Abstract
Variation in the foraging niche and parental provisioning behaviors of breeding seabirds have the potential to affect population dynamics (e.g. foraging success, breeding productivity, and ultimately population size). We sampled blood plasma of family’ groups (females, males, and chicks) of Magellanic penguins (Spheniscus magellanicus) from Martillo Island, Argentina. We used stable isotope analyses on plasma samples to examine food provisioning, isotopic niche, trophic position, and diet composition of penguins between the early and late chick-rearing periods. We found clear differences in the isotopic niches of penguins between the two stages of the chick-rearing period related to shifts in foraging habitat and/or diet composition between stages. We found no evidence of individual consistency in isotopic niches or sex-specific selective provisioning by adults. In addition, we found high variability within family groups (accounting for 90% of the total isotopic variability). This study improves our understanding of the age, sex, individual, and breeding stage-specific trophic niches of Magellanic penguins, which may be helpful in projecting how they may respond to future environmental change (e.g., changes that affect prey availability).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding differences in resource partitioning between organisms is central to ecology. Optimal foraging theory has long been used to understand and predict prey choice and patch use in animal populations (Stephens and Krebs 1986). One of the key predictions of this theory is that variation in prey choices is driven by intraspecific competition which is expected to increase both population and individual niche breadth (Araújo et al. 2008).
In this context, the foraging behavior of seabirds is affected by the distribution and abundance of prey such that inter-annual and seasonal variation in resource availability can influence their foraging strategies and fitness (Watanuki 1992; Votier et al. 2008; Sanz-Aguilar et al. 2009). In addition, seabirds also exhibit intra-specific variation in foraging strategies due to factors such as sex, age, morphology, breeding status, and individual specialization (Bolnick et al. 2003; Raya Rey et al. 2012b; Le Vaillant et al. 2013; Ceia and Ramos 2015). Quantifying the degree of temporal and intra-specific variation of foraging niches and identifying their drivers is relevant, as they have the potential to affect foraging and breeding success, and ultimately population trends (Lynch et al. 2012; Waluda et al. 2012; Horswill et al. 2017).
Colonial seabirds, such as penguins, are central place foragers (Orians and Pearson 1979), and therefore are easily accessible when breeding. During this period, they are obliged to capture enough food to provision their chicks and themselves (Croxall and Davis 1999; Boersma et al. 2015). This tradeoff may preference chicks, with adults provisioning chicks with higher quality food items in respect to those they consume for themselves (Forero et al. 2002; Dehnhard et al. 2016). Alternatively, adults may favor self-provisioning and prioritize survival over reproductive success (Ballard et al. 2010; Booth and McQuaid 2013). This balance could also be influenced by sexual differences in the foraging behaviors that are driven by morphological differences between parents (Rosciano et al. 2019).
In sexually dimorphic and diving species, the body size is allometrically correlated with diving capacities (i.e., larger-bodied individuals can dive deeper) (Noren et al. 2001). In turn, this could affect the amount and type of food provided by parents of different sexes. In penguins, past studies have examined differential investment in food provisioning by parents. Adelie penguin (Pygoscelis adeliae) females made on average longer foraging trips than males, resulting in a lower provisioning rate compared to males (Clarke et al. 1998). Meanwhile, Macaroni penguins (Eudyptes chrysolophus) females provision their chicks throughout the rearing period and at higher rates than males, which only participate in later stages (Barlow and Croxall 2002). Similarly, southern rockhopper penguins (Eudyptes chrysocome) females provide most of the food during chick rearing and they feed their chicks with higher trophic level prey than they consume for themselves (Rosciano et al. 2019). Magellanic penguins from North Patagonia have selective food provisioning since chicks have a more similar diet to their male parent and this pattern was more evident at colonies and seasons where penguins had a more diverse diet (Ciancio et al. 2018).
Magellanic penguins (Spheniscus magellanicus) are moderately sexually dimorphic, males have a larger size and are heavier than females (Boersma et al. 2013) and are opportunistic and pelagic foragers, depending on the season and prey species abundance and availability in the region (Scolaro et al. 1999; Clausen and Pütz 2002; Scioscia et al. 2014; Dodino et al. 2020). Magellanic penguins typically lay two eggs, and hatching success could be variable among colonies and years (Boersma et al. 2013). Brood reduction takes place after hatching, and the larger of the two chicks, usually the first hatchling, receives more food (Blanco et al. 1996; Boersma et al. 2013). Tierra del Fuego colonies represent the southernmost breeding range and their individuals are known to feed on key regulatory species of the food web in the Beagle Channel (e.g. Grimothea gregaria, Sprattus fuegensis) (Scioscia et al. 2014; Diez et al. 2016, 2018; Dodino et al. 2020). Particularly, the pelagic aggregations of both species, G. gregaria and S. fuegensis, occur all year throughout the channel but during winter the latter increases its availability in the inner sector of the channel (Diez et al. 2018). In addition, an expansion in the distribution and abundance of pelagic swarms of G. gregaria was reported for the Beagle Channel in recent years (Diez et al. 2016).
In Martillo Island, the diet and foraging ecology of breeding Magellanic penguins have been studied in terms of the type of food, using stomach content methodology (Scioscia et al. 2014), in terms of their isotopic niche during early chick-rearing (Dodino et al. 2022) and the diving behavior using time and depth recorders and GPS-TD loggers during early chick-rearing (Raya Rey et al. 2010, 2012a; Harris et al. 2020). All these studies have reported different degrees of variability in the feeding ecology of the species related to their generalist habitat. For example, (Scioscia et al. 2014) reported variability in the diet composition among different reproductive stages and years, and suggest a potential relationship with changes in the distribution and abundance of their main prey. While Raya Rey et al. (2012a) reported sex-related differences in diving foraging behavior and suggested that those differences could be related to changes in S. fuegensis’ availability. However, those studies have not explored potential differences in self-provisioning and chick-provisioning behaviors.
Stable isotope analyses (SIA) of carbon (δ13C) and nitrogen (δ15N) provide the ability to assess animals’ diet and habitat use over both short and longer-term time scales (Inger and Bearhop 2008; Layman et al. 2012; Cherel et al. 2014). δ13C and δ15N values act as measures of the scenopoetic (i.e. habitat) and bionomic (e.g. diet) components of a consumer’s trophic niche, referred to as the “isotopic niche” (Newsome et al. 2007). The isotopic niche framework is a useful tool to assess foraging habitat and diet shifts between stages of species' annual cycle. Blood plasma in penguins integrates dietary information over an approximately 15-day period (Barquete et al. 2013; Jenkins et al. 2019). As such, blood plasma collected during the middle of the early and late chick-rearing stages, which each have a duration of approximately 30 days in Magellanic penguins (Boersma et al. 2013), would facilitate isotopic niche comparisons between these two stages. Moreover, collecting plasma samples from adults of both sexes and their chicks provides a robust methodology to analyze parental-provisioning, sex-specific, and individual-based strategies across the early and late chick-rearing stages.
In this context, the main objective of this study was to evaluate temporal, individual, and sex-specific variation in the trophic niche and food provisioning of Magellanic penguins between early and late chick-rearing stages (ECR and LCR, respectively), at Martillo Island, Beagle Channel, Argentina, using stable isotope analysis of blood plasma. We defined “seasonal consistency” as reflecting population-level metrics of penguin diets (i.e., isotopic niche, trophic position, diet composition) that remain consistent between the two stages. In contrast, we define “individual consistency” as intra-individual differences in diets that remain consistent between stages (i.e., same values of stable isotopes) even if there is an overall shift in diet at the population level (e.g., Herman et al. 2017). In addition, we defined “selective provisioning” as when one adult sex (male or female) selectively feeds the chicks more than the other adult (e.g., Rosciano et al. 2019). Taking into account these definitions, we hypothesized that (1) at the population level, the isotopic niches of adults and the chicks they provision will differ between ECR and LCR stages reflecting seasonal shifts in diet composition and/or foraging habit use between these two stages, (2) inter-individual differences in diets will be inconsistent between stages similar to Rosciano et al. (2020) reflecting a lack of individual consistency, (3) breeding adults have sex-specific selective provisioning based on previous studies on the species (Ciancio et al. 2018) and as a consequence of differences in prey selection between parents.
Methods
Study area and sample collection
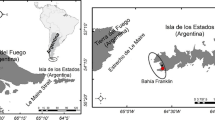
We conducted fieldwork at the Magellanic penguin colony on Martillo Island (54° 54ʹ S, 67° 23ʹ W). This hammer-shaped island is located in the eastern section of the Beagle Channel (Tierra del Fuego, Argentina) and holds ca. 4900 active nests (Raya Rey unpubl. data). We sampled 13 family groups during the ECR (chicks with 30 days old, late-November to early-December 2016) and during the LCR (chicks with 60 days old, mid-January 2017). We collected samples from 13 females, 13 males and 21 chicks in each stage, totalling 94 samples. All the nests had two chicks at the beginning of the season, but five chicks died before the late-chick rearing stage, consequently, the early samples of those chicks were not considered. In each breeding stage, we collected 3 ml of whole-blood from the tarsal vein for each member of the family group (female, male and chicks). Adults’ sex determination was based on differences in beak widths and lengths (Gandini et al. 1992). Chicks were identified by making a small cut in the interdigital webbing to one of them. Each blood sample was centrifuged in the laboratory (1000 rpm, 30 min) on the same day it was collected to separate the plasma from the blood cells. Each fraction was preserved in hermetic tubes at − 80 °C until further carbon and nitrogen isotope processing in the laboratory (Hobson et al. 1997).
Stable isotope analysis
We lyophilized plasma samples for 12 h then homogenized and weighed samples (0.6 mg ± 0.1 mg) and into tin cups. Sample was flash-combusted (Costech ECS 4010 elemental analyzers) and analyzed for carbon (δ13C), nitrogen (δ15N) stable isotope values, and C:N ratios via an interfaced Thermo Scientific Delta XP continuous-flow stable isotope ratio mass spectrometer at Louisiana State University. USGS 40 and USGS 41 glutamic acid reference materials were used to normalize sample values. Sample precision based on the repeated sample and reference material was 0.1‰ for both δ13C and δ15N values. Stable isotope values are expressed in δ notation in per mil units (‰), according to the following equation:
where X represents either 13C and 15N and R the ratio between 15N/14N or 13C/12C. Rstandard for δ15N was based on atmospheric N2 while for δ13C was based on Vienna Pee Dee Belemnite (V-PDB). Average plasma C:N values ranged from 3.58 to 5.11 (Table 1) indicating variable lipid content among samples (Post et al. 2007). Therefore, prior to statistical analysis, we normalized plasma δ13C values using the equation of Post et al. (2007) for aquatic organisms:
Isotopic niche analysis and consistency at population level
At population level, we used plasma δ13C and δ15N values to characterize isotopic niche overlap and differences in isotopic niche area among adults and their chicks as a proxy of the trophic niche overlap and size (Newsome et al. 2007). Specifically, we calculated standardized ellipse areas (SEA) corrected for small sample sizes (SEAC) for adult females, adult males, and chicks separately for each breeding state (i.e. ECR and LCR) and between sexes/ages within each stage (Jackson et al. 2011). We estimated isotopic niche overlap as the proportion of SEAC for each group that overlaps with a comparison group's SEAC. In addition, we constructed Bayesian SEAs (SEAB) using the Stable Isotopes Bayesian Ellipses (SIBER, Jackson et al. 2011) package in R version 3.4.0 (R Core Team 2021) to compare two-dimensional isotopic niche areas among groups. We then used the resulting Bayesian posterior probability distributions of SEAB to calculate the pairwise probabilities that isotopic niche areas from one group are different than a comparison group (Jackson et al. 2011).
We examined consistencies between stages using a generalized lineal model (GLM) with Gaussian distribution (nlme package, Pinheiro et al. 2015) where the response variable was the δ15N and δ13C values for each sex/age group and the explanatory variable was the stage (ECR and LCR) (6 models totally).
Trophic position estimates (population level)
We estimated the trophic position (TP) of adult females, adult males, and chicks separately for each breeding state (ECR and LCR) using the Bayesian approach of the tRophicPosition package in R (version 4.0.3, Quezada-Romegialli et al. 2018). We selected the jagsOneBaseline model and we selected as baseline the values of mussels previously reported at Martillo Island (δ13C = − 17.1 ± 0.4 ‰, δ15N = 12.2 ± 0.3 ‰; Dodino et al. 2020). We assumed mussels incorporate the isotopic signal of the entire water column and were completely herbivorous and occupied a TP of 2. We used the trophic discrimination factor (TDF) of 2.6 ± 0.5 ‰ for δ15N values estimated for plasma tissue from Magellanic penguins (Jenkins et al. 2019). We evaluated differences in TP between sex/age groups using the compareTwoDistributions function (Quezada-Romegialli et al. 2018).
Mixing model analyses (population level)
We used a Bayesian stable isotope mixing model to compare the diet composition of the sexes/ages between stages (‘MixSiar’ in R, Stock et al. 2018). Taking into account previous information on adults Magellanic penguins’ diet (Schiavini et al. 2005; Scioscia et al. 2014; Dodino et al. 2020) and their foraging areas during the ECR stage i.e., close to the colony, within the Beagle Channel (Raya Rey et al. 2010; Harris et al. 2020), and during the LCR stage, i.e. eastward feeding areas, near to Picton Island (55° 4.5ʹ S 66° 53.3ʹ W) (Raya Rey unpubl. data), we selected published stable isotope data of potential prey items collected along the Beagle Channel (e.g., the squat lobster Grimothea gregaria, the Fuegian sprat Sprattus fuegensis) (Riccialdelli et al. 2020).
Following the approach of Dodino et al. (2020), we combined prey species into three statistically and ecologically relevant prey groups based on their TPs estimated in previous works to reduce model uncertainty and to aid in interpretation: (1) low-trophic level species (TP ~ 2): pelagic form of the squat lobster G. gregaria (2) mid-trophic level species (TP ~ 3) with a mix of small pelagic, bentho-pelagic species such as the benthic form of G. gregaria, fuegian sprat S. fuegensis, and notothenioid Patagonotothen tessellata and P. ramsayi, (3) mid-trophic level species (TP ~ 3–4) with coastal species such as Eleginops maclovinus, P. cornucola, and Odontesthes smitti (Table S1).
Given the fact that the years in which prey group stable isotope values were available did not fully coincide with the year in which penguin plasma were collected, the use of these prey values has the potential to add uncertainty to our model predictions. For this reason, we applied the same correction factor used by Riccialdelli et al. (2020) in all δ13C values of prey items and penguin samples to account for the Suess effect (Francey et al. 1999; Indermühle et al. 1999) and to further reduce the potential for temporal biases in mixing model results. We modeled the contribution of each food source to the synthesis of Magellanic penguin blood using the TDF of − 0.6 ± 0.5 ‰ for δ13C and 2.6 ± 0.5 ‰ for δ15N (Jenkins et al. 2019). We ran the model over 3 Markov Chain Monte Carlo chains of 3,000,000 iterations and discarded the first 1,500,000 (Gelman and Rubin 1992). We used a prior of 25% for group 1, 25% for group 2, and 3% for group 3. The prior was selected based on previous information on diet composition data for Magellanic penguins (Scioscia et al. 2014; Dodino et al. 2020).
Individual consistency
To test whether individual diets and foraging habitats are consistent between stages, we tested for relationships between individual’s ECR and LCR δ15N and δ13C values using Pearson correlations for normally distributed population.
Family groups
We evaluated differences in δ13C and δ15N values between members of the family groups (females, males, and chicks), using generalized linear mixed effect models (GLMMs). We used one model for each response variable (δ13C, δ15N), the explanatory variables were sex/age group and the chick-rearing stage, and the random factor was the family identity to take into account the variability within family groups. We provided the estimate, the standard errors (SE), the t value, the p value, and the confidence interval (CI) for each model. Then, we conducted a posteriori Tukey HSD test for multiple comparisons (multicomp package; Hothorn et al. 2008).
To evaluate a possible relationship between chicks’ stable isotope values (δ13C, δ15N) and their parents’ stable isotope values, we ran GLM with Gaussian distribution using chick’s stable isotope values as a response variable and parents’ stable isotope values as an explanatory variable. A separate model was made by stage for each isotope, that is, a total of eight models.
Finally, we chose the heuristic Euclidean distances (ED) (Phillips 2001) between chicks and parent as a proxy of diet similarity. This is given by:
where ED is the isotopic Euclidean distance and the other terms are the isotope values for chick and adults, respectively. If we assume that if parents have different stable isotope values due to individual or sex-specific differences in diet, chick’s stable isotope values will be most similar to the individual parent that provisions them with the most food (i.e., have the smallest ED between parent and chick). To evaluate the effect of adult’ sex on ED, we run GLMMs with gamma distribution and log link function using the ED as a response variable, sex as an explanatory variable, and family identity as a random variable.
Results
Seasonal and isotopic niche consistency (population level)
Females, males, and chicks did not overlap their isotopic niches between the two chick-rearing stages (0% overlap in all comparisons, Fig. 1). In contrast, within each stage, we found a large degree of niche overlap among sexes/ages (Fig. 2). During the ECR females-males overlap’ corresponded to 58% and 65% of their isotopic niches, respectively (Fig. 2). Between females and chicks, the overlap represented 73% and 92% of their isotopic niches, respectively (Fig. 2). Moreover, the overlap between males-chicks represented 52% and 60% of their isotopic niches, respectively (Fig. 2). During the LCR females-males overlap’, corresponding to 25% and 43% of their isotopic niches, respectively (Fig. 2). Between females-chicks, the overlap corresponded to a 43% and 54% of their isotopic niches, respectively (Fig. 2). Males-chicks overlap’, corresponding to 56% and 40% of their isotopic niches, respectively (Fig. 2).
Standard ellipses corrected for small sample size (SEAC) estimated from δ13C and δ15N values of plasma samples collected from Magellanic penguins of Martillo Island during the chick-rearing period (ECR early-chicks rearing in red, LCR late-chicks rearing in purple) and grouped by sex/age (females, males, chicks)
SEAB predicted different niche widths between ages/stages (Fig. 3). Between stages, the three sexes/ages showed the largest isotopic niche during the ECR than the LCR (Pairwise-probabilities, females-ECR > females-LCR: 95%, males-ECR > males-LCR: 70%, chicks-ECR > chicks-LCR: 75%). During the ECR, females showed the largest isotopic niche and the males the narrowest (Pairwise-probabilities, females > males: 63%; females > chicks: 77%, chicks > males: 65%). During the LCR females, again, showed the largest isotopic niche, but now, the chicks showed the narrowest (Pairwise-probabilities, females > males: 78%; females > chicks: 94%, males > chicks: 79%).
Bayesian standard ellipse areas (SEAB, presented in ‰2) for Magellanic penguins’ plasma samples from Martillo Island by sex/age (adult females, adult males and chicks) during the chicks rearing period (ECR early-chicks rearing in red, LCR late-chicks rearing in purple). Black dots correspond to the mode SEAB for each sex/age group, shaded boxes represent the 50%, 75%, and 95% credible intervals from dark to light color
For δ15N values, we found no support for seasonal consistency in females and chicks (δ15Nfemales: F1,24 = 13.45, p < 0.01; δ15Nchicks: F1,41 = 9.52, p < 0.01) while for males we found consistency between the early and late chick-rearing stages (δ15Nmales: F1,24 = 0.31, p = 0.58). For δ13C values, we found no support for seasonal consistency between the early and late chick-rearing stages for any sex/age group (δ13Cfemales: F1,24 = 62.07, p < 0.01; δ13Cmales: F1,24 = 52.96, p < 0.01; δ13Cchicks: F1,41 = 123.87, p < 0.01).
Trophic position estimates (population level)
Estimates of TP differed between breeding stages (Fig. 4). For all sex/age group comparisons, we found generally higher TP distribution during early chick rearing relative to the late chick-rearing stage (in 87%, 57%, and 86% of models runs within females, males, and chicks, respectively). Within the ECR stage, adult females generally had higher TP distribution than adult males or chicks (in 68% and 67% of model runs; respectively), and adult males only had higher TP estimates relative to chicks in 50% of model runs. Within the LCR stage, males had higher TP relative to females and chicks in 71% and 81% of model runs; respectively, and females had higher TP relative to chicks in 66% of model runs.
Trophic Position of adult females, adult males and chicks separated by stage (ECR early-chicks rearing in red, LCR late-chicks rearing in purple). Black dots correspond to the mode trophic position for each age/colony in each stage; shaded boxes represent the 50%, 75%, and 95% credible intervals from dark to light color
Mixing model analyses (population level)
During the ECR stage, mixing model analyses indicated that prey group 2 (i.e. mid-trophic level, pelagic, and bentho-pelagic prey species) represented 100% of the diet of all three sex/age groups (Fig. 5, Table 2). During the LCR stage groups continue to consume prey group 2 (20–50% of diets on average) while increasing the consumption of group 1 (i.e., low-trophic level prey species; 30–50% of diets on average; Fig. 5, Table 2). For both breeding stages, the predicted dietary proportion of prey group 3 (i.e., mid-trophic level coastal prey species) was relatively low (0–30% of diets on average) in all three sex/age groups (Fig. 5, Table 2).
Isospace plot based on individual δ13C and δ15N values of chicks, adult females and adult males of Magellanic penguins at Martillo Island during chicks-rearing (early and late stages) in 2016 and of their prey sources (average ± SD): group 1 (Grimothea gregaria—pelagic), group 2 (G. gregaria—benthic, Sprattus fuegensis, Patagonototen tessellata, P. ramsayi) and group 3 (Eleginops maclovinus, Odontesthes smitti, P. cornucola). Prey stable isotope values are corrected by the trophic discrimination factors estimated by Magellanic plasma samples (Jenkins et al. 2019). References: ECR early-chicks rearing, LCR late-chicks rearing
Individual consistency
We found no support for individual consistency, and no support for seasonal consistency for the three sex/ages, with no significant correlations in individual penguin δ15N (Females: t11 = − 0.02 r = − 0.01, p = 0.98; Males: t11 = − 0.12, r = − 0.04, p = 0.91; Chicks: t19 = 1.74, r = 0.24, p = 0.30) and δ13C values (Females: t11 = 1.76, r = 0.47, p = 0.1; Males: t11 = 0.68, r = 0.20, p = 0.50; Chicks: t19 = 1.07, r = 0.24, p = 0.30) between stages (Fig. 6).
Family groups
GLMMs indicated a high percentage of variability explained by the random family ID parameter, which represents a high intra-family variability (Table 1). We found higher δ13C and δ15N values during ECR relative to LCR in all three sex/age groups (Table 1). The linear relationship between chicks and their parents showed significant differences between them for δ13C values during the ECR stage but the adjusted R2 value was relatively weak (Table 3). For δ15N, we did not find a linear relationship for any stage between the chicks and their parents indicating that there was no intra-familiar consistency (Table 3).
Finally, Euclidean distance (ED), which is a proxy of diet similarity between chicks and parent, ranged from 0.07 to 2.48 ‰ (mean ± SD: 1.38 ± 0.62) and from 0.04 to 2.35 ‰ (mean ± SD: 1.25 ± 0.59) for chick-female and chick-male pairs during the ECR stage, respectively. While during the LCR stage, ED ranged from 0.17 to 1.90 ‰ (mean ± SD: 1.00 ± 0.44) and from 0.37 to 2.23 ‰ (mean ± SD: 0.98 ± 0.44) for chick-female and chick-male pairs, respectively. For both chick-rearing stages, we found no effect of adult sex on ED (GLMMECR, estimate = 1.07, SE = 0.17, t = 5.38, p = 0.52, CI: [0.87–1.31]; GLMMLCR, estimate = 1.04, SE = 0.13, t = 8.48, p = 0.77, CI: [0.81–1.32]).
Discussion
Our study provides insights into temporal variation in the trophic niche, seasonal and individual consistency, and parental provisioning behaviors in Magellanic penguins during chick-rearing at a breeding colony in the southernmost range of their distribution. Optimal foraging theory suggests that adults should adapt their strategies to maximize foraging efficiency, and therefore, fitness (Pyke et al. 1977). Although the flexibility of foraging is described as a mechanism that increases its efficiency in a variable environment (e.g. Grémillet et al. 2012), there is evidence that individual specialization and behavioral consistency may also be adaptive (Wakefield et al. 2015; Patrick and Weimerskirch 2017). While we found that the isotopic niches of Magellanic penguins differed seasonally between the ECR and LCR stages, there was little to no evidence of individual or sex-specific selective provisioning but high variability within family groups (accounting for 90% of the total isotopic variability).
The results obtained partially refute our hypotheses. Specially, we found support for our predictions of seasonal differences in adults’ trophic niche between chick-rearing stages and a lack of seasonal individual trophic consistency. However, we did not find evidence of selective provisioning as was previously reported for this species at colonies in North Patagonia (Ciancio et al. 2018). Magellanic penguins share parental care duties during the brooding period (Boersma et al. 2013) and are opportunistic foragers depending on the season and prey species abundance and availability in the region (Scolaro et al. 1999; Clausen and Pütz 2002). Indeed, at this southern colony, Scioscia et al. (2014) using stomach content reported an inconsistent or irregular difference in diet between sexes within each year and breeding stage, which could explain the high isotopic variability we found within family groups. However, this comparison should be taken with caution due to the mismatch of methodology between Scioscia et al. (2014) and our study (stomach content only provides a snapshot of diet during one foraging trip while stable isotope values of plasma integrate diet over 15 days period).
Seasonal shifts in foraging habitat were previously reported using SIA in other penguins’ species between pre-breeding and breeding season, or between breeding and post-breeding season (e.g., little penguins, Kowalczyk et al. 2014; gentoo and Adelie penguins, Herman et al. 2017). However, studies exploring isotopic variation within the breeding season are less common (Gorman et al. 2021). In our study, shifts in foraging habitat use between the ECR and LCR stages, in addition to changes in diet composition, may have influenced the isotopic niches of Magellanic penguins.
Baseline δ13C and δ15N values (e.g., phytoplankton and zooplankton) can change between inshore/benthic and offshore/pelagic habitats and isotopic differences between consumers have also been linked to foraging habitats in space and time (France 1995; Cherel and Hobson 2007; Graham et al. 2010). The inconsistency found in the δ13C and δ15N values of females and chicks between ECR and LCR, and only in δ13C for males could be related to a change in the foraging area used by adults (inshore vs. offshore areas), a change in diet composition, or a change in both foraging area and diet composition. Considering that during the LCR stage chicks are larger and require food with less frequency, adults could undertake longer and further away foraging trips (Boersma et al. 2013). Previous GPS studies in this colony have found that adults during this time can use eastward feeding areas outside the Beagle Channel (Raya Rey unpubl. data), also foraging trip duration is used as a proxy of foraging distance which proved to be longer for the LCR (Scioscia et al. 2010), with this region characterized by lower δ13C and δ15N values typically from open waters (Riccialdelli et al. 2020). A change in δ13C and δ15N values between stages may support this explanation for chick and females.
Moreover, our mixing model results suggest variation in diet composition. Adults fed on a mixture of low- and mid-trophic level species during the LCR stage and predominantly on mid-trophic level species during ECR. Specifically, during LCR, we found a shift in both females’ and chicks’ isotopic niches towards lower isotopic values, lower TP, and a predicted shift in the diet composition increasing the consumption of the pelagic form of G. gregaria. This prey species has lower δ13C and δ15N values and occupy lower TPs in comparison to mid-trophic species (group 2, such as the benthic form of G. gregaria, S. fuegensis, Patagonotothen spp) (TP ~ 2.0 vs TP ~ 3.0, respectively, Riccialdelli et al. 2020), consequently, this change in the prey choice also explained the trophic shift observed during the LCR. For males, the consistency in δ15N values and TPs between stages complicates the interpretation. Although the shift in δ13C values may reflect a change in foraging areas, as supported by previous GPS data, we can also assume a consistency in the type of prey chosen by them during each stage, mobile and high trophic level prey. However, when we explore the diet composition, we found the same differences reported for females and chicks. We acknowledge that we may have some important prey missed in our study, such as squids (e.g., Doryteuthis gahi), which are common prey for Magellanic penguin (Scioscia et al. 2014). This squid species exhibits inshore-offshore migrations and can enter into the channel through the east mouth for reproduction (Rosenfeld et al. 2014) and larval development (Presta et al. 2023). Their contribution to the diet of adults may have implications for mixing model results.
However, with our data is it not possible to conclusively determine if a change in the foraging area, change in the diet composition, or a combination of these two factors are the most significant drivers of isotopic variation. To address this limitation, future studies should combine SIA with GPS tracking at each stage and sample a more comprehensive list of prey taxa.
The plasma stable isotope-based mixing model results for the ECR differ from those reported in a previous analysis using whole blood stable isotope values during the chick-rearing period at the same colony. Using a whole blood stable isotope-based mixing model, Dodino et al. (2022) suggest that adults and chicks from Martillo Island fed mainly on G. gregaria-pelagic (prey group 1) during the ECR period, similar to what our plasma stable isotope-based mixing model results suggest for the LCR period. This is likely related to the fact that the whole blood integrates diets over a ~ 30 days period (Hobson and Clark 1992; Barquete et al. 2013), and consequently when collected during the ECR stage could also integrate dietary information from the late incubation stage. However, despite these differences between studies using plasma vs. whole blood stable isotope values, both studies indicate that adults feed their offspring the same diets compositions that they consume for themselves. In addition, we found broadly similar isotopic niches among sexes in both stages suggesting that sexual dimorphism and different diving capacities of Magellanic penguins from Martillo Island do not influence diets and food provisioning during chick-rearing as previously found for the diving behavior in the same colony (Raya Rey et al. 2012a).
Prey availability could influence adults’ prey choice and provisioning behaviors (Waluda et al. 2012; Horswill et al. 2017; Dodino et al. 2020). In addition, the size and the energy density of prey item can be a factor, since larger prey items could be more difficult for a chick to digest than a smaller one and also chick growth rates is influenced by the caloric content of prey (Van Heezik and Davis 1990). Diez et al. (2018) reported that G. gregaria is present all year round with similar abundance in the Beagle Channel. In addition, G. gregaria is the smallest and the highest energy density prey (50–70 mm, 11.0 kJ g−1, respectively, Ciancio et al. 2007) in comparison with many of the prey species within group 2 (e.g., S. fuegensis: 35–140 mm, 7.15 kJ g−1; P. tesellata: 118–120 mm, 4.0 kJ g−1; P. ramsayi: 60–140 mm, 4.8 kJ g−1; size and energy density respectively, Ciancio et al. 2007). In this sense, it is interesting to notice with this study that adults only selected prey group 2 during the LCR. One explanation of these new founds could be related to a potential lower digestibility of this prey, which could affect breeding success during an early chick-rearing stage. In this sense, it is suggested that chicks grow at a reduced rate with a diet based on this prey item (Thompson 1993). However, the change in the diet composition during the LCR stage may not have as large of an impact on chick survival since the chicks are bigger and their energy requirements are lower relative to the ECR stage.
Individual niche variation is frequently found within generalist populations and is commonly assessed by quantifying the degree of inter-individual foraging variation and the presence of individual consistency over time (Bolnick et al. 2003; Sargeant et al. 2007). Magellanic penguins are a generalist population and at Tierra del Fuego showed different foraging behavior between neighboring colonies (related to chicks’ requirements and the spatial distribution of prey, Dodino et al. 2022) and between years (related to changes in prey availability, Dodino et al. 2020). In this sense, it was expected that Magellanic penguins’ population at Martillo Island would have little to no individual consistency in their trophic niche and a high variability within family groups. Future research on individual foraging specialization in this species across their breeding distribution is needed to identify the potential environmental mechanisms that may act to mediate the degree of individual variation within these generalist populations.
Finally, for a better understanding of trophic consistency Magellanic, future studies could extend the present work over years to evaluate inter-annual consistency in the feeding sites chosen during each chick-rearing stage. While not possible to assess using our current dataset, if resources are spatiotemporally correlated a ‘win-stay, lose-shift’ tactic could be optimal such that potential fidelity to feeding areas in each stage could be associated with prior success (Switzer 1993; Schmidt 2001; Piper 2011; Spencer 2012).
Implications
Site-based conservation is a key strategy for protecting biodiversity worldwide (Watson et al. 2014). Identifying the spatial use of Magellanic penguins at the Beagle Channel during the chick-rearing period highlights the critical habitats used by this species, which is crucial for planning marine protected areas networks.
Identifying the prey items that Magellanic penguins chose for themselves and their chicks is important in the context of global climate change. Since changes in environmental conditions (i.e., variability in sea surface temperature, chlorophyll a, etc.) could affect the availability and/or the contamination exposure of important penguin prey item. For example, harmful algal blooms that can cause massive fish to die off, contaminate seafood with toxins, or detrimentally alter ecosystem function have been increasing in frequency, magnitude, and duration worldwide (Glibert et al. 2014) and the Beagle Channel is not an exception (Almandoz et al. 2019). The effects of climate change on Magellanic penguins’ prey can have important consequences on penguins foraging and breeding success, and ultimately population growth. Knowledge of the plasticity of Magellanic penguins foraging habitats during the chick-rearing period is crucial to understand how they could respond to these increasingly frequent events.
Finally, the application of the ED method, proposed by Ciancio et al. (2018), to quantify the sex-related differences in parental provisioning provide a quick and easy way to quantify sex-related differences in species that share parental duties as Magellanic penguins. Information about their plasticity food provisioning along their breeding distribution is little known and could have conservation implications. For example, it can allow us to assess their adaptive capacity in the face of changes in the availability of their prey due to natural global changes or anthropogenic effects, such as fishing.
Conclusion
This study improves our understanding of chick provisioning behaviors and age, sex, and breeding stage-specific trophic niches of Magellanic penguins. Despite Magellanic penguins being considered as less concern in the red list of the IUCN, as long-lived species living in an unpredictable environment, knowledge of species diets and foraging plasticity is central for projecting how they may respond to future environmental change (i.e., changes in prey availability, change in the contamination exposure of their key prey items, etc.). Finally, knowledge of the potential prey and areas that adult penguins choose to feed themselves and their offspring could have key implications for the marine spatial planning at the Beagle Channel ecosystem, which currently does not have regulation.
Data availability
Penguins’ stable isotope raw data could find available here: https://ri.conicet.gov.ar/handle/11336/197811
References
Almandoz G, Cefarelli A, Diodato S, Montoya N, Benavides HR, Carignan M, Hernando M, Fabro E, Metfies K, Lundholm N, Schloss I, Álvarez M, Ferrario M (2019) Harmful phytoplankton in the Beagle Channel (South America) as a potential threat to aquaculture activities. Mar Pollut Bull 145:105–117. https://doi.org/10.1016/j.marpolbul.2019.05.026
Araújo MS, Guimarães PR, Svanbäck R, Pinheiro A, Guimarães P, dos Reis SF, Bolnick DI (2008) Network analysis reveals contrasting effects of intraspecific competition on individual vs. population diets. Ecology 89:1981–1993. https://doi.org/10.1890/07-0630.1
Ballard G, Dugger KM, Nur N, Ainley DG (2010) Foraging strategies of Adélie penguins: adjusting body condition to cope with environmental variability. Mar Ecol Prog Ser 405:287–302
Barlow K, Croxall J (2002) Provisioning behaviour of Macaroni Penguins Eudyptes chrysolophus. Ibis 144:248–258
Barquete V, Strauss V, Ryan PG (2013) Stable isotope turnover in blood and claws: a case study in captive African Penguins. J Exp Mar Biol Ecol 448:121–127. https://doi.org/10.1016/j.jembe.2013.06.021
Blanco D, Yorio P, Boersma P (1996) Feeding behavior, size asymmetry, and food distribution in magellanic penguin (Spheniscus magellanicus) chicks. Auk 113:496–498. https://doi.org/10.2307/4088917
Boersma PD, Frere E, Kane O, Pozzi L, Pütz K, Raya Rey A, Rebstock G, Simeone A, Smith J, Van Buren A, Yorio P, Garcia Borboroglu P (2013) Magellanic Penguin (Spheniscus magellanicus). In: Borboroglu PG, Boersma PD (eds) Penguin biology. University of Washington Press, Seattle, pp 232–263
Boersma PD, Rebstock GA, Garcia Borboroglu JP (2015) Marine protection is needed for Magellanic penguins in Argentina based on long-term data. Biol Conserv 182:197–204
Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML (2003) The ecology of individuals: incidence and implications of individual specialization. Am Nat 161:1–28
Booth JM, McQuaid CD (2013) Northern rockhopper penguins prioritise future reproduction over chick provisioning. Mar Ecol Prog Ser 486:289–304. https://doi.org/10.3354/meps10371
Ceia FR, Ramos JA (2015) Individual specialization in the foraging and feeding strategies of seabirds: a review. Mar Biol 162:1923–1938. https://doi.org/10.1007/s00227-015-2735-4
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287. https://doi.org/10.3354/meps329281
Cherel Y, Connan M, Jaeger A, Richard P (2014) Seabird year-round and historical feeding ecology: blood and feather δ13C and δ15N values document foraging plasticity of small sympatric petrels. Mar Ecol Prog Ser 505:267–280. https://doi.org/10.3354/meps10795
Ciancio JE, Pascual MA, Beauchamp DA (2007) Energy density of Patagonian aquatic organisms and empirical predictions based on water content. Trans Am Fish Soc 136:1415–1422. https://doi.org/10.1577/t06-173.1
Ciancio J, Yorio P, Wilson R, Frere E (2018) Food provisioning in Magellanic penguins as inferred from stable isotope ratios. Rapid Commun Mass Spectrom 32:489–494. https://doi.org/10.1002/rcm.8065
Clarke J, Manly B, Kerry K, Gardner H, Franchi E, Corsolini S, Focardi S (1998) Sex differences in Adelie penguin foraging strategies. Polar Biol 20:248–258
Clausen AP, Pütz K (2002) Recent trends in diet composition and productivity of Gentoo, Magellanic and Rockhopper penguins in the Falkland Islands. Aquat Conserv Mar Freshw Ecosyst 12:51–61. https://doi.org/10.1002/aqc.476
Croxall JP, Davis LS (1999) Penguins: paradoxes and patterns. Mar Ecol Prog Ser 27:1–12
Dehnhard N, Eens M, Sturaro N, Lepoint G, Demongin L, Quillfeldt P, Poisbleau M (2016) Is individual consistency in body mass and reproductive decisions linked to individual specialization in foraging behavior in a long-lived seabird? Ecol Evol 6:4488–4501. https://doi.org/10.1002/ece3.2213
Diez M, Cabreira A, Madirolas A, Lovrich G (2016) Hydroacoustical evidence of the expansion of pelagic swarms of Munida gregaria (Decapoda, Munididae) in the Beagle Channel and the Argentine Patagonian Shelf, and its relationship with habitat features. J Sea Res 114:1–12. https://doi.org/10.1016/j.seares.2016.04.004
Diez M, Cabreira A, Madirolas A, De Nascimento J, Scioscia G, Schiavini A, Lovrich G (2018) Winter is cool: spatio-temporal patterns of the squat lobster Munida gregaria and the Fuegian sprat Sprattus fuegensis in a sub-Antarctic estuarine environment. Polar Biol 41:2591–2605. https://doi.org/10.1007/s00300-018-2394-2
Dodino S, Riccialdelli L, Polito M, Pütz K, Raya Rey A (2020) Inter-annual variation in the trophic niche of Magellanic penguins Spheniscus magellanicus during the pre-molt period in the Beagle Channel. Mar Ecol Prog Ser 655:215–225. https://doi.org/10.3354/meps13518
Dodino S, Riccialdelli L, Polito M, Pütz K, Raya Rey A (2022) Intraspecific trophic variation during the early chick-rearing period in Magellanic penguins Spheniscus magellanicus: influence of age and colony location. Mar Biol 2:169–116
Forero MG, Hobson KA, Bortolotti GR, Donázar JA, Bertellotti M, Blanco G (2002) Food resource utilisation by the Magellanic penguin evaluated through stable-isotope analysis: segregation by sex and age and influence on offspring quality. Mar Ecol Prog Ser 234:289–299. https://doi.org/10.3354/meps234289
France R (1995) Differentiation between littoral and pelagic food webs in lakes using stable carbon isotopes. Limnol Oceanogr 40:1310–1313. https://doi.org/10.4319/lo.1995.40.7.1310
Francey RJ, Allisoni CE, Etheridgei DM, Trudingeri CM (1999) A 1000-year high precision record of δ13C in atmospheric CO. Tellus 51:170–193
Gandini PA, Frere E, Holik TM (1992) Implicancias de las diferencias en el tamaño corporal entre colonias para el uso de medidas morfométricas como método de sexado en Spheniscus magellanicus. El Hornero 13:211–213
Gelman A, Rubin D (1992) Inference from iterative simulation using multiple sequences. Stat Sci 7:457–472
Glibert P, Icarus Allen J, Artioli Y, Beusen A, Bouwman L, Harle J, Holmes R, Holt J (2014) Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Glob Change Biol 20:3845–3858. https://doi.org/10.1111/gcb.12662
Gorman K, Ruck K, Williams T, Fraser W (2021) Advancing the sea ice hypothesis: trophic interactions among breeding Pygoscelis penguins with divergent population trends throughout the western Antarctic Peninsula. Front Mar Sci 8:526092. https://doi.org/10.3389/fmars.2021.526092
Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D (2010) Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. Isoscapes: understanding movement, pattern, and process on Earth through isotope mapping. Springer, Netherlands, pp 299–318
Grémillet D, Welcker J, Karnovsky NJ, Walkusz W, Hall ME, Fort J, Brown ZW, Speakman JR, Harding AMA (2012) Little auks buffer the impact of current Arctic climate change. Mar Ecol Prog Ser 454:197–206. https://doi.org/10.3354/meps09590
Harris S, Scioscia G, Pütz K, Mattern T, Raya Rey A (2020) Niche partitioning between coexisting gentoo Pygoscelis papua and Magellanic penguins Spheniscus magellanicus at Martillo Island Argentina. Mar Biol. https://doi.org/10.1007/s00227-020-03722-w
Herman R, Valls F, Hart T, Petry M, Trivelpiece W, Polito M (2017) Seasonal consistency and individual variation in foraging strategies differ among and within Pygoscelis penguin species in the Antarctic Peninsula region. Mar Biol 164:115. https://doi.org/10.1007/s00227-017-3142-9
Hobson KA, Clark RG (1992) Assessing avian diets using stable isotopes I: Turnover of 13C in tissues. The Condor 94:181–188
Hobson KA, Gloutney ML, Gibbs HL (1997) Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can J Zool 75:720–1723
Horswill C, Trathan PN, Ratcliffe N (2017) Linking extreme interannual changes in prey availability to foraging behaviour and breeding investment in a marine predator, the macaroni penguin. PLoS ONE. https://doi.org/10.1371/journal.pone.0184114
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363
Indermühle A, Stocker TF, Joos F, Fischer H, Smith HJ, Wahlen M, Deck B, Mastroianni D, Tschumi J, Blunier T, Meyer R, Stauffer B (1999) Holocene carbon-cycle dynamics based on CO2 trapped in ice at Taylor Dome, Antarctica. Nature 398:121–126
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461
Jackson A, Inger R, Parnell A, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER - Stable Isotope Bayesian Ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jenkins E, Gulka J, Yurkowski DJ, Davoren GK, Gonzalez L (2019) Diet–tissue discrimination factors (δ15N and δ13C values) for blood components in Magellanic (Spheniscus magellanicus) and southern rockhopper (Eudyptes chrysocome) penguins. Rapid Commun Mass Spectrom. https://doi.org/10.1002/rcm.8612
Kowalczyk N, Chiaradia A, Preston T, Reina R (2014) Linking dietary shifts and reproductive failure in seabirds: a stable isotope approach. Funct Ecol 28:755–765. https://doi.org/10.1111/1365-2435.12216
Layman CA, Araujo MS, Boucek R, Hammerschlag-Peyer CM, Harrison E, Jud ZR, Matich P, Rosenblatt AE, Vaudo JJ, Yeager LA, Post DM, Bearhop S (2012) Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol Rev 87:545–562. https://doi.org/10.1111/j.1469-185X.2011.00208.x
Le Vaillant M, Le Bohec C, Onésime P, Wienecke B, Le Maho Y, Kato A, Ropert-Coudert Y (2013) How age and sex drive the foraging behaviour in the King penguin. Mar Biol 160:1147–1156. https://doi.org/10.1007/s00227-013-2167-y
Lynch HJ, Naveen R, Trathan PN, Fagan WF (2012) Spatially integrated assessment reveals widespread changes in penguin populations on the Antarctic Peninsula. Ecology 93:1367–1377
Newsome S, Martinez del Rio C, Bearhop S, Phillips D (2007) A niche for stable isotope ecology. Front Ecol Environ 5:429–436. https://doi.org/10.1890/060150.01
Noren SR, Williams TM, Pabst DA, McLellan WA, Dearolf JL (2001) The development of diving in marine endotherms: preparing the skeletal muscles of dolphins, penguins, and seals for activity during submergence. J Comp Physiol Biochem 171:127–134. https://doi.org/10.1007/s003600000161
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Colombus
Patrick SC, Weimerskirch H (2017) Reproductive success is driven by local site fidelity despite stronger specialisation by individuals for large-scale habitat preference. J Anim Ecol 86:674–682. https://doi.org/10.1111/1365-2656.12636
Phillips DL (2001) Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia 127:166–170. https://doi.org/10.1007/s004420000571
Pinheiro J, Bates DM, Saikat D, Sarkar D, Team RC (2015) Linear and Nonlinear Mixed Effects Models: 3.2.5. R package version 3.1–131
Piper WH (2011) Making habitat selection more “familiar”: a review. Behav Ecol Sociobiol 65:1329–1351
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. https://doi.org/10.1007/s00442-006-0630-x
Presta M, Riccialdelli L, Bruno D, Castro L, Fioramonti N, Florentín O, Berghoff C, Capitanio F, Lovrich G (2023) Mesozooplankton community structure and trophic relationships in an austral high-latitude ecosystem (Beagle Channel): the role of bottom-up and top-down forces during springtime. J Mar Syst. https://doi.org/10.1016/j.jmarsys.2023.103881
Pyke GH, Pulliam HR, Charnov EL (1977) Optimal foraging: a selective review of theory and tests. Q Rev Biol 52:137–154
Quezada-Romegialli C, Jackson AL, Hayden B, Kahilainen KK, Lopes C, Harrod C (2018) tRophicPosition, an r package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol Evol 9:1592–1599. https://doi.org/10.1111/2041-210X.13009
R Core Team (2021) R: A language and evironment for statistical computing, version 4.1.0.
Raya Rey A, Bost C, Schiavini A, Pütz K (2010) Foraging movements of Magellanic penguins Spheniscus magellanicus in the Beagle Channel, Argentina, related to tide and tidal currents. J Ornithol 151:933–943. https://doi.org/10.1007/s10336-010-0531-y
Raya Rey A, Pütz K, Scioscia G, Lüthi B (2012a) Sexual differences in the foraging behaviour of Magellanic Penguins related to stage of breeding. Emu 112:90–96
Raya Rey A, Polito M, Archuby D, Coria N (2012b) Stable isotopes identify age- and sex-specific dietary partitioning and foraging habitat segregation in southern giant petrels breeding in Antarctica and southern Patagonia. Mar Biol 2:1317–1326
Riccialdelli L, Becker Y, Fioramonti N, Torres M, Bruno D, Raya Rey A, Fernández D (2020) Trophic structure of southern marine ecosystems: a comparative isotopic analysis from the Beagle Channel to the oceanic Burdwood Bank area under a wasp-waist assumption. Mar Ecol Prog Ser 655:1–27
Rosciano N, Polito M, Raya Rey A (2019) What’s for dinner mom? Selective provisioning in southern rockhopper penguins (Eudyptes chrysocome). Polar Biol 42:1529–1535. https://doi.org/10.1007/s00300-019-02538-9
Rosciano N, Polito M, Raya Rey A (2020) Seasonally persistent foraging niche segregation between sympatric Southern Rockhopper and Magellanic penguins breeding at Isla de los Estados, Argentina. J Ornithol 161:1093–1104. https://doi.org/10.1007/s10336-020-01800-w
Rosenfeld S, Ojeda J, Hüne M, Mansilla A, Contador T (2014) Egg masses of the Patagonian squid Doryteuthis (Amerigo) gahi attached to giant kelp (Macrocystis pyrifera) in the sub-Antarctic ecoregion. Polar Res. https://doi.org/10.3402/polar.v33.21636
Sanz-Aguilar A, Massa B, Lo Valvo F, Oro D, Minguez E, Tavecchia G (2009) Contrasting age-specific recruitment and survival at different spatial scales: a case study with the European storm petrel. Ecography 32:637–646. https://doi.org/10.1111/j.1600-0587.2009.05596.x
Sargeant B, Wirsing A, Heithaus M, Mann J (2007) Can environmental heterogeneity explain individual foraging variation in wild bottlenose dolphins (Tursiops sp.)? Behav Ecol Sociobiol 61:679–688
Schiavini A, Yorio P, Gandini PA, Raya Rey A, Boersma PD (2005) Los pingüinos de las costas argentinas: estado poblacional y conservación. El Hornero 20:5–23
Schmidt KA (2001) Site fidelity in habitats with contrasting levels of nest predation and brood parasitism. Evol Ecol Res 2:633–648
Scioscia G, Rey A, Favero M, Schiavini A (2010) Factores que afectan el éxito reproductivo y la calidad de la nidada del pingüino patagónico (Spheniscus magellanicus) en el Canal Beagle, Tierra del Fuego, Argentina. El Hornero 25:17–25
Scioscia G, Raya Rey A, Saenz Samaniego RA, Florentín O, Schiavini A (2014) Intra- and interannual variation in the diet of the Magellanic penguin (Spheniscus magellanicus) at Martillo Island, Beagle Channel. Polar Biol 37:1421–1433. https://doi.org/10.1007/s00300-014-1532-8
Scolaro JA, Wilson RP, Laurenti S, Kierspel M, Gallelli H, Upton JA (1999) Feeding preferences of the Magellanic Penguin over its breeding range in Argentina. Waterbirds 22:104–110. https://doi.org/10.2307/1521999
Spencer WD (2012) Home ranges and the value of spatial information. J Mammal 93:929–947. https://doi.org/10.1644/12-MAMM-S-061.1
Stephens DW, Krebs JR (1986) Foraging theory. Princeton University Press, Princeton
Stock BC, Jackson AL, Ward EJ, Parnell AC, Phillips DL, Semmens BX (2018) Analyzing mixing systems using a new generation of Bayesian tracer mixing models. PeerJ. https://doi.org/10.7717/peerj.5096
Switzer PV (1993) Site fidelity in predictable and unpredictable habitats. Evol Ecol 7:533–555. https://doi.org/10.1007/BF01237820
Thompson KR (1993) Variation in magellanic penguin Spheniscus magellanicus diet in the Falkland Islands. Mar Ornithol 21:57–67
Van Heezik Y, Davis L (1990) Effects of food variability on growth rates, fledging sizes and reproductive success in the Yellow-eyed Penguin Megadyptes antipodes. Ibis 132:354–365
Votier S, Bearhop S, Fyfe R, Furness R (2008) Temporal and spatial variation in the diet of a marine top predator—links with commercial fisheries. Mar Ecol Prog Ser 367:223–232. https://doi.org/10.3354/meps07621
Wakefield E, Cleasby I, Bearhop S, Bodey T, Davies R, Miller P, Newton J, Votier S, Hamer K (2015) Long-term individual foraging site fidelity-why some gannets don’t change their spots. Ecology 96:3058–3074
Waluda CM, Hill SL, Peat HJ, Trathan PN (2012) Diet variability and reproductive performance of macaroni penguins Eudyptes chrysolophus at Bird Island, South Georgia. Mar Ecol Prog Ser 466:261–274
Watanuki Y (1992) Individual diet difference, parental care and reproductive success in slaty-backed gulls. Condor 94:159–171. https://doi.org/10.2307/1368805
Watson JEM, Dudley N, Segan DB, Hockings M (2014) The performance and potential of protected areas. Nature 515:67–73. https://doi.org/10.1038/nature13947
Acknowledgements
We would like to thank Maria Eugenia Lopez, Analía San Martin and Hernan Sacristan for their help during the fieldwork. Also, we thank Hayat Bennadji, Ulises Balza, and Nicolás A. Lois for technical help with stable isotope analysis. We acknowledge Andres and Alejandro Greco from Piratur SA for transportation to Martillo Island; and the Centro Austral de Investigaciones Científicas (CADIC), Museo Acatushún de Aves y Mamíferos Marinos Australes, and Ea. Harberton, which provided logistical support.
Funding
This study was financially supported by the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012-1832, PICT 2014-1870), Wildlife Conservation Society, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), and the Antarctic Research Trust. Research by S.D. was carried out under a Ph.D. fellowship from CONICET.
Author information
Authors and Affiliations
Contributions
SD: conceptualization, methodology, formal analysis and investigation, writing-original draft preparation; LR: conceptualization, methodology, investigation, resources, writing-review and editing, supervision; MJP: methodology, resources, writing-review and editing; KP: resources, writing-review and editing, funding acquisition; ARR: conceptualization, methodology, investigation, resources, writing-review and editing, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dodino, S., Riccialdelli, L., Polito, M.J. et al. Variation in the trophic niche and food provisioning between the early and late chick-rearing stages in Magellanic penguins Spheniscus magellanicus at Martillo Island, Tierra del Fuego, Argentina. Mar Biol 170, 96 (2023). https://doi.org/10.1007/s00227-023-04242-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04242-z