Abstract
In vertebrates little is known about causes and correlates of differences in individual body condition. This is, however, essential if we are to gain an understanding of environmental influences on wild populations. To track changes in individual physiological state we analysed blood metabolite concentrations in breeding common terns Sterna hirundo. We selected birds to contrast sex, breeding experience, sampling year and nutritional state and collected repeated samples from individuals during incubation. Unlike cholesterol, triglyceride and uric acid levels strongly correlated with nutritional state. While cholesterol levels in males remained stable throughout incubation, comparatively lower levels were measured in females directly after egg-laying. In 2006, only highly experienced females showed increased cholesterol concentrations by mid-incubation, whereas less experienced females maintained reduced levels and were apparently unable to recover. In 2007, all females showed an increase in cholesterol by mid-incubation. Triglyceride levels did not differ between sexes, but concentrations were significantly higher in more experienced birds and were overall higher in 2007 than in 2006. Uric acid concentrations did not differ significantly. The variation in cholesterol and triglyceride levels can be explained by differences in foraging conditions, as food abundance, onset of breeding and mean clutch size indicate 2007 to be the superior year. Thus, environmental conditions and breeding experience play an important role for cholesterol and triglyceride levels as well as the physiological state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The period of reproduction requires high investments and induces costs in many organisms, thus, influencing the physiological state and determining reproductive success and survival (Drent and Daan 1980; Partridge and Harvey 1985; Clutton-Brock 1991; Williams 2005; Harshman and Zera 2007). Consequently, high variability in individual quality leads to a significantly disproportional contribution of offspring to a population (Wooller et al. 1992). However, knowledge of physiological processes that reflect these individual differences is limited. Sometimes costs can be masked by individual variation like body condition, age, reproductive history and experience (Clutton-Brock et al. 1996; Hamel et al. 2009). Thus, some individuals might be able to compensate the costs, e.g. by increasing food intake, whereas others cannot. Alternatively, if individuals with a tight resource budget are able to adapt to moderate environmental changes only, costs of reproduction may only become detectable under unfavourable environmental conditions. In long-lived vertebrates reproductive performance and output generally increase with age (Clutton-Brock 1988; Forslund and Pärt 1995; Jones et al. 2008). This increase in breeding performance might be due to a gain in experience (e.g. improvement of foraging efficiency) rather than to age per se (Saether 1990; Wooller et al. 1992). Thus, individuals of different degrees of experience may suffer from unfavourable foraging conditions to a different extent as can be expressed, e.g. in arrival date (Ezard et al. 2007), onset of breeding and reproductive output (Arnold et al. 2004) and body condition (Kitaysky et al. 1999; Wendeln and Becker 1996).
Analysis of blood metabolites, being a minor invasive method, has found broad application in ecological research and veterinary diagnosis, providing information on the underlying physiological mechanisms that reflect physiological state, body condition, performance (activity, productivity and ability to cope with environmental conditions), change of nutritional state and health status (Brown 1996).
Plasma cholesterol level has been shown to be linked to body condition in several studies. In black ducks Anas rubripes chicks in poor condition had significantly lower cholesterol levels (Rattner et al. 1987). A positive correlation of cholesterol with body mass was found in white-crowned sparrows Zonotrichia leucophrys gambelii (de Graw et al. 1979), where cholesterol values paralleled seasonal variation in body mass. However, this was not found to be the case in black-headed gulls Larus ridibundus (Cantos et al. 1994). Fasting experiments also resulted in different findings: whereas Totzke et al. (1999) measured similar cholesterol levels in fasting and control herring gulls Larus argentatus, remaining fairly constant during the food-controlled experiment, Alonso-Alvarez and Ferrer (2001) found a strong relationship between cholesterol level in the plasma of yellow-legged gulls Larus cachinnans and their body mass change. Compared with several other blood parameters Alonso-Alvarez et al. (2002a) identified cholesterol concentration as the best indicator of individual body condition.
Triglyceride concentrations in the plasma increase during resorption and are transported via the blood stream to adipose tissues and energy consuming organs. The concentration of plasma triglycerides correlated positively with the amount of body fat (Dabbert et al. 1997; Quillfeldt et al. 2004) or increased with body mass gain in several bird species (Jenni-Eiermann and Jenni 1994; Jenni and Schwilch 2001) as well as in insectivorous bats (McGuire et al. 2009). In contrast to cholesterol, triglyceride levels were involved in the seasonal variation associated with migration in black-headed gulls (Cantos et al. 1994).
In birds, being uricotelic animals, uric acid represents the main end product of nitrogen excretion and has thus been used as an index for protein catabolism. Concentrations in the plasma can be elevated either during resorption, indicating the breakdown of nutritional protein and decrease post-feeding (Wilson and Miles 1988) or can be increased during fasting due to the mobilisation of body protein reserves, when animals reach the limit of their fasting ability (Boismenu et al. 1992; Hollmén et al. 2001).
Although several studies have used blood metabolites as a measure of physiological condition, inconsistencies persist. Effects of individual dissimilarity and environmental influences remain poorly understood, especially in studies on free-living animals. Most studies were restricted in age range of sampled individuals (Alonso-Alvarez 2005), are mainly based on cross-sectional analyses (Alonso-Alvarez et al. 2002b; Hario et al. 1991) and/or considered 1 year only (Alonso-Alvarez et al. 2002b; Alonso-Alvarez and Velando 2003; Hario et al. 1991). Long-term data on reproductive history to distinguish individual differences and their influence on physiological state are scarce.
In this respect, our study population of common terns Sterna hirundo, a long-lived seabird, under investigation since 1984, is a promising model, providing detailed individual-based longitudinal data. In common terns both mates of a breeding pair incubate the clutch at similar proportions (Wiggins and Morris 1987). Independent of size, mass per se represents a measure of body condition (Wendeln et al. 1997), which is directly affected by food availability (Frank and Becker 1992). Body mass in females decreases after egg-laying and in both sexes during the chick rearing period (Wendeln and Becker 1996; see also Limmer and Becker 2007). While body mass remains constant during incubation in males, inconsistent patterns are found in females: their body mass decreased significantly in 1992 and 1993, but not in 1991, presumably because of inter-year differences in foraging conditions. Common terns with their expensive lifestyle have been found to work near the limits of their capacity during reproduction (Pearson 1968; Becker and Ludwigs 2004).
In this paper, we present data on cholesterol, triglyceride and uric acid levels in the blood as indicators of the physiological state of common terns during the incubation period. Our results are based on a dataset of repeated measurements of blood metabolite concentrations of breeding common terns. In each case both mates of a pair were sampled three times over the course of incubation in 2006 and 2007. In either year individuals were selected with respect to their breeding experience to contrast birds of low and high breeding experience. All individuals were of known sex, and 68 of 78 birds were of known age, since they were fitted with transponders before fledging, which allows individual identification and recording of breeding attempts during their entire life-time (Becker and Wendeln 1997; Ezard et al. 2007). Additionally, we analysed blood metabolite levels with respect to their nutritional state (hungry, satiated) in order to assess the direct influence of food intake. Data characterising the breeding season, e.g. arrival and egg-laying date, clutch size and body mass change, from marked individuals of the entire colony were considered to explore effects of inter-year differences in environmental conditions.
We address the following questions in this study: (1) Do the sexes show differences in blood metabolite concentrations during incubation owing to different roles of mates in reproduction? (2) How do metabolite levels in the blood change during incubation? We want to go into this question in more detail, considering also environmental conditions and breeding experience: (3) How does breeding experience interact with environmental conditions in determining blood metabolite levels?
Materials and methods
Study site
Fieldwork was carried out at a common tern colony of about 450 nesting pairs at Banter See, Wilhelmshaven, Germany. Fledglings have been ringed at this colony since 1984 and since 1992 additionally marked with passive transponders of unlimited lifespan (TROVAN ID 100; Becker and Wendeln 1997). Furthermore, 101 adult birds were caught and fitted with transponders between 1992 and 1995. The breeding site consists of 6 artificial islands, surrounded by a low concrete wall where 44 elevated platforms were installed for common terns to land and rest on. All resting places were equipped with antennae, 16 of which additionally had integrated balances allowing remote and automatic recording and weighing of transponder-marked individuals (Wendeln and Becker 1996; Limmer and Becker 2007) with high repetition and reliability. Arrival date of an individual was defined as the first day of its registration at the colony site (Ezard et al. 2007). For details about the evaluation of mass data see Limmer and Becker (2007). To identify breeding birds, an antenna was placed around each nest for 1–2 days during incubation period. Reproductive parameters, e.g. egg-laying date (laying date of first egg of clutch), clutch size (no. of eggs per clutch), were obtained from checks every 2 or 3 days and could be assigned to individuals. On some nests where both partners were fitted with transponders a balance combined with an antenna was installed temporarily below the clutch. Nest attendance together with mass recordings allowed assigning the nutritional state of individuals as hungry or satiated (Becker and Frank 1990).
Given a high return probability to the natal colony and high site fidelity with an adult return rate of about 90%, the electronic system together with manually collected data provides information (e.g. breeding attempts) for every breeding season over the life-time for marked birds of known age in this colony (Becker et al. 2001). Birds were sexed by observation of copulations and since 1998 using standard molecular methods (Becker and Wink 2002).
Blood sampling
Blood samples were taken using Triatomine bugs Dipetalogaster maximus (larvae stage 3; von Helversen et al. 1986; Becker et al. 2006) according to the modified protocol of Arnold et al. (2008). Transponder detections (every 5–10 s) by nest antennae and additional supervision ensured that the target bird was incubating. After about 30 min the bugs had usually successfully completed their blood meal (about 300 μl) and were taken out of the nest. The success rate for blood sampling in 2006 and 2007 using this method was 86.1 and 82.2%, respectively. Within 30 min after the blood meal we punctured the bug and drew the blood into a syringe, which was placed on ice immediately. Our validation experiments proved a strong correlation between cholesterol values in the blood collected via bug and via needle in common terns [R = 0.77, n = 9; paired t test: t = −3.495, P < 0.01; cholesterol levels in blood sampled with the bug (190.9 ± 7.2 mg dL−1) were slightly higher than in blood sampled with the needle (171.9 ± 8.5 mg dL−1)]. Further validations for cholesterol as well as for triglyceride and uric acid concentrations were performed on captive herring gulls and revealed no significant differences either (personal unpublished data). In addition, the method has been validated for the determination of corticosterone concentration in common terns (Arnold et al. 2008), hormone concentrations (Voigt et al. 2004), doubly labelled water experiments (Voigt et al. 2003, 2005) and antibodies (Voigt et al. 2006) in mammals.
Sampling design
In 2006 and 2007 we took repeated blood samples during the incubation period from 34 and 44 adult common terns, respectively. Birds were sampled three times over the course of incubation—early (day 3–5 after clutch completion), mid (day 9–14 after clutch completion) and late (day 0–1 after hatching of the first chick). All blood samples were collected between 5 and 10 a.m. Central European Summer Time, when motivation to incubate was highest (unpublished data).
We always sampled both mates of a breeding pair. In common terns, like in many other long-lived species, reproductive output (Nisbet et al. 2002; Ezard et al. 2007) and body condition (Limmer and Becker 2007) increase with age and experience throughout the lifespan. To compare blood metabolite levels between young, less experienced, and old, highly experienced birds, we selected breeding pairs with no or only little breeding experience (0–3 breeding attempts, age 3–6 years; 2006: 8 pairs, 2007: 9 pairs; hereinafter referred to as inexperienced breeders) and experienced breeders (at least 7 breeding attempts, age 10–20 years; 2006: 9 pairs, 2007: 13 pairs). In consecutive years different individuals were sampled. Common terns mate with high probability assortatively with individuals of the same age as recruits (Ludwigs and Becker 2005) as well as later in life (Ludwig and Becker 2008) and demonstrate high mate fidelity (González-Solís et al. 1999). In our analyses we therefore included samples of a few unknown pair partners, which were with high probability of the same age as their partner (2006: 4 males, 1 female; 2007: 3 males, 2 females) in addition to 29 (2006) and 39 (2007) transponder-marked birds.
Additional samples were taken in 2008 and 2009 to investigate the direct influence of food intake, preferably fish, on blood parameter concentration. We collected samples of 28 and 19 individuals, respectively, in different nutritional state, satiated (immediately after a bird returned with a high body mass from a foraging trip) and hungry (after an incubation stint and drop in body mass). A subsample of 16 and 14 individuals, respectively, provided repeated samples of either nutritional state for at least one parameter, other individuals were only sampled once. Nest reliefs in incubating common tern pairs occur every 1.6–2.7 h and the mass gain per foraging trip lies between 13.2 and 15.8 g (Frank and Becker 1992). Thus, in our data set, individuals were defined and sampled as “hungry” after a mean incubation stint of 1.7 ± 0.2 h (time between nest relief and sampling; daytime stints only; n = 35) and a mean mass loss of 14.4 g (paired t test: t = −11.514, P < 0.001, n = 39). Birds in this colony are used to manipulations at the nest and thus always continued incubating immediately after the interruption was over.
Serum chemistry analyses
Blood samples were placed on ice and centrifuged within the next 7 h of sampling. The plasma was removed and stored at −20°C. Cholesterol, triglyceride and uric acid levels were determined using a dry-chemistry blood serum analyser (EKTACHEM DT60, Kodak). It required an amount of 10 μl sample (cholesterol 1:1, triglycerides 1:2, uric acid 1:4, diluted with 0.9% NaCl) per measurement (Calorimetric test slides, Vitros®, Ortho-Clinical Diagnostics, Johnson & Johnson Company). The validity of this method for birds has been shown previously (Bairlein and Totzke 1992; Totzke and Bairlein 1998). Samples were assayed in one series per year. The serum analyser was calibrated using a commercial calibrator kit and control serum (DT Calibrator Kit and Kodatrol Control Serum, Vitros®, Ortho-Clinical Diagnostics, Johnson & Johnson Company).
Statistical analyses
Blood metabolites, nutritional state and body mass
Pearson correlations were applied to test correlations between blood metabolite concentrations as well as to investigate relationships between blood parameter concentrations originating from birds in different nutritional state (dataset 2008/2009). Triglyceride data were log-transformed for normal distribution of data. We used general linear model (GLM) statistics to explore the influence of nutritional state on blood parameter concentrations. Body mass was included as a covariate.
Blood metabolites over the course of incubation
To investigate changes in blood metabolites over the course of incubation we collected three repeated blood samples from most individuals (dataset 2006/2007). From some birds, however, we had no third sample (at hatching) owing to clutch desertion (as it happens frequently in young and less experienced breeding pairs), unfertilized eggs or if the male did not brood its chicks long enough for the bug to suck blood, but mainly fed them. In three cases the first or second sample was missing as the bug did not ingest enough blood. In order to include samples of birds which we sampled only twice we selected the linear mixed effects model (LME) with restricted maximum likelihood estimation for unbalanced and repeated measures data. We alternatively introduced cholesterol, triglyceride or uric acid level as dependent variable with repeated measurements, each sample taken early, mid and late during the incubation period. Sampling year, sex and breeding experience were added as fixed factors and bird identity as random factor. Uric acid level, which revealed a highly significant correlation with nutritional state (in our dataset 2008/2009), was introduced as a covariate in the models with cholesterol or triglycerides to control for the effect of nutrition. A backward stepwise procedure was used to select the respective minimal model of best fit. Residuals were tested for normal distribution. To test for differences in triglyceride level between individuals of different degrees of breeding experience within years, we applied the independent samples t test. Differences in cholesterol level between mates of a pair within a sampling period and in females between incubation periods were compared using the paired t test.
Inter-year differences in reproductive performance
Data collected from all marked females of the study colony, being present in 2006 and in 2007, or alternatively, data from the subset of sampled females (dataset 2006/2007) were included in the analysis of inter-year differences in reproductive performance. For statistics on the marked females of the study colony, except for tests on body mass, repeated data of the same individuals in 2 years were used. Wilcoxon signed-rank test was applied to test inter-annual differences in clutch size and onset of egg-laying, respectively, paired t test for time interval from arrival to egg-laying date, t test for independent samples to compare body mass increase from arrival to incubation. The subset of sampled birds comprised different individuals in consecutive years. Inter-annual differences in onset of egg-laying and clutch size, respectively, were tested using the Mann–Whitney U test, the t test for independent samples to analyse differences in time interval from arrival to egg-laying date.
All statistics were calculated using SPSS® 17.0 and values are presented as mean ± standard error (SE). The level of significance was set at P ≤ 0.05.
Results
Blood metabolites, nutritional state and body mass
Cholesterol concentrations were neither correlated with triglyceride (R = −0.142, P = 0.287, n = 58) nor with uric acid concentrations (R = −0.185, P = 0.161, n = 59). However, the values of triglycerides and uric acid revealed a strongly positive correlation with each other (R = 0.674, P < 0.001, n = 61). The latter were both strongly influenced by nutritional state. Uric acid levels of satiated birds were significantly increased (\( \Updelta \bar{x} \) = 24.7 mg dL−1, 242.5%, n = 22, repeated samples) compared to hungry birds, as indicated by body mass (\( \Updelta \bar{x} \) = 14.4 g, 11.8%, n = 39, repeated samples; GLM: nutritional state, F 1,64 = 36.417, P < 0.001; body mass, F 1,64 = 7.087, P = 0.010). Triglyceride levels of satiated birds were also significantly increased (\( \Updelta \bar{x} \) = 91.6 mg dL−1, 93.5%, n = 22, repeated samples), unrelated to body mass, however (GLM: nutritional state, F 1,66 = 20.219, P < 0.001; body mass, F 1,67 = 0.011, P = 0.918). On the other hand, individual cholesterol values remained comparatively stable and independent of nutritional state and were not significantly related to body mass (\( \Updelta \bar{x} \) = −15.5 mg dL−1, −7.2%, n = 22, repeated samples; GLM: nutritional state, F 1,64 = 4.746, P = 0.033; body mass, F 1,64 = 3.082, P = 0.084). Correlations between repeated samples of different nutritional state revealed to be considerably stronger for cholesterol (R = 0.762, P < 0.001, n = 22) than for triglyceride (R = 0.446, P = 0.037, n = 22) and uric acid levels (R = 0.475, P = 0.025, n = 22).
Blood metabolite levels over the course of incubation
Cholesterol
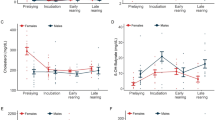
The application of a LME model revealed that cholesterol levels were significantly influenced by a three-way interaction of year, sex and breeding experience and a two-way interaction of sex with incubation period (Table 1). While cholesterol levels in males remained fairly constant over the incubation period, females showed low levels directly after egg-laying, being significantly different between the sexes for experienced breeders in both years, and for inexperienced breeders only in 2006 (Fig. 1). In 2006, experienced females reached cholesterol levels similar to males by mid-incubation. The increase was significant between early- and mid- as well as early- and late-incubation. In inexperienced females, however, we measured constantly low cholesterol values during incubation in 2006. Inexperienced mates showed significantly different cholesterol levels throughout incubation in 2006. In contrast, in 2007 inexperienced as well as experienced females reached high cholesterol concentrations by the end of incubation. Uric acid concentration was introduced into the model as a covariate to account for the influence of nutritional state on cholesterol level.
Cholesterol concentrations (mean ± SE) in common terns of different degrees of experience during incubation in 2006 and 2007 (filled circle males, open circle females, n no. of individuals). For statistics see Table 1. In addition, paired samples statistics (t test) are presented for differences in cholesterol levels between mates of a pair (top of graphs) and in females between incubation periods (bottom of graphs) (*P ≤ 0.05, **P ≤ 0.01)
Triglycerides
In contrast to cholesterol, triglyceride concentrations showed no relationship with sex, which was therefore excluded from the model (Table 1). However, as in cholesterol levels, breeding experience was found to be a factor of major influence. The interactions of experience with year as well as experience with incubation period revealed significant results. Over the course of incubation triglyceride levels remained at a relatively low level in 2006 irrespective the birds’ degree of breeding experience (Fig. 2). In 2007 we measured significantly higher levels in experienced than in inexperienced birds early during incubation. In experienced birds, triglyceride levels decreased over the course of incubation, whereas inexperienced birds showed a slight increase towards the end of incubation, finally equalling the levels of experienced birds.
Triglyceride concentrations (mean ± SE) in common terns of different degrees of experience during incubation in 2006 and 2007 (filled diamond experienced breeders, open diamond inexperienced breeders, n no. of individuals). For statistics see Table 1. In addition, t test statistics are presented for differences in triglyceride levels between individuals of different degrees of breeding experience (top of graphs) (*P ≤ 0.05)
Uric acid
We found no significant effects of year, sex, breeding experience or incubation period on uric acid concentrations (year: F 1,73 = 1.235, P = 0.270; sex: F 1,74 = 0.963, P = 0.330; breeding experience: F 1,73 = 0.234, P = 0.630; incubation period: F 2,89 = 0.223, P = 0.800). In 2007 mean uric acid concentration was slightly higher than in 2006 (2006: 21.9 ± 1.7 mg dL−1; 2007: 24.4 ± 1.4 mg dL−1). This difference was based on low levels in 2006 of inexperienced breeders, but was not significant (2006, inexperienced: 19.4 ± 2.6 mg dL−1; 2007, inexperienced: 25.6 ± 2.2 mg dL−1; 2006, experienced: 24.3 ± 2.1 mg dL−1; 2007, experienced: 23.6 ± 1.9 mg dL−1).
Inter-year differences in reproductive performance
Conditions during breeding seasons in 2006 and 2007 were reflected by significant differences in several parameters of reproduction in our study colony and in the subset of sampled individuals (Table 2). Breeding season 2006 was characterised by a delayed onset of egg-laying (egg-laying date per se as well as a prolonged time period between arrival and egg-laying date). Breeding birds showed a lower increase in body mass from arrival to incubation in 2006 and clutch size was significantly reduced.
Discussion
Reproduction implicates sex specific consequences on body resources. This applies to body mass (Wendeln and Becker 1996; Moe et al. 2002) as well as underlying physiological traits such as blood metabolites and energy expenditure (Fairbrother et al. 1990; Hollmén et al. 2001; Alonso-Alvarez et al. 2002b; Williams and Vézina 2001).
Do the sexes show differences in blood metabolite concentration during incubation owing to different roles of mates in reproduction?
We measured significantly lower cholesterol levels in female than male common terns after egg-laying. Low cholesterol concentrations could be a consequence of egg production, which requires significant investment of body reserves in birds (Gayathri et al. 2004; Nager et al. 2001). Costs of egg production are particularly high among Laridae and Sternidae, laying relatively large eggs for their body size compared to other avian taxa (Moore et al. 2000). A typical three-egg clutch represents about 50% of female body mass in common terns, with a mean egg mass of about 20 g (19.74 ± 0.02 g, n = 983, eggs weighed between 1993 and 2001 in our study colony; see also Nisbet 1978).
Expenditures of egg production relate to cholesterol in two ways, in terms of hormonal adaptations using cholesterol as precursor of steroids, and with respect to lipid investment in egg formation. In domestic chicken Gallus gallus high cholesterol levels have been measured in the plasma during the pre-breeding period when cholesterol is synthesized in the liver and transported via lipoprotein complexes to the ovary, where oestrogen secretion is elevated and lipids are deposited in follicles (Kern et al. 1972; Christie and Moore 1972). Most, if not all of the cholesterol in the egg yolk originates from the maternal blood stream (Connor et al. 1965). Nisbet (1978) determined the amount of lipids in the yolk of common tern eggs between 31 and 35%. This corresponds to the findings of Senser and Scherz (1991) for chicken eggs with 32%. If we assume the cholesterol/lipid ratio also to be the same in chicken (3.95% according to Senser and Scherz 1991) and common terns, one egg of the latter would contain about 74 mg cholesterol. This corresponds to the considerable 2.8-fold amount of cholesterol circulating in the blood of a female common tern (estimation based on a body mass of 130 g (Wendeln and Becker 1996), 10% blood (Bezzel and Prinzinger 1990) and 200 mg dL−1 cholesterol (see “Results”)).
For methodological reasons, we sampled birds only during the incubation period itself and not prior to egg-laying. But the low levels shortly after egg-laying compared to cholesterol levels of females at the end of incubation and the relatively constant mean cholesterol level of males during incubation suggest a recovery pattern to be a plausible explanation. This is supported by results of Gayathri and Hedge (1994), who found no significant difference in cholesterol levels between male and female pigeons during non-breeding period. Furthermore Gayathri et al. (2004) measured a drop in cholesterol levels following egg-laying in female pigeons. Our results are also in line with the findings of Alonso-Alvarez et al. (2002b) in yellow-legged gulls: female gulls and terns showed significantly lower cholesterol concentrations than males during the first 10 days of incubation. Furthermore, in this study we could show on the individual level, that in female common terns cholesterol levels were increasing again during the further course of incubation and finally conforming with the levels of the male mates. This pattern was found to be affected by complex interactions between the foraging conditions of the year and the breeding experience of females.
The inter-year difference could be caused by contrasting foraging conditions in the 2 years of data collection, as indicated by reduced food abundance in 2006 in their foraging area “Jadebusen” in the German Wadden Sea (Dänhardt and Becker, unpublished data). One consequence of the poor food supplies during courtship feeding in 2006 was a significantly later onset of egg-laying, due to an increased time interval between arrival date and clutch initiation, a lowered clutch size and body mass increase from arrival to incubation in our study colony compared to 2007. The importance of food supply for timing of breeding is supported by experimental studies, in which food supplementation resulted in a significantly advanced laying date (reviewed in Williams 2005). In 2007 the mean clutch size in the colony was significantly higher (see above) and all the experienced breeders of our subsample had a clutch of three eggs. Lower cholesterol concentrations in females with larger clutches might be the result of a trade-off in allocation of resources. Individuals that laid only two eggs in 2007 despite favourable foraging conditions showed higher cholesterol levels compared to 2006. The effect of the interaction of year and clutch size with cholesterol concentration during early incubation period was significant (F 1,35 = 7.032, P = 0.012, n = 39 females).
Triglycerides are the most abundant lipids found in the egg yolk, but in contrast to cholesterol, we did not find differences in concentrations between the sexes. This is in accordance with results of Alonso-Alvarez et al. (2002b) in yellow-legged gulls. Unlike cholesterol, triglyceride levels during early-incubation did not reveal any significant association with clutch size or year in common terns. Differences in the effect on egg-laying females between cholesterol and triglyceride concentrations might be explained by the fact that cholesterol levels remained relatively stable independent of nutritional state, due to cholesterol possibly not being easily and abundantly available. Triglyceride concentrations, in contrast, varied to a much higher extent in the blood stream. Triglyceride and uric acid concentrations were strongly and positively correlated and were both increased in satiated birds. The similarity of uric acid as well as triglyceride concentrations in both sexes indicated males and females to be equally nourished. This is consistent with the behaviour of shared incubation by both partners in common terns (Frank and Becker 1992).
How do blood metabolite concentrations change during incubation?
While the variation of cholesterol levels over the course of incubation in females was dependent on year and breeding experience, levels in males remained fairly constant throughout incubation in both years. Thus, cholesterol concentrations followed the same course during incubation as body mass in male common terns (Wendeln and Becker 1996), kittiwakes Rissa tridactyla (Moe et al. 2002) and yellow-legged gulls (Alonso-Alvarez et al. 2002b).
Triglyceride levels were positively correlated with nutritional state. We measured comparatively low levels of triglycerides throughout incubation in 2006. On average concentrations were slightly higher in experienced birds, especially during early-incubation. In 2007, highly experienced birds showed very high triglyceride concentrations at the beginning, which decreased over the course of incubation to levels confirming the ones of birds with low breeding experience. In a year of good feeding conditions, highly experienced birds might profit from the first half of incubation to maintain or regain a good physiological state. The subsequent decrease in triglyceride concentrations might be a result of decreasing foraging trip durations towards hatching date of the chicks (unpublished data). At the end of incubation, our last sampling period, the first chick had already hatched and lower triglyceride levels might thus reflect stress associated with parental care of chicks. Levels were significantly lower during early-incubation in inexperienced birds than in experienced ones, but towards the end they reached comparable concentrations.
Uric acid concentrations did not reveal significant changes during incubation in common terns. Alonso-Alvarez et al. (2002b) measured an increase in uric acid in both sexes during incubation. This might be explained by a change in food availability, thus being an intra-seasonal effect.
How does breeding experience interact with environmental conditions in determining blood metabolite levels?
In our study, we investigated for the first time individual blood metabolite concentrations throughout incubation not only in relation to sex, but considered breeding experience and inter-annual variability of foraging conditions. In line with the predictions, our results revealed a distinctive course of cholesterol concentration during incubation period dependent on year and breeding experience. As described by Alonso-Alvarez et al. (2002b) for yellow-legged gulls and consistent with findings on other measures of body condition (Hario et al. 1991; Moe et al. 2002), in 2007 we found an increase of cholesterol in the plasma in females by mid-incubation towards a level comparable to males, independent of the degree of experience. In 2006, however, only experienced females reached high cholesterol levels, whereas inexperienced females retained low cholesterol concentrations throughout incubation. This might reflect a better recovery process of females with high breeding experience. The reason for this inter-year difference in cholesterol level patterns over the course of the incubation period might be contrasting foraging conditions in these 2 years interacting with the level of experience. This explanation is supported by a prolonged time interval between arrival and egg-laying date, differences in clutch size and individual mass change from arrival to incubation between the 2 years mentioned above. Small seabirds, such as common terns, with a tight energy budget have been shown to react very sensitively to changes in food abundance and availability, which directly affect their condition (Pearson 1968; Frank and Becker 1992) and also the cholesterol level in females, constrained by egg production. Our results show that experience becomes relevant as soon as environmental conditions are suboptimal. Although we did not measure cholesterol levels before egg-laying (see above), the findings suggest a recovery in the physiological state of females as revealed by increasing cholesterol concentrations over the course of incubation.
In triglycerides experience also matters: concentrations were significantly higher in experienced birds than in inexperienced ones. Increased breeding experience can involve a higher foraging efficiency and/or a better cooperation with the mate. Our results are in line with findings of Ferrer and Dobado-Berrios (1998), who measured higher triglyceride concentrations with increased age in Spanish imperial eagles Aquila adalberti. In contrast to cholesterol, the inter-experience differences became prominent in 2007, a year of higher food availability.
Triglyceride concentration varied with nutritional state, with birds showing increased levels after food intake. This is supported by studies on garden warblers Sylvia borin (Jenni-Eiermann and Jenni 1998) and insectivorous bats (McGuire et al. 2009), who show that levels of triglycerides are closely related to nutrition. Levels paralleled seasonal variation in body mass in relation to migration in several bird species (Cantos et al. 1994; Totzke and Bairlein 1998). Triglyceride levels turning out to be significantly higher in 2007 concurs with the hypothesis of 2007 being a year of superior food availability.
Uric acid concentrations revealed a close relationship to nutritional state and correlated positively with body mass at sampling. Although parameters like egg-laying date and clutch size indicate 2007 to be a year of superior food availability, uric acid concentrations measured were marginally, but not significantly higher.
Conclusion
Our results underline the importance of considering individual quality, indicated by experience and age, to disentangle the effects that cause variation in physiological parameters during reproduction. Regarding nutritional state, cholesterol levels remained relatively stable compared to triglyceride and uric acid levels. Triglyceride levels turned out to be a measure of nutritional index, which is supported by findings of McGuire et al. (2009) and were higher in a year of increased food availability. Inter-individual differences in the cholesterol level in females become apparent as soon as environmental conditions are suboptimal. It would be of further interest to measure cholesterol and triglyceride levels prior to and after the incubation period. Moreover, investigating cholesterol concentrations under different foraging conditions could contribute to a more comprehensive understanding of the resource allocation trade-off in birds during egg production and incubation.
References
Alonso-Alvarez C (2005) Age-dependent changes in plasma biochemistry of yellow-legged gulls (Larus cachinnans). Comp Biochem Physiol A 140:512–518
Alonso-Alvarez C, Ferrer M (2001) A biochemical study of fasting, subfeeding, and recovery processes in yellow-legged gulls. Physiol Biochem Zool 74:703–713
Alonso-Alvarez C, Velando A (2003) Female body condition and brood sex ratio in yellow-legged gulls Larus cachinnans. Ibis 145:220–226
Alonso-Alvarez C, Ferrer M, Velando A (2002a) The plasmatic index of body condition in yellow-legged gulls Larus cachinnans: a food-controlled experiment. Ibis 144:147–149
Alonso-Alvarez C, Velando A, Ferrer M, Veira JAR (2002b) Changes in plasma biochemistry and body mass during incubation in the yellow-legged gull. Waterbirds 25:253–258
Arnold JM, Hatch JJ, Nisbet ICT (2004) Seasonal declines in reproductive success of the common tern Sterna hirundo: timing or parental quality? J Avian Biol 35:33–45
Arnold JM, Oswald SA, Voigt CC, Palme R, Braasch A, Bauch C, Becker PH (2008) Taking the stress out of blood collection: comparison of field blood-sampling techniques for analysis of baseline corticosterone. J Avian Biol 39:588–592
Bairlein F, Totzke U (1992) New aspects on migratory physiology of trans-Saharan passerine migrants. Ornis Scand 23:244–250
Becker PH, Frank D (1990) Kontinuierliche Wägung brütender Seevögel zur Analyse der Ernährungssituation. In: Proceedings of the international 100 DO-G meeting, 1988, Bonn, pp 173–179
Becker PH, Ludwigs J-D (2004) Sterna hirundo common tern. BWP Update 6(1,2):91–137
Becker PH, Wendeln H (1997) A new application for transponders in population ecology of the common tern. Condor 99:534–538
Becker PH, Wink M (2002) Geschlechtsabhängige Größenunterschiede von Flügglingen der Flussseeschwalbe (Sterna hirundo). J Ornithol 143:51–56
Becker PH, Wendeln H, González-Solís J (2001) Population dynamics, recruitment, individual quality and reproductive strategies in common terns marked with transponders. Ardea 89:241–252
Becker PH, Voigt CC, Arnold JM, Nagel R (2006) A non-invasive technique to bleed incubating birds without trapping: a blood-sucking bug in a hollow egg. J Ornithol 147:115–118
Bezzel E, Prinzinger R (1990) Ornithologie. Ulmer, Stuttgart
Boismenu C, Gauthier G, Larochelle J (1992) Physiology of prolonged fasting in greater snow geese (Chen caerulescens atlantica). Auk 109:511–521
Brown ME (1996) Assessing body condition in birds. In: Nolan V Jr, Ketterson ED (eds) Current ornithology, vol 13. Plenum Press, New York, pp 67–135
Cantos FJ, Alonso-Gómez AL, Delgado MJ (1994) Seasonal changes in fat and protein reserves of the black-headed gull, Larus ridibundus, in relation to migration. Comp Biochem Physiol A 108:117–122
Christie WW, Moore JH (1972) The lipid components of the plasma, liver and ovarian follicles in the domestic chicken (Gallus gallus). Comp Biochem Physiol B 41:287–295
Clutton-Brock TH (1988) Reproductive success: studies of individual variation in contrasting breeding systems. University of Chicago Press, Chicago
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
Clutton-Brock TH, Stevenson IR, Marrow P, MacColl AD, Houston AI, McNamara JM (1996) Population fluctuations, reproductive costs and life-history tactics in female Soay sheep. J Anim Ecol 65:675–689
Connor WE, Osborne JW, Marion WL (1965) Incorporation of plasma cholesterol-4-C14 into egg yolk cholesterol. Proc Soc Exp Biol Med 118:710–713
Dabbert CB, Martin TE, Powell KC (1997) Use of body measurements and serum metabolites to estimate the nutritional status of mallards wintering in the Mississippi Alluvial Valley, USA. J Wildl Dis 33:57–63
de Graw WA, Kern MD, King JR (1979) Seasonal changes in the blood composition of captive and free-living white-crowned sparrows. J Comp Physiol B 129:151–162
Drent RH, Daan S (1980) The prudent parent: energetic adjustments in avian breeding. Ardea 68:225–252
Ezard THG, Becker PH, Coulson T (2007) Correlations between age, phenotype, and individual contribution to population growth in common terns. Ecology 88:2496–2504
Fairbrother A, Craig MA, Walker K, O’Loughlin D (1990) Changes in mallard (Anas platyrhynchos) serum chemistry due to age, sex, and reproductive condition. J Wildl Dis 26:67–77
Ferrer M, Dobado-Berrios P (1998) Factors affecting plasma chemistry values of the Spanish Imperial Eagle, Aquila adalberti. Comp Biochem Physiol A 120:209–217
Forslund P, Pärt T (1995) Age and reproduction in birds—hypotheses and tests. TREE 10:374–378
Frank D, Becker PH (1992) Body mass and nest reliefs in common terns Sterna hirundo exposed to different feeding conditions. Ardea 80:57–69
Gayathri KL, Hedge SN (1994) Sexual differences in blood values of the pigeon, Columba livia. Comp Biochem Physiol B 109:219–224
Gayathri KL, Shenoy KB, Hedge SN (2004) Blood profile of pigeons (Columba livia) during growth and breeding. Comp Biochem Physiol A 138:187–192
González-Solís J, Wendeln H, Becker PH (1999) Within and between season nest-site and mate fidelity in common terns (Sterna hirundo). J Ornithol 140:491–498
Hamel S, Côté SD, Gaillard J-M, Festa-Bianchet M (2009) Individual variation in reproductive costs of reproduction: high-quality females always do better. J Anim Ecol 78:143–151
Hario M, Kilpi M, Selin K (1991) Parental investment by the sexes in the herring gull: the use of energy reserves during early breeding. Ornis Scand 22:308–312
Harshman LG, Zera AJ (2007) The cost of reproduction: the devil in the details. TREE 22:80–86
Hollmén T, Franson JC, Hario M, Sankari S, Kilpi M, Lindstrom K (2001) Use of serum biochemistry to evaluate nutritional status and health of incubating common eiders (Somateria mollissima) in Finland. Physiol Biochem Zool 74:333–342
Jenni L, Schwilch R (2001) Plasma metabolite levels indicate change in body mass in reed warblers Acrocephalus scirpaceus. Avian Sci 1:55–65
Jenni-Eiermann S, Jenni L (1994) Plasma metabolite levels predict individual body-mass changes in a small long-distance migrant, the garden warbler. Auk 111:888–899
Jenni-Eiermann S, Jenni L (1998) What can plasma metabolites tell us about the metabolism, physiological state and condition on individual birds? An overview. Biol Conserv Fauna 102:312–319
Jones OR, Gaillard J-M, Tuljapurkar S, Alho JS, Armitage KB, Becker PH, Bize P, Brommer J, Charmantier A, Charpentier M, Clutton-Brock TH, Dobson FS, Festa-Bianchet M, Gustafsson L, Jensen H, Jones CG, Lillandt B-G, McCleery R, Merilä J, Neuhaus P, Nicoll MAC, Norris K, Madan K, Pemberton J, Pietiäinen H, Ringsby TH, Roulin A, Saether B-E, Setchell JM, Sheldon BC, Thompson PM, Weimerskirch H, Wickings EJ, Coulson T (2008) Senescence rates are determined by ranking on the fast-slow life-history continuum. Ecol Lett 11:664–673
Kern MD, de Graw WA, King JR (1972) Effects of gonadal hormones on the blood composition of white-crowned sparrows. Gen Comp Endocrinol 18:43–53
Kitaysky AS, Wingfield JC, Piatt JF (1999) Dynamics of food availability, body condition and physiological stress response in breeding black-legged kittiwakes. Funct Ecol 13:577–584
Limmer B, Becker PH (2007) The relative role of age and experience in determining variation in body mass during the early breeding career of the common tern (Sterna hirundo). Behav Ecol Sociobiol 61:1885–1896
Ludwig SC, Becker PH (2008) Supply and demand: causes and consequences of assortative mating in common terns Sterna hirundo. Behav Ecol Sociobiol 62:1601–1611
Ludwigs JD, Becker PH (2005) What do pairing patterns in common tern, Sterna hirundo, recruits reveal about the significance of sex and breeding experience? Behav Ecol Sociobiol 57:412–421
McGuire LP, Fenton BM, Faure PA, Guglielmo CG (2009) Determining feeding state and rate of mass change in insectivorous bats using plasma metabolite analysis. Physiol Biochem Zool 82:812–818
Moe B, Langseth I, Fyhn M, Gabrielsen GW, Bech C (2002) Changes in body condition in breeding kittiwakes Rissa tridactyla. J Avian Biol 33:225–234
Moore DJ, Williams TD, Morris RD (2000) Mate provisioning, nutritional requirements for egg production, and primary reproductive effort of female common terns Sterna hirundo. J Avian Biol 31:183–196
Nager RG, Monaghan P, Houston DC (2001) The cost of egg production: increased egg production reduces future fitness in gulls. J Avian Biol 32:159–166
Nisbet ICT (1978) Dependence of fledging success on egg-size, parental performance and egg-composition among common and roseate terns, Sterna hirundo and S. dougallii. Ibis 120:207–215
Nisbet ICT, Apanius V, Friar MS (2002) Breeding performance of very old common terns. J Field Ornithol 73:117–124
Partridge L, Harvey PH (1985) Evolutionary biology: costs of reproduction. Nature 316:20
Pearson TH (1968) The feeding biology of sea-bird species breeding on the Farne Islands, Northumberland. J Anim Ecol 37:521–552
Quillfeldt P, Masello JF, Möstl E (2004) Blood chemistry in relation to nutrition and ectoparasite load in Wilson’s storm-petrels Oceanites oceanicus. Polar Biol 27:168–176
Rattner BA, Haramis GM, Chu DS, Bunck CM, Scanes CG (1987) Growth and physiological condition of black ducks reared on acidified wetlands. Can J Zool 65:2953–2958
Saether B-E (1990) Age-specific variation in reproductive performance of birds. In: Power DM (ed) Current ornithology, vol 7. Plenum Press, New York, pp 251–283
Senser F, Scherz H (1991) Der kleine ‘Souci-Fachmann-Kraut’, Lebensmitteltabelle für die Praxis. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart
Totzke U, Bairlein F (1998) The body mass cycle of the migratory garden warbler (Sylvia borin) is associated with changes of basal plasma metabolite levels. Comp Biochem Physiol A 121:127–133
Totzke U, Fenske M, Hüppop O, Raabe H, Schach N (1999) The influence of fasting on blood and plasma composition of herring gulls (Larus argentatus). Physiol Biochem Zool 72:426–437
Voigt CC, von Helversen O, Michener RH, Kunz TH (2003) Validation of a non-invasive blood-sampling technique for doubly-labelled water experiments. J Exp Zool A 296:87–97
Voigt CC, Faßbender M, Dehnhard M, Wibbelt G, Jewgenow K, Hofer H, Schaub GA (2004) Validation of a minimally invasive blood-sampling technique for the analysis of hormones in domestic rabbits, Oryctolagus cuniculus (Lagomorpha). Gen Comp Endocrinol 135:100–107
Voigt CC, Michener R, Wibbelt G, Kunz TH, von Helversen O (2005) Blood-sucking bugs as a gentle method for blood-collection in water budget studies using doubly labelled water. Comp Biochem Physiol A 142:318–324
Voigt CC, Peschel U, Wibbelt G, Frölich K (2006) An alternative, less invasive blood sample collection technique for serologic studies utilizing Triatomine bugs (Heteroptera; Insecta). J Wildl Dis 42:466–469
von Helversen O, Volleth M, Núnez J (1986) A new method for obtaining blood from a small mammal without injuring the animal: use of Triatomid bugs. Experientia 42:809–810
Wendeln H, Becker PH (1996) Body mass change in breeding common terns Sterna hirundo. Bird Stud 43:85–95
Wendeln H, Becker PH, Wagener M (1997) Beziehungen zwischen Körpermasse und Körpergröße bei Paarpartnern der Flussseeschwalbe (Sterna hirundo). Vogelwarte 39:141–148
Wiggins DA, Morris RD (1987) Parental care of the common tern Sterna hirundo. Ibis 129:533–540
Williams TD (2005) Mechanisms underlying the costs of egg production. Bioscience 55:39–48
Williams TD, Vézina F (2001) Reproductive energy expenditure, intraspecific variation and fitness in birds. In: Nolan V Jr, Thompson CF (eds) Current ornithology, vol 16. Plenum Press, New York, pp 355–392
Wilson HR, Miles RD (1988) Plasma uric acid of broiler breeder and Leghorn male chickens: effect of feeding time. Poult Sci 67:345–347
Wooller RD, Bradley JS, Croxall JP (1992) Long-term population studies of seabirds. TREE 7:111–114
Acknowledgments
We are grateful for assistance and support to all helpers involved in this study: especially J. J. Riechert, who primarily held responsibility for taking the samples from birds of different nutritional state. A. Braasch, T. H. G. Ezard, A. Ostendorp, G. Wagenknecht and S. Weitekamp for their help in the field, R. Nagel, S. A. Oswald, G. Scheiffarth and J. Trauernicht for technical support and G. A. Schaub for providing the blood-sucking bugs. K. L. Szostek, F. Bairlein and anonymous reviewers helped to improve the paper. This study was done under licence of Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Oldenburg (33.42502/18-03.05) and financially supported by Deutsche Forschungsgemeinschaft (BE 916/8 and 9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Bauch, C., Kreutzer, S. & Becker, P.H. Breeding experience affects condition: blood metabolite levels over the course of incubation in a seabird. J Comp Physiol B 180, 835–845 (2010). https://doi.org/10.1007/s00360-010-0453-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-010-0453-2