of its relevance for systematics. Ophelia 14:346–370
Coleman CO (1994) Comparative anatomy of the alimentary canal of hyperid amphipods. J Crust Biol 4:346–370
Coruzzi L, Witkus R, Vernon GM (1982) Function-related structural characters and their modifications in the hindgut epithelium of two terrestrial isopods, Armadillidium vulgare and Oniscus asellus. Exp Cell Biol 50:229–240
Davis LE, Burnett AL (1964) A study of growth and cell differentiation in the hepatopancreas of the crayfish. Dev Biol 10:122–153
De Grave S, Pentcheff ND, Ahyong ST, Chan TY, Crandall KA, Dworschak PC, Felder DL, Feldmann RM, Fransen CHJM, Goulding LYD, Lemaitre R, Low MEY, Martin JW, Ng PKL, Schweitzer CE, Tan SH, Tshudy D, Wetzer R (2009) A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool (Suppl 21:1–109
Del Ramo J, Pastor A, Torreblanca A, Medina J, Díaz-Mayans J (1989) Cadmium-binding proteins in midgut gland of freshwater crayfish Procambarus clarkii. Bull Environ Contam Toxicol 42:241–246
Diaz AC, Fernandez Gimenez AV, Velurtas SM, Fenucci JL (2008) Ontogenetic changes in the digestive system of Pleoticus muelleri (Decapoda, Penaeoidea). Invertebr Reprod Dev 52:1–12
Eberl R (2012) Distribution, habitat and food preferences of sympatric high intertidal isopod species Ligia occidentalis and Ligia pallasii (Ligiidae: Oniscidea). J Nat Hist 46:29–30
Factor RJ (1981) Development and metamorphosis of the digestive system of larval lobsters, Homarus americanus (Decapoda: Nephropidae). J Morphol 169:225–242
Factor RJ (1982) Development and metamorphosis of the feeding apparatus of the stone crab, Menippe mercenaria (Brachyura, Xanthidae). J Morphol 172:299–312
Friesen JA, Mann KH, Willison JHM (1986) Gross anatomy and fine structure of the gut of marine mysid shrimp Mysis stenolepis. Can J Zool 64:431–441
Garm A (2004) Mechanical functions of setae from the mouth apparatus of seven species of decapod crustaceans. J Morphol 260:85–100
Gerberding M, Browne WE, Patel NH (2002) Cell lineage analysis of the amphipod crustacean Parhyale hawaiensis reveals an early restriction of cell fates. Development 129:5789–5801
Gherardi F, Souty-Grosset C, Vogt G, Diéguez-Uribeondo J, Crandall KA (2010) Infraorder Astacidea Latreille, 1802 p.p.: the freshwater crayfish. In: Schram FR, von Vaupel Klein JC (eds) Treatise on zoology – anatomy, taxonomy, biology. The Crustacea. Vol 9, part A: Eucarida: Euphausiacea, Amphionidacea, and Decapoda (partim). Brill, Leiden, pp 269–423
Graf F, Michaut P (1980) Fine structure of the midgut posterior caeca in the crustacean Orchestia in intermolt: recognition of two distinct segments. J Morphol 165:261–284
Greenaway P (1985) Calcium balance and moulting in the Crustacea. Biol Rev 60:425–454
Gruner H-E (1993) 1. Klasse Crustacea. In: Gruner H-E (Hrsg) Lehrbuch der Speziellen Zoologie. Band 1: Wirbellose Tiere, 4. Teil: Arthropoda (ohne Insecta). Gustav Fischer Verlag, Stuttgart, pp 448–1030
Hall M, Wang R, van Antwerpen R, Sottrup-Jensen L, Söderhäll K (1999) The crayfish plasma clotting protein: a vitellogenin-related protein responsible for clot formation in crustacean blood. Proc Natl Acad Sci U S A 96:1965–1970
Hames CAC, Hopkin SP (1989) The structure and function of the digestive system of terrestrial isopods. Zoology 217:599–627
Hames CAC, Hopkin SP (1991) A daily cycle of apocrine secretion by the B cells in the hepatopancreas of terrestrial isopods. Can J Zool 69:1931–1937
Hartenstein R (1964) Feeding, digestion, glycogen, and the environmental conditions of the digestive system in Oniscus asellus. J Insect Physiol 10:611–612
Hassall M, Jennings JB (1975) Adaptive features of gut structure and digestive physiology in the terrestrial isopod Philoscia muscorum (Scopoli) 1763. Biol Bull 149:348–364
Herring P (1976) Bioluminescence in decapod Crustacea. J Mar Biol Assoc UK 56:1029–1047
Herring P (1982) The comparative morphology of hepatic photophores in decapod Crustacea. J Mar Biol Assoc UK 61:723–737
Hinton DJ, Corey S (1979) The mouthparts and digestive tract in the larval stages of Homarus americanus. Can J Zool 57:1413–1423
Holdich DM, Mayes KR (1975) A fine-structural re-examination of the so-called ‘midgut’ of the isopod Porcellio. Crustaceana 29:186–192
Hopkin S, Martin M (1982) The distribution of zinc, cadmium, lead and copper within the hepatopancreas of a woodlouse. Tissue Cell 14:703–715
Hornung E (2011) Evolutionary adaptation of oniscidean isopods to terrestrial life: structure, physiology and behaviour. Terr Arthropod Rev 4:95–130
Hryniewiecka-Szyfter Z, Storch V (1986) The influence of starvation and different diets on the hindgut of isopoda (Mesidotea entomon, Oniscus asellus, Porcellio scaber). Protoplasma 134:53–59
Hu KJ, Leung PC (2007) Food digestion by cathepsin L and digestion-related rapid cell differentiation in shrimp hepatopancreas. Comp Biochem Physiol B 146:69–80
Icely JD, Nott JA (1984) On the morphology and fine structure of the alimentary canal of Corophium volutator (Pallas) (Crustacea: Amphipoda). Phil Trans R Soc Lond B 306:49–78
Icely JD, Nott JA (1992) Digestion and absorption: digestive system and associated organs. In: Harrison FW, Humes AG (ed) Microscopic anatomy of invertebrates. Vol 10: Decapod Crustacea. Wiley-Liss, New York, pp 147–201
James MO, Boyle SM (1998) Cytochromes P450 in Crustacea. Comp Biochem Physiol 121C:157–172
Kao D, Lai AG, Stamataki E, Rosic S, Konstandinides N, Jarvis E, Di Donfrancesco A, Pouchkina-Stancheva N, Sémon M, Grillo M, Bruce H, Kumar S, Siwanowicz I, Le A, Lemire A, Eisen MB, Extavour C, Browne WE, Wolff C, Averof M, Patel NH, Sarkies P, Pavlopoulos A, Aboobaker A (2016) The genome of the crustacean Parhyale hawaiensis, a model for animal development, regeneration, immunity and lignocellulose digestion. eLife 5:e20062
Khayat M, Funkenstein B, Tietz A, Lubzens E (1995) In vivo, in vitro and cell-free synthesis of hemocyanin in the shrimp Penaeus semisulcatus (de Haan). Comp Biochem Physiol B 112:31–38
Kobusch W (1998) The foregut of the Mysida (Crustacea, Peracarida) and its phylogenetic relevance. Phil Trans R Soc Lond B 353:559–581
Köhler H-R, Hüttenrauch K, Berkus M, Gräff S, Alberti G (1996) Cellular hepatopancreatic reactions in Porcellio scaber (Isopoda) as biomarkers for the evaluation of heavy metal toxicity in soils. Appl Soil Ecol 3:1–15
Kostanjšek R, Štrus J, Avguštin G (2002) Genetic diversity of bacteria associated with the hindgut of the terrestrial crustacean Porcellio scaber (Crustacea: Isopoda). FEMS Microbiol Ecol 40:171–179
Kostanjšek R, Avguštin G, Drobne D, Štrus J (2003) Morphological and molecular examination of bacteria associated with the wall of the papillate region of the gut in Porcellio scaber. In: Sfenthourakis S, Araujo PB, Hornung E, Schmalfuss H, Taiti S, Szlavecz K (eds) The biology of terrestrial isopods. Crustaceana monographs 2. Brill, Leiden, pp 103–120
Kostanjšek R, Štrus J, Lapanje A, Avguštin G, Rupnik M, Drobne D (2006) Intestinal microbiota of terrestrial isopods. In: König H, Varma A (ed) Soil biology, Vol 6: intestinal microorganism in soil invertebrates. Springer, Berlin, pp 115–131
Kostanjšek R, Štrus J, Avguštin G (2007) “Candidatus Bacilloplasma”, a novel lineage of Mollicutes associated with the hindgut wall of the terrestrial isopod Porcellio scaber (Crustacea: Isopoda). Appl Environ Microbiol 73:5566–5573
Kostanjšek R, Milatovič M, Štrus J (2010) Endogenous origin of endo-β-1,4-glucanase in common woodlouse Porcellio scaber (Crustacea, Isopoda). J Comp Physiol B 180:1143–1153
Kozlovskaja LS, Striganova BR (1977) Food digestion and assimilation in desert woodlouse and their relations to the soil microflora. Ecol Bull 25:240–245
Kummer G, Keller R (1993) High-affinity binding of crustacean hyperglycemic hormone (CHH) to hepatopancreatic plasma membranes of the crab Carcinus maenas and the crayfish Orconectes limosus. Peptides 14:103–108
Lehnert SA, Johnson SE (2002) Expression of hemocyanin and digestive enzyme messenger RNAs in the hepatopancreas of the Black Tiger Shrimp Penaeus monodon. Comp Biochem Physiol B 133:163–171
Lemmens JWTJ, Knott B (1994) Morphological changes in external and internal feeding structures during the transition phyllosoma-puerulus-juvenile in the western rock lobster (Panulirus cygnus, Decapoda: Palinuridae). J Morphol 220:271–280
Lešer V, Drobne D, Vilhar B, Kladnik A, Žnidaršič N, Štrus J (2008) Epithelial thickness and lipid droplets in the hepatopancreas of Porcellio scaber (Crustacea: Isopoda) in different physiological conditions. Zoology 111:419–432
Linton SM, Greenaway P, Towle DW (2006) Endogenous production of endo-beta-1,4-glucanase by decapod crustaceans. J Comp Physiol B 176:339–348
Loizzi RF (1971) Interpretation of crayfish hepatopancreatic function based on fine structural analysis of epithelial cell lines and muscle network. Z Zellforsch Mikrosk Anat 113:420–440
Lovett DL, Felder DL (1989) Ontogeny of gut morphology in the white shrimp Penaeus setiferus (Decapoda, Penaeidae). J Morphol 201:253–272
Loya-Javellana GN, Fielder DR, Thorne MJ (1995) Foregut evacuation, return of appetite and gastric fluid secretion in the tropical freshwater crayfish, Cherax quadricarinatus. Aquaculture 134:295–306
Manship BM, Walker AJ, Davies AJ (2011) Brooding and embryonic development in the crustacean Paragnathia formica (Hesse, 1864) (Peracarida: Isopoda: Gnathiidae). Arthropod Struct Dev 40:135–145
Martin JW, Davis GE (2001) An updated classification of the recent Crustacea. Science Series 39. Natural History Museum of Los Angeles County, Los Angeles
McGaw IJ, Curtis DL (2013) A review of gastric processing in decapod crustaceans. J Comp Physiol B 183:443–465
Mickėnienė L (1999) Bacterial flora in the digestive tract of native and alien species of crayfish in Lithuania. Freshwater Crayfish 12:279–287
Mikami S, Greenwood JG, Takashima F (1994) Functional morphology and cytology of the phyllosomal digestive system of Ibacus ciliatus and Panulirus japonicus (Decapoda, Scyllaridae and Palinuridae). Crustaceana 67:212–225
Milatovič M, Kostanjšek R, Štrus J (2010) Ontogenetic development of Porcellio scaber: staging based on microscopic anatomy. J Crust Biol 30:225–235
Möhrlen F, Baus S, Gruber A, Rackwitz H-R, Schnölzer M, Vogt G, Zwilling R (2001) Activation of pro-astacin. Immunological and model peptide studies on the processing of immature astacin, a zinc-endopeptidase from the crayfish Astacus astacus. Eur J Biochem 268:2540–2546
Mrak P, Bogataj U, Štrus J, Žnidaršič N (2015) Formation of the hindgut cuticular lining during embryonic development of Porcellio scaber (Crustacea, Isopoda). In: Taiti S, Hornung E, Štrus J, Bouchon D (eds) Trends in terrestrial isopod biology. ZooKeys, vol 515, pp 93–109
Muhammad F, Zhang ZF, Shao MY, Dong YP, Muhammad S (2012) Ontogenesis of digestive system in Litopenaeus vannamei (Boone, 1931) (Crustacea: Decapoda). Ital J Zool 79:77–85
Mykles DL (1979) Ultrastructure of alimentary epithelia of lobsters, Homarus americanus and H. gammarus, and crab, Cancer magister. Zoomorphol 92:201–215
Nair SG (1956) On the embryology of the isopod Irona. J Embryol Exp Morph 4:1–33
Nair GA, Attia FA, Saeid NH (1994) Food preference and growth-rates of the woodlouse Porcellio scaber Latreille, 1804 (Isopoda, Oniscidea, Porcellionidae). Afr J Ecol 32:80–84
Nakamura K, Seki K (1990) Organogenesis during metamorphosis in the prawn Penaeus japonicus. Nippon Suisan Gakkaishi 56:1413–1417
Nishida S, Quigley BD, Booth BD, Nemoto T, Kittaka J (1990) Comparative morphology of the mouthparts and foregut of the final-stage phyllosoma, puerulus, and postpuerulus of the rock lobster Jasus edwardsii (Decapoda: Palinuridae). J Crust Biol 10:293–305
Nishida S, Takahashi Y, Kittaka J (1995) Structural changes in the hepatopancreas of the rock lobster, Jasus edwardsii (Crustacea: Palinuridae) during development from the puerulus to post-puerulus. Mar Biol 123:837–844
Odendaal JP, Reinecke AJ (2003) Quantifying histopathological alterations in the hepatopancreas of the woodlouse Porcellio laevis (Isopoda) as a biomarker of cadmium exposure. Ecotoxicol Environ Saf 56:319–325
Pakes MJ, Weis AK, Mejia-Ortiz L (2014) Arthropods host intracellular chemosynthetic symbionts, too: cave study reveals an unusual form of symbiosis. J Crust Biol 34:334–341
Palackal T, Faso L, Zung JL, Vernon G, Witkus R (1984) The ultrastructure of the hindgut epithelium of terrestrial isopods and its role in osmoregulation. Symp Zool Soc Lond 53:185–198
Phupet B, Pitakpornpreecha T, Baowubon N, Runsaeng P, Utarabhand P (2018) Lipopolysaccharide- and β-1,3-glucan-binding protein from Litopenaeus vannamei: purification, cloning and contribution in shrimp defense immunity via phenoloxidase activation. Dev Comp Immunol 81:167–179
Prosi F, Dallinger R (1988) Heavy metals in the terrestrial isopod Porcellio scaber Latreille. I. Histochemical and ultrastructural characterization of metal-containing lysosomes. Cell Biol Toxicol 4:81–96
Queiroz LD, Abrunhosa FA, Maciel CR (2011) Ontogenesis and functional morphology of the digestive system of the freshwater prawn, Macrobrachium amazonicum (Decapoda: Palaemonidae). Zoologia 28:395–402
Reedy AR (1935) The structure, mechanism and development of the gastric armature in Stomatopoda with a discussion as to its evolution in Decapoda. Proc Natl Acad Sci India B Biol Sci 1:650–675
Richter S, Scholtz G (2001) Phylogenetic analysis of the Malacostraca (Crustacea). J Zool Syst Evol Res 39:113–136
Rode J, Drašlar K (1998) The structure of the digestive system of woodlouse Cylisticus convexus de Geer (Crustacea: Isopoda: Oniscoidea): hindgut and hepatopancreas. Acta Biol Slov 42:25–37
Roldan BM, Shivers RR (1987) The uptake and storage of iron and lead in cells of the crayfish (Orconectes propinquus) hepatopancreas and antennal gland. Comp Biochem Physiol 86C:201–214
Saborowski R (2015) Nutrition and digestion. In: Chang ES, Thiel M (eds) The natural history of the Crustacea. Vol 4: physiological regulation. Oxford University Press, New York, pp 285–319
Santos EA, Nery LEM, Keller R, Gonçalves AA (1997) Evidence for the involvement of the crustacean hyperglycemic hormone in the regulation of lipid metabolism. Physiol Zool 70:415–420
Schmitz EH (1992) Amphipoda. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates. Vol 9: Crustacea. Wiley-Liss, New York, pp 43–528
Schram FR (1986) Crustacea. Oxford University Press, New York
Schultz TW (1976) The ultrastructure of the hepatopancreatic caeca of Gammarus minus (Crustacea, Amphipoda). J Morphol 149:383–400
Shechter A, Berman A, Singer A, Freiman A, Grinstein M, Erez J, Aflalo ED, Sagi A (2008) Reciprocal changes in calcification of the gastrolith and cuticle during the molt cycle of the red claw crayfish Cherax quadricarinatus. Biol Bull 214:122–134
Skiebe P (2003) Neuropeptides in the crayfish stomatogastric nervous system. Microsc Res Tech 60:302–312
Sonakowska L, Włodarczyk A, Poprawa I, Binkowski M, Śróbka J, Kamińska K, Kszuk-Jendrysik M, Chajec L, Zajusz B, Rost-Roszkowska MM (2015) Structure and ultrastructure of the endodermal region of the alimentary tract in the freshwater shrimp Neocaridina heteropoda (Crustacea, Malacostraca). PLoS One 10:e0126900
Soroka Y, Milner Y, Sagi A (2000) The hepatopancreas as a site of yolk protein synthesis in the prawn Macrobrachium rosenbergii. Invert Reprod Dev 37:61–68
Spitzner F, Meth R, Krüger C, Nischik E, Eiler S, Sombke A, Torres G, Harzsch S (2018) An atlas of larval organogenesis in the European shore crab Carcinus maenas L. (Decapoda, Brachyura, Portunidae). Front Zool 15:27
Stöcker W, Raeder U, Bijlholt MMC, Wichertjes T, van Bruggen EFJ, Markl J (1988) The quaternary structure of four crustacean two-hexameric hemocyanins: immunocorrelation, stoichiometry, reassembly and topology of individual subunits. J Comp Physiol B 158:271–289
Storch V (1982) Der Einfluß der Ernährung auf die Ultrastruktur der großen Zellen in den Mitteldarmdrüsen terrestrischer Isopoda (Armadillidium vulgare, Porcellio scaber). Zoomorphol 100:131–142
Storch V (1984) The influence of nutritional stress on the ultrastructure of the hepatopancreas of terrestrial isopods. Symp Zool Soc Lond 53:167–184
Storch V (1987) Microscopic anatomy and ultrastructure of the stomach of Porcellio scaber (Crustacea: Isopoda). Zoomorphol 106:301–311
Storch V, Anger K (1983) Influence of starvation and feeding on the hepatopancreas of larval Hyas araneus (Decapoda, Majidae). Helgol Meeresunters 36:67–75
Storch V, Štrus J (1989) Microscopic anatomy and ultrastructure of the alimentary canal in terrestrial isopods. Monit Zool Ital Monogr 4:105–126
Storch V, Štrus J (2004) Comparative electron microscopic study of the stomach of Orchestia cavimana and Arcitalitrus sylvaticus (Crustacea: Amphipoda). J Morphol 259:340–346
Storch V, Bluhm BA, Arntz WE (2001) Microscopic anatomy and ultrastructure of the digestive system of three Antarctic shrimps (Crustacea: Decapoda: Caridea). Polar Biol 24:604–614
Storch V, Štrus J, Brandt A (2002) Microscopic anatomy and ultrastructure of the digestive system of Natatolana obtusata (Vanhöffen, 1914) (Crustacea, Isopoda). Acta Zool 83:1–14
Stromberg JO (1964) On the embryology of the isopod Idotea. Arkiv för Zoologi 17:421–473
Stromberg JO (1967) Segmentation and organogenesis in Limnoria lignorum (Rathke) (Isopoda). Arkiv för Zoologi 20:91–139
Štrus J (1987) The effects of starvation on the structure and function of the hepatopancreas in the isopod Ligia italica. Inv Pesq 51:505–514
Štrus J, Blejec A (2001) Microscopic anatomy of the integuments and digestive system in the moult cycle of Ligia italica (Oniscidea). In: Kensley B, Brusca RC (eds) Isopod systematics and evolution. AA Balkema, Rotterdam, pp 343–352
Štrus J, Drašlar K (1988) Ultrastructural evidence of the midgut cells in the isopod Ligia italica (Isopoda: Crustacea). Inst Phys Conf Ser 93:149–150
Štrus J, Storch V (1991) Moulting of the alimentary canal in Ligia italica Fab. and Porcellio scaber L. (Crustacea, Oniscoidea). In: Juchault P, Mocquard JP (ed) Proceedings of the Third International Symposium on the Biology of Terrestrial Isopods, Poitiers, pp. 189–194
Štrus J, Burkhardt P, Storch V (1985) The ultrastructure of the midgut glands in Ligia italica under different nutritional conditions. Helgol wiss Meeresunters 39:367–374
Štrus J, Drobne D, Ličar P (1995) Comparative anatomy and functional aspects of the digestive system in amphibious and terrestrial isopods (Isopoda: Oniscidea). In: Alikhan MA (ed) Terrestrial isopod biology. AA Balkema, Rotterdam, pp 15–23
Štrus J, Klepal W, Repina J, Tušek-Žnidarič M, Milatovič M, Pipan Ž (2008) Ultrastructure of the digestive system and the fate of midgut during embryonic development in Porcellio scaber (Crustacea: Isopoda). Arthropod Struct Dev 37:287–298
Suh H, Toda T, Hong S (1994) Ontogeny of foregut morphology in the euphausiid Euphausia pacifica. J Crust Biol 14:47–53
Szyfter Z (1966) The correlation of moulting and changes occurring in the hepatopancreas of Porcellio scaber Latr. (Crustacea, Isopoda). Bull Soc Amis Sci Lett Poznan 7:95–114
Takahashi Y, Hishida S, Kittaka J (1994) Histological characteristics of fat bodies in the puerulus of the rock lobster Jasus edwardsii (Hutton, 1875) (Decapoda, Palinuridae). Crustaceana 66:318–325
Talbot P, Clark WH, Lawrence AL (1972) Fine structure of the midgut epithelium in the developing brown shrimp, Penaeus aztecus. J Morphol 138:467–485
To TH, Brenner TL, Cavey MJ, Wilkens JL (2004) Histological organization of the intestine in the crayfish Procambarus clarkii. Acta Zool 85:119–130
Trevisan M, Leroy D, Decloux N, Thomee JP, Compere P (2014) Moult-related changes in the integument, midgut and digestive gland in the freshwater amphipod Gammarus pulex. J Crust Biol 34:539–551
Tziouveli V, Bastos-Gomez G, Bellwood O (2011) Functional morphology of mouthparts and digestive system during larval development of the cleaner shrimp Lysmata amboinensis (de Man, 1888). J Morphol 272:1080–1091
Tzuc JT, Escalante DR, Herrera RR, Cortés GG, Ortiz MLA (2014) Microbiota from Litopenaeus vannamei: digestive tract microbial community of Pacific white shrimp. SpringerPlus 3:280
Ullrich B, Storch V (1993) Development of the stomach in Euphausia superba Dana (Euphausiacea). J Crust Biol 13:423–431
Van den Oord A (1966) The biosynthesis of the emulsifiers of the crab Cancer pagurus L. Comp Biochem Physiol 17:715–718
Vannier J, Liu J, Lerosey-Aubril R, Vinther J, Daley AC (2014) Sophisticated digestive systems in early arthropods. Nat Commun 5:3641
Vernon GM, Herold L, Witkus ER (1974) Fine structure of the digestive tract epithelium in the terrestrial isopod Armadillidium vulgare. J Morphol 144:337–359
Vogt G (1985) Histologie und Cytologie der Mitteldarmdrüse von Penaeus monodon (Decapoda). Zool Anz 215:61–80
Vogt G (1993) Differentiation of B-cells in the hepatopancreas of the prawn Penaeus monodon. Acta Zool 74:51–60
Vogt G (1994) Life-cycle and functional cytology of the hepatopancreatic cells of Astacus astacus (Crustacea, Decapoda). Zoomorphol 114:83–101
Vogt G (2002) Functional anatomy. In: Holdich DM (ed) Biology of freshwater crayfish. Blackwell Science, Oxford, pp 53–151
Vogt G (2008a) Investigation of hatching and early post-embryonic life of freshwater crayfish by in vitro culture, behavioral analysis, and light and electron microscopy. J Morphol 269:790–811
Vogt G (2008b) The marbled crayfish: a new model organism for research on development, epigenetics and evolutionary biology. J Zool 276:1–13
Vogt G, Quinitio ET (1994) Accumulation and excretion of metal granules in the prawn, Penaeus monodon, exposed to water-borne copper, lead, iron and calcium. Aquat Toxicol 28:223–241
Vogt G, Štrus J (1998) Diseases of the shrimp Palaemon elegans (Crustacea, Decapoda) in the Bay of Piran, Adriatic Sea. J Nat Hist 32:1795–1806
Vogt G, Štrus J (1999) Hypogean life-style fuelled by oil. Naturwiss 86:43–45
Vogt G, Storch V, Quinitio ET, Pascual FP (1985) Midgut gland as monitor organ for the nutritional value of diets in Penaeus monodon (Decapoda). Aquaculture 48:1–12
Vogt G, Stöcker W, Storch V, Zwilling R (1989a) Biosynthesis of Astacus protease, a digestive enzyme from crayfish. Histochem 91:373–381
Vogt G, Quinitio ET, Pascual FP (1989b) Interaction of the midgut gland and the ovary in vitellogenesis and consequences for the breeding success: a comparison of unablated and ablated spawners of Penaeus monodon. In: De Pauw N, Jaspers E, Ackefors H, Wilkins N (eds) Aquaculture – a biotechnology in progress. European Aquaculture Society, Bredene, pp 581–592
Wägele J-W (1992) Isopoda. In: Harison FW, Humes AG (eds) Microscopic anatomy of invertebrates. Vol 9: Crustacea. Wiley-Liss, New York, pp 529–617
Wägele J-W, Welsch U, Müller W (1981) Fine structure and function of the digestive tract of Cyathura carinata (Krøyer) (Crustacea, Isopoda). Zoomorphol 98:69–88
Wang YC, Chang PS, Chen HY (2007) Tissue expressions of nine genes important to immune defence of the Pacific white shrimp Litopenaeus vannamei. Fish Shellfish Immunol 23:1161–1177
Warburg MR (1987) Isopods and their terrestrial environment. Adv Ecol Res 17:187–242
Warburg MR, Rosenberg M (1989) Ultracytochemical identification of NA+, K+-ATPase activity in the isopodan hindgut epithelium. J Crust Biol 9:525–528
Watling L (2013) Feeding and digestive system. In: Watling L, Thiel M (eds) The natural history of the Crustacea. Vol 1: functional morphology and diversity. Oxford University Press, New York, pp 237–260
Wieser W (1966) Cooper and the role of isopods in degradation of organic matter. Science 153:67–69
Wieser W, Klima J (1969) Compartmentalization of copper in the hepatopancreas of isopods. Mikroskopie 24:1–9
Wolfe SH, Felgenhauer BE (1991) Mouthpart and foregut ontogeny in larval, postlarval, and juvenile spiny lobster, Panulirus argus Latreille (Decapoda, Palinuridae). Zool Scr 20:57–75
Wolff C (2009) The embryonic development of the malacostracan crustacean Porcellio scaber (Isopoda, Oniscidea). Dev Genes Evol 219:545–564
Zhang N, Song C, Wang M, Liu Y, Hui M, Cui Z (2017) Diversity and characterization of bacteria associated with the deep-sea hydrothermal vent crab Austinograea sp. comparing with those of two shallow-water crabs by 16S ribosomal DNA analysis. PLoS One 12:e0187842
Zimmer M (2002) Nutrition in terrestrial isopods (Isopoda: Oniscidea): an evolutionary-ecological approach. Biol Rev 77:455–493
Zimmer M, Topp W (1998) Microorganisms and cellulose digestion in the gut of the woodlouse Porcellio scaber. J Chem Ecol 24:1397–1408
Žnidaršič N, Štrus J, Drobne D (2003) Ultrastructural alterations of the hepatopancreas in Porcellio scaber under stress. Environ Toxicol Pharmacol 13:161–174
Funding
This work was supported and financed by the Slovenian Research Agency (ARRS), MR grants Nos. 1000-11-310087 and 1000-15-0510 and the research program Integrative Zoology and Speleobiology No. P1-0184.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Štrus, J., Žnidaršič, N., Mrak, P. et al. Structure, function and development of the digestive system in malacostracan crustaceans and adaptation to different lifestyles. Cell Tissue Res 377, 415–443 (2019). https://doi.org/10.1007/s00441-019-03056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-03056-0