Abstract
Altricial mammals and birds become endothermic at about half the size of adults and presumably would benefit energetically from entering torpor at that time. Because little is known about torpor during development in endotherms, we investigated whether after the establishment of endothermic thermoregulation (i.e. the ability to maintain a high body temperature during cold exposure), Sminthopsis macroura, a small (∼25 g) insectivorous marsupial, is capable of entering torpor and whether torpor patterns change with growth. Endothermic thermoregulation was established when the nest young reached a body mass of ∼10 g, and they were capable of entering torpor early during development at ∼10–12 g, lending some support to the view that torpor is a phylogenetically old mammalian trait. Torpor bout length shortened significantly and the minimum metabolic rate during torpor increased as juveniles approached adult size, and consequently total daily energy expenditure increased steeply with age. Relationships between total daily energy expenditure and body mass during development of S. macroura (slope ∼1.3) differed substantially from the relationship between basal metabolism and body mass in adult endotherms (slope ∼0.75) suggesting that the energy expenditure–size relationship during the development differs substantially from that in adults under thermo-neutral conditions. Our study shows that while torpor can substantially reduce energy expenditure during development of endotherms and hence is likely important for survival during energy bottlenecks, it also may enhance somatic growth when food is limited. We therefore hypothesize that torpor during the development in endotherms is far more widespread than is currently appreciated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that torpor in mammals and birds is characterized by substantial reductions of body temperature (T b) and metabolic rate (MR), and it is an important strategy for survival, especially in small species (Morrison 1960; French 1986; Geiser and Ruf 1995; Barnes and Carey 2004). Small endotherms have large surface to volume ratios resulting in high loss of heat during cold exposure, high mass-specific basal MR (BMR) in thermo-neutrality, high mass-specific energy expenditures during locomotion, and a relatively small capacity for fat storage. Consequently, the energetic cost to maintain constant homeothermy, especially during cold exposure and/or periods of food shortage, may be prohibitively high. Employing torpor and reducing energy expenditure during certain parts of the day or the year can minimize the impacts of such energetic bottlenecks. Thus, while species that enter torpor are endothermic, they do not remain homeothermic all of the time, and so are widely referred to as heterothermic endotherms to reflect the variability in their MR and T b.
While it is generally accepted that body size is one important factor that determines whether or not a species is heterothermic, little is known about the impact of size and growth on the development of both endothermy and the development of heterothermic responses by endotherms. Altricial mammals and birds are poikilothermic at birth or hatching but become endothermic well before they reach adult body mass, often at about half adult size (Morrison and Petajan 1962; Dawson and Hudson 1970; Schleucher 1999). The period when endothermic thermoregulation develops is critical in the life of mammals and birds. High energy costs are likely to provide a strong selection pressure for heterothermy because individuals would presumably profit from entering torpor to help alleviate the energetic disadvantages of small size. However, little work has been conducted on the timing of torpor development as a survival strategy. The few published data about the development of torpor include studies of kowaris (Dasyuroides byrnei), shrews (Crocidura russula), hamsters (Phodopus sungorus), and martins (Delichon urbica; Nagel 1977; Geiser et al. 1986; Prinzinger and Siedle 1988; Bae et al. 2003). These species are capable of entering torpor soon after endothermic thermoregulation is established or when they are weaned, and also use torpor as adults. Even laboratory rats (Rattus norvegicus), which are considered to be homeothermic endotherms as adults, exhibit “torpor-like” fluctuations in T b at body mass of ∼20 g (Nuesslein-Hildesheim et al. 1995). Since these species belong to taxonomically diverse and species-rich groups (marsupials, insectivores, rodents, passerines, >8,000 species) it is possible that the importance of torpor for energy conservation and thus survival during the developmental stages of endotherms is not fully recognized.
Given that little is known about the development of torpor, our purpose was to assess the development of endothermic thermoregulation in the stripe-faced dunnart (Sminthopsis macroura). Specifically we evaluated whether endothermy and torpor develop concurrently and whether the expression of torpor is modified during growth. Sminthopsis macroura is an insectivorous/carnivorous marsupial of the order Dasyuromorphia. The species was selected for the study because, unlike most placental mammals, its development is slow and developmental changes can be investigated in detail, energetics can be examined beginning from small size without damaging growing individuals, and because it enters daily torpor when adult (Godfrey 1969; Geiser and Baudinette 1987). We use the results from our study to address the implications of energy expenditure during development on energy expenditure–size relationships in adult endotherms as well as implications in relation to the evolution of heterothermy.
Methods
Six S. macroura young (2 females, 4 males) from two litters bred from a laboratory colony at the University of New England were used to examine the development of endothermic thermoregulation. The colony was housed under natural photoperiod and an ambient temperature (T a) of 22°C. Dasyurids are prone to kill their young when stressed. As young are permanently attached to the mother’s nipple for 4–5 weeks after birth and, after removal from the nipple, young cannot be re-attached during early development, measurements could not be conducted from birth. Measurements began when young were no longer permanently attached to a nipple in the pouch and were left in the nest (beginning at a body mass of ∼3 g, approximately 50 days of age and still poikilothermic). As an index of size, we measured body mass to the nearest 0.1 g for the remainder of the development.
We used a Gompertz 3-parameter equation as in Zullinger et al. (1984), to describe growth data:
Where ‘A’ is the asymptotic body mass, ‘K’ is the growth rate constant in days−1 and ‘I’ is the age at the inflection point in days (maximum growth rate).
To quantify the development of the capacity for endothermic thermoregulation, we measured the MR as the rate of oxygen consumption of young animals weekly using open-flow respirometry from ∼3 g body mass (∼50 days) for one hour each at T a 33.0±0.5°C and 20±0.5°C until animals reached ∼9 g when endothermic thermoregulation was established as indicated by a near constant T b during cold exposure. It is probable that these small individuals were not post-absorptive during MR measurements. To quantify further changes of resting MR (RMR) within the thermo-neutral zone (TNZ) (RMRTNZ) we continued to measure oxygen consumption until the individuals reached ∼16.5 g (∼130 days) as well as in adults ∼ 25 g (>200 days) at T a 33.0±0.5°C, which approximates the pouch temperature (33.6±0.7°C, measured with an infrared thermometer) and is within the TNZ of adults (Song et al. 1995). All MR measurements at T a 20 and 33°C were conducted during the daytime (inactive period given that the species is nocturnal) and were completed within about 5 h. As RMRTNZ (juveniles >9 g) and BMR (adults) readings were taken >7 h after feeding, these individuals were post-absorptive. T b was measured rectally immediately after removal from the nest and at the end of MR measurements (at T a 20°C after exposure for 1 h) using a fine thermocouple probe calibrated to the nearest 0.1°C that was read with a digital thermometer (Omega HH-71 T).
Eleven additional juvenile S. macroura (6 females, 5 males) were used to assess the potential for developmental changes in the expression of torpor patterns. These young were the offspring of four females captured with neonate pouch young in south-west Queensland in November (late Spring) 2003. Data for a twelfth juvenile were excluded from our analyses because it failed to thrive during the measurement period and eventually died while still a juvenile. Beginning in January 2004, when juveniles reached ∼12 g body mass at ∼75–80 days, the point when endothermic thermoregulation was well established, to ∼210 days, MR of the young animals was measured over periods of ∼23.5 h beginning in the late afternoon (i.e. ∼10 h or more after feeding). We measured the duration and depth of torpor bouts as well as changes in RMR and average daily MR (ADMR) at T a 16.0 ±0.5°C. We selected T a 16°C for our measurements because torpor use at this T a is frequent in adult S. macroura, the torpor MR (TMR) approximates the minimum for the species, and torpor bouts are typically long ∼5–6 h (Geiser and Baudinette 1987). Measurements were made once per month over 4 months for all eleven individuals and for a fifth month for six of the eleven individuals (i.e. during summer and autumn). Mean values reported in the text reflect values for all eleven individuals over the first 4 months of measurements. For our regression analyses, we also used the data from month 5.
For both experiments, food and water were not available during MR measurements, but were available ad libitum at all other times. Food consisted of a mixture of moistened cat chow and canned dog food and was provided fresh daily. Animals were weighed before and after each MR measurement period and a linear decrease of body mass was assumed for calculation of mass-specific MR during MR measurements. A single-channel oxygen analyser (Ametek Applied Electrochemistry S-3A, Pittsburgh, PA, USA; resolution 0.01%) fitted with a high output board was used to make the short-term measurements, and a second single channel oxygen analyser (FOX, Sable Systems, Las Vegas, NV; resolution 0.001%) was used for the 1-day measurements including the low MRs during torpor bouts. Variables for two or three individuals and outside air as a reference were measured in sequence for 3 min each using solenoid valves (i.e. each individual and outside air were measured every 9–12 min). Initially MR of nest young was measured in 50 ml respirometers (flow rate 200 ml min−1), young at >12 g body mass were measured in 500 ml respirometers (flow rate ∼400 ml min−1) and adults in 750 ml respirometers (flow rate ∼450 ml min−1). The flow-rate of dry air passing through the respirometry chamber was controlled with rotameters (7908, Aarlborg, New York, NY, USA) and measured with mass flowmeters (FMA-5606, Omega, Stamford, CT, USA). For both RMR and TMR, at least three consecutive readings (i.e. over at least 27–36 min) when MR was stable and minimal were assumed to represent steady-state values and were averaged. ADMR was calculated by integrating the data over the entire ∼23.5 h, extrapolating to 24 h and converting to Joules (20.083 J ml O −12 ). For comparison, a second estimate of daily energy expenditure in thermo-neutrality was derived by extrapolating RMRTNZ measurements of the developing young and BMR in adults to 24 h. Calculations of MR were performed according to Eq. 3a from Withers (1977) assuming a RQ of 0.85. Torpor was defined as a reduction of MR below 75% RMR at T a 16°C and torpor bout duration was calculated as the time MR remained below 75% RMR.
T a was measured every 3 min during the period when MR was determined and the mean values were calculated as described for MR. T a in the respirometry chambers was measured to the nearest 0.1°C via calibrated thermocouple probes that were inserted 1 cm into the chamber and recorded with a digital thermometer (Omega DP116). Outputs from the flowmeter, oxygen analyser (S-3A) and digital thermometer were interfaced to a personal computer using an A/D 14-bit converter card or a serial port for the FOX oxygen analyser.
Numerical values are presented as mean ± 1 standard deviation (SD) for “n” the number of individuals and “N” represents the total number of measurements. We used repeated-measures ANOVA to assess whether variables of individuals differed over the 4-month measurement period. Linear regressions were fitted using the method of least squares for all data including the fifth month. We minimized pseudo-replication by employing the repeated measures analysis and because each individual was represented by a similar number of data points for regressions.
Results
Development of thermoregulation
Growth of young S. macroura was slow (Fig. 1). At birth, they weigh ∼10 mg (Tyndale-Biscoe and Renfree 1987). Only after 30 –40 days, when they reached a body mass of ∼2–3 g, were they left unattended in the nest when the mother foraged. Weaning occurred at ∼70 days as reported previously (Godfrey 1969) at a body mass of ∼8–9 g. Young attained adult mass after >200 days.
The change of body mass during the development of S. macroura. Growth is described by a Gompertz 3-parameter equation: \(\hbox{body mass (g)} = 19.07 {\rm e}^{-{\rm e}^{{-0.027({\rm age}-63.7)}}} r^{2} = 0.87.\) Body mass at birth (10 mg) was taken from Tyndale-Biscoe and Renfree (1987). At an age of >100 days, the upper individuals are males (n=4), the lower two females (n=2)
Initially nest young at ∼3 g body mass were poikilothermic. When exposed to T a 20°C for 1 h, T b fell from T b ∼33°C immediately after removal from the nest to T b ∼23°C (Fig. 2a). The ability to regulate T b at T a 20°C for a period of 1 h improved substantially with age and body size. By the time of weaning at about 70 days of age and a body mass of 8–9 g, T b fell only slightly when young were exposed to T a 20°C. The intersection of the two regressions of T b versus mass occurred at 9 –10 g suggesting that at that mass, T b can be maintained during mild cold exposure (i.e. endothermic thermoregulation is established). The improvement in thermoregulatory ability at T a 20°C was accompanied by a substantial increase in MR from ∼1 ml O2 g−1 h−1 at 3 g to ∼7 ml O2 g−1 h−1 at 8–9 g. The RMRTNZ of young at T a 33°C also increased slightly during this period of growth (Fig. 2b).
The relationship between RMRTNZ at T a 33°C and body mass is illustrated further in Fig. 3, where the two variables are plotted on a double-log plot in comparison to the BMR of adult insectivorous/carnivorous marsupials, including the 95% confidence interval. At 3.5 g, when young S. macroura were poikilothermic, the mean RMRTNZ was 80% of the BMR predicted for an insectivorous/carnivorous marsupial of that mass and below the 95% confidence interval. At 5.1 g, RMRTNZ increased to 112% of that predicted reflecting increased metabolism required for growth (Fig. 1), even though they were still poikilothermic. Between a body mass of 7 and 12 g, mean RMRTNZ varied from 134 to 173% of the predicted BMR, well above the 95% confidence interval. These values reflect maximum growth but at this time also a fully developed endothermic metabolism. In 16.5 g young and in adults at 24.8 g, RMRTNZ and BMR was 89 and 86% respectively of the predicted BMR, and both values were near or slightly below the 95% confidence interval for the BMR of carnivorous marsupials. The SD of MR measurements (Fig. 3) changed substantially from large to smaller values with growth indicating substantial individual differences especially at the two smallest body masses measured and smaller individual differences in bigger individuals as has been reported in previous developmental studies (Dawson and Hudson 1970). However, metabolic differences early during the growth phase do not appear to be due to differences in body mass because an increase in sexual dimorphism of size (Fig. 1) was accompanied by a decrease in the variation of RMRTNZ.
Double-log plot of resting metabolic rate at T a 33°C (RMRTNZ) of S. macroura during development (filled circles) and BMR (circle) in adults at 24.8 g. Values are means (n=5 individuals >8 g, n=3–5 individuals <8 g) with SD. The regression line with 95% confidence intervals represents the BMR of insectivorous/carnivorous marsupials [BMR (ml O2 g−1 h−1)= 2.39 body mass (g)−0.262, r 2 =0.88, n=28 species; Geiser 2003]
Development of torpor and energy expenditure
Torpor depth and length changed substantially during the development and growth of S. macroura (illustrated for a representative individual#106 M/03, Fig. 4). During months 1 and 2, entry into torpor occurred near midnight and torpor bouts lasted until the early afternoon (Fig. 4a, b). In months 3 and 4 (Fig. 4c, d), entry into torpor occurred later in the measurement period, typically around the time when the lights were switched on, and spontaneous arousal also occurred earlier, around noon. The pattern of torpor entry also differed between early and late nest young. In the early nest young, torpor usually commenced after a steady decline of MR during the night Fig. 4a, b), in older young, a MR peak preceded torpor entry (Fig. 4c, d). Loss of body mass during the 23.5-h MR measurements was 1.66±0.37 g (month 1), 2.10±0.44 g (month 2), 2.38±0.56 g (month 3) and 2.49±0.44 g (month 4). Interestingly, the % loss of body mass was similar among the measurements: 13.2% (month 1 and 2), 14.4% (month 3), and 13.7% (month 4). Torpor use was high (96% of measurement periods) and on only two out of a total of 50 23.5-h measurements did two individuals (N=n=2) not enter torpor and both of these individuals had a body mass of >15.5 g.
Oxygen consumption traces as a direct measure of metabolic rate of a representative juvenile S. macroura (#106 M/03) measured for one day over 4 months at T a 16°C. The steep decline in oxygen consumption indicates torpor entry and the steep rise depicts rewarming from torpor. The black bars indicate the periods of darkness
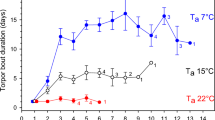
Torpor bout length varied significantly (ANOVA, F 3,30 =15.0, P<0.0001) over the 4 month measurement period when data for all eleven individuals were available. Torpor bouts (excluding the two zero values) lasted 11.75±2.67 h at a body mass of 12.6±1.2 g (month 1), 7.08±3.23 h at a body mass of 15.9±1.5 g (month 2), 5.76±3.13 h at a body mass of 16.5±1.3 g (month 3), and 3.32±1.66 h at a body mass of 18.1±2.2 g (month 4). Mean torpor bout length in month 4 was only 26% of that in month 1. Torpor bout length decreased significantly with increasing body mass (r 2 =0.42) over the 5-month period (Fig. 5a).
Physiological variables of endothermic S. macroura as a function of body mass during growth at T a 16°C (filled circles). All regressions at T a 16°C were significant (P<0.01). a Torpor bout length (h) = 21.46–0.91 body mass (g), r 2 =0.42. b Torpor metabolic rate, TMR (ml O2 g−1 h−1)= 0.25+0.36 body mass (g), r 2 =0.2. c Resting metabolic rate, RMR (ml O2 g−1 h−1)=7.31−0.20, r 2 =0.71. d Average daily metabolic rate, ADMR (kJ day−1)=−5.03+2.14 body mass (g), r 2 =0.63. The circles in d indicate daily energy expenditure as derived from mean RMRTNZ and BMR at T a 33°C in Fig. 3 (ADMRTNZ); this regression was not significant (P>0.5). Equation: ADMRTNZ (kJ day−1)=8.64+0.056 body mass (g), r 2 =0.13
Minimum TMR during torpor bouts increased significantly (ANOVA, F 3,30=4.3, P=0.012) from 0.176±0.131 ml O2 g−1 h−1 in month 1, to 0.335±0.214 ml O2 g−1 h−1 in month 2, to 0.368±0.206 ml O2 g−1 h−1 in month 3 and finally to 0.378±0.126 ml O2 g−1 h−1 in month 4. TMR was weakly but positively related (r 2 =0.20) to body mass (Fig. 5b). However, TMR was negatively related to torpor bout length [TMR (ml O2 g−1 h−1 =0.55–0.034 torpor bout length (h), r 2 =0.35].
In contrast to TMR, mass-specific RMR at T a 16°C decreased significantly (ANOVA, F 3,30=39.5, P<0.0001) as expected. RMR fell from 5.00±0.33 ml O2 g−1 h−1 in month 1, to 4.01±0.29 ml O2 g−1 h−1 in month 2, to 3.96±0.45 ml O2 g−1 h−1 in month 3, and 3.50±0.29 ml O2 g−1 h−1 in month 4. RMR decreased as a linear function (r 2 =0.71) of body mass (Fig. 5c).
Despite the decrease in RMR with growth, mass-specific ADMR increased significantly (ANOVA, F 3,30=5.4, P<0.005) from 3.28±0.69 ml O2 g−1 h−1 in month 1, to 3.66±0.54 ml O2 g−1 h−1 in month 2, to 4.01±0.59 ml O2 g−1 h−1 in month 3, and finally to 4.13±0.52 ml O2 g−1 h−1 in month 4. Total ADMR increased substantially (19.92±5.05 kJ day−1 month 1; 28.09±5.16 kJ day−1 month 2; 31.95±5.51 kJ day−1 month 3; 35.67±3.76 kJ day−1 month 4) and significantly (ANOVA, F 3,30=24.8, P<0.0001) with age and body mass (r 2 =0.63; Fig. 5d). In contrast to the ADMR derived from 1-day measurements at T a 16°C, the daily energy expenditure extrapolated from RMRTNZ measurements during development (from Fig. 3, at >8 g) did not change significantly (P>0.5) with growth (Fig. 5d) and underestimated ADMR by a factor of ∼1.7× (at body mass ∼12 g) increasing to ∼4× (at body mass ∼22 g).
Discussion
We found that, as in other marsupials, slow growth and development coincide with a slow development of endothermic thermoregulation in S. macroura. The data demonstrate that torpor use is frequent and pronounced shortly after endothermy is established, and that torpor expression during this time is deeper and longer than observed in independent, larger young, and adults. If our findings reflect responses occurring in the wild they suggest that employment of torpor during development is an important adaptation that allows some growing endotherms to balance energy expenditure during periods of food unavailability.
The growth rate of S. macroura was slow. The growth constant K was 0.027, which is similar to that predicted for other marsupials, but less than half that of placental mammals (Lee and Cockburn 1985). Consequently, the approximately 82-day conception to weaning time in S. macroura is about 1.5-times that predicted for a 25-g placental mammal (Lee and Cockburn 1985). Growth and development in S. macroura were accompanied by an enormous increase (∼7-fold) in MR during cold exposure, which was associated by a substantial growth of fur. When measurements began, fur was thin, whereas at weaning, fur was thick and similar in appearance to that in adults. It is likely that the increase in heat production observed during the development in S. macroura was to a large extent due to an increase in muscle mass and shivering, similar to that found in birds, because both groups, in contrast to placental mammals, appear to lack functional brown fat (Nicol et al. 1997; Schleucher 1999).
Torpor in small juvenile S. macroura was pronounced. However, with time, torpor bouts became shorter and torpor depth was shallower. At the age of 5–6 months (late autumn), bout lengths were somewhat shorter than those measured in adults in winter but TMR was almost identical to that of adults (Geiser and Baudinette 1987). This suggests that once growth is complete and adult body mass is attained, torpor expression in this species, apart from seasonal adjustments and perhaps those associated with ageing, changes little.
Our laboratory observations raise the question of whether torpor during development is likely to be used in the wild. We know that single individuals or groups of young marsupial are left in the nest when they are small and may be without their mother for prolonged periods (Holloway and Geiser 2000 and personal observations). While huddling may help individuals keep warm for short periods, attempting to remain homeothermic during this time would likely be impossible early during development, and very costly, and perhaps detrimental to survival later during the development. While it is known that torpor during development and reproduction reduces the growth rate of young (Racey 1973), which may be problematic in species that require fast growth and maturation, this is likely to be of less consequence for marsupials, which have a slow rate of development. An extension of the time by several days of torpor when adult body mass is reached is unlikely to be critical given the extremely long developmental period of marsupials. Our data suggest that by employing torpor extensively during growth, energy expenditure can be reduced substantially. This is likely an important survival strategy for young marsupials, but also for other altricial mammals and birds in the wild.
Torpor bouts during the growth phase in S. macroura became shorter and shallower from summer to late autumn. These observations are interesting because in S. macroura and similarly in Antechinus spp. (Geiser 1988), torpor expression declines as photoperiod shortens in autumn. In many northern hemisphere species, a shortening in photoperiod has the opposite effect, resulting in a substantial increase in torpor expression (Lynch et al. 1978; Ruf et al. 1991). These observations imply that the effect of photoperiod on seasonal changes in torpor in S. macroura, as well as in S. crassicaudata (Holloway and Geiser 1996), differs from that reported in northern hemisphere species. It appears that body mass and perhaps maturation are the major influences on the change of torpor patterns during development in S. macroura.
A decline in torpor bout length as the juvenile S. macroura grew and increased in size supports the view that torpor bouts in small heterothermic species are longer than in larger species (French 1985). Small heterotherms have higher relative energy costs for thermoregulation and locomotion than large species coupled with a limited capacity for fat storage. We argue that prolonged torpor bouts are essential for survival of small species or individuals during periods with a limited food supply. While food limitations will affect adults as well as growing young, energy constraints on juveniles are likely to be more pronounced than in adults. This is because high energy expenditure in juveniles shortly after weaning will be coupled with inexperience in finding food and replenishing energy resources. This may be the reason why the relationship between size and torpor bout length during development of S. macroura (∼6-fold difference in torpor bout length over a ∼2-fold difference in body mass) is more pronounced than that found between adults of different species (∼2-fold difference in torpor bout length over a ∼350-fold difference in body mass, French 1985).
Deep and prolonged bouts of torpor by young animals had a substantial influence on energy expenditure. ADMR (kJ day−1) in small (body mass ∼12 g) juvenile S. macroura was only about 55% of that in larger, older (body mass ∼18 g) juveniles, even though the RMR and consequently energy costs for thermoregulation in small individuals was about 170% of that in larger young. The substantial increase in ADMR with growth is best explained by the longer torpor bouts and the lower TMR in the smaller juveniles (Fig. 6). ADMR was negatively related to torpor bout length (r 2 =0.86) in developing S. macroura, suggesting that increased duration of torpor bouts due to earlier entry as well as later arousals form the most important part of the strategy to adjust daily energy expenditure when energy is limited. Nevertheless, the depth of torpor bouts also contributed significantly to lowering daily energy expenditure in small individuals, as the log TMR (best fit) explained 59% of the observed variance in ADMR (Fig. 6b). These observations suggest that adjustments to both the depth and length of torpor bouts during the development of young provide growing offspring with an effective avenue for minimizing daily energy expenditure. Torpor should also enhance the availability of nutrients and energy for somatic growth, and this approach is similar to that used by adult hummingbirds for fat accumulation during migration (Hiebert 1993).
Our study has implications for the evolution of endothermy and torpor. As most functional variables cannot be quantified from the study of fossil material, developmental data are often used as a proxy for functional changes during phylogeny (Seymour et al. 2004). Thus our developmental data, demonstrate that S. macroura use torpor soon after the establishment of endothermic thermoregulation, and a continued high thermal tolerance (eurythermy) throughout their development and as adults. This finding is supportive of a view that torpor in mammals occurred early in their evolutionary history, perhaps via initial facultative endothermy (Grigg et al. 2004). However, our study does not resolve the argument about whether torpor is a plesiomorphic or a derived trait because our experiment was conducted on a single species and it is possible that torpor evolved independently in different taxa. Moreover, we did not quantify whether a torpor-like state is entered before endothermic thermoregulation was well established (i.e. when animals were poikilothermic or only partially endothermic with a low thermogenic capacity and a MR intermediate between reptiles and mammals). If it could be shown experimentally that before individuals achieve endothermic thermoregulation at low T a, they are capable of entering torpor at somewhat higher T a (e.g. ∼25°C), and are capable of endothermic rewarming, perhaps with a little help from solar radiation or other form of external heat (Geiser et al. 2002), the hypothesis that torpor is “almost certainly plesiomorphic” (Grigg et al. 2004), would receive further support.
A common statement in the literature is that while BMR should not be equated with total energy expenditure of an animal, it is a good substitute for total energy expenditure because BMR as well as metabolic rates measured in the field (FMR) increase with size (e.g. McNab 2002). Our results allowed us to examine the relationship between RMRTNZ during development after endothermy was largely established (i.e. BMR except that it was measured in developing juveniles rather than in adults) in relation to ADMR over that time. Whereas ADMR increased substantially with mass, daily energy expenditure calculated from RMRTNZ changed little similar to the RMRTNZ versus mass relationship observed in rats during mid to late development (Brody 1945). RMRTNZ in S. macroura also always underestimated ADMR, and this error increased with size (Fig. 5d). This shows that resting energy expenditure in thermo-neutrality does not reflect the change in the total daily energy expenditure during growth.
Moreover, the slope of the increase in ADMR with body mass during development in S. macroura on a double-log plot can be compared with that of BMR versus body mass in adult endotherms. The slope for ADMR versus body mass during growth [log ADMR (kJ day−1)=−0.113+1.305 log body mass (g), r 2 =0.62] was almost twice (∼1.3) that of the commonly accepted slope of ∼0.75 between BMR and body mass. The latter is often assumed to be a reasonable reflection of the increase in costs of living as a function of size. However, our analysis demonstrates that energy expenditure in the TNZ is a poor predictor for total energy expenditure during development, which, especially in marsupials, represents a substantial part of their total life span (Lee and Cockburn 1985). Given that ADMR at low T a incorporates costs for various physiological activities, such as thermoregulation and movement, it is clearly a more ecologically meaningful measure of energy expenditure than a measurement of RMR in thermo-neutrality. BMR and its mass exponents obviously remain an interesting scientific challenge. However, the lack of a relationship between ADMR and RMRTNZ (present study), the vastly different slopes for ADMR versus body mass during growth and BMR versus body mass in adults (present study), the lack of correlation between RMRTNZ and reproductive output in mice (Speakman et al. 2004), and discrepancies between FMR and BMR in adult mammals especially at small body masses (Degen and Kam 1995), bring into question (see also Frappell and Butler 2004) whether the ecological value of BMR and its mass exponents is not commonly overstated.
Low energy expenditure by endotherms in the TNZ is often associated with torpor use in adult mammals, likely because both torpor and a low BMR can contribute to a reduction in daily energy expenditure (Lovegrove 1996; Geiser and Brigham 2000). However, this is not always the case. At a body mass ∼12 g at which torpor was most pronounced in juvenile S. macroura (Figs. 4, 5), RMRTNZ was 34% above that predicted for the BMR of adult insectivorous/carnivorous marsupials at the same body mass (Fig. 3). In contrast, the RMRTNZ of juveniles was 11% below the BMR predicted for insectivorous/carnivorous marsupials at a body mass of 16.5 g when torpor depth and length were substantially reduced. Our finding of a relative high RMRTNZ (Fig. 3) associated with long and deep torpor in small juvenile S. macroura (Figs. 4, 5) and a low RMRTNZ associated with shorter and shallower bouts of torpor in larger juveniles (Figs. 3, 5) indicates that a low RMRTNZ (i.e. BMR in adults) is not the cause of torpor expression. Our results do not support McNab’s (2002) view, derived from some species when adult, that a low energy expenditure in the TNZ requires that a species must enter torpor and a high energy expenditure in the TNZ is always associated with strict homeothermy.
Small mammals and birds represent the vast majority of all endothermic species (Smith et al. 2003). Many, especially small mammals (Prinzinger 1993; Wilkinson and South 2002), are short lived with the development and growth period making up a relatively large proportion of the life cycle. It is therefore essential to better understand the energetics of the growth process and to establish whether torpor, as employed in the species we studied, is a common mechanism used by small endotherms for overcoming energy constraints during development.
References
Bae HH, Larkin JE, Zucker I (2003) Juvenile Siberian hamsters display torpor and modified locomotor activity and body temperature rhythms in response to reduced food availability. Physiol Biochem Zool 76:858–867
Barnes BM, Carey HV (eds)(2004) Life in the cold: evolution, mechanisms, adaptation, and application. Twelveth international hibernation symposium. biological papers, University of Alaska#27. Institute of Arctic Biology, University of Alaska, Fairbanks
Brody S (1945) Bioenergetics and growth. Reinhold Publishing Corporation, New York
Dawson WR, Hudson JW (1970) Birds. In: Whittow GC (ed) Comparative physiology of thermoregulation, vol 1. Academic Press, New York, pp 223–310
Degen AA, Kam M (1995) Scaling of field metabolic rate to basal metabolic ratio in homeotherms. Ecoscience 2:48–54
Frappell PB, Butler PJ (2004) Minimal metabolic rate, what is it, its usefulness, and its relationship to the evolution of endothermy: a brief synopsis. Physiol Biochem Zool 77:865–868
French AR (1985) Allometries of the duration of torpid and euthermic intervals during mammalian hibernation: a test of the theory of metabolic control of the timing of changes in body temperature. J Comp Physiol B 156:13–19
French AR (1986) Patterns of thermoregulation during hibernation. In: Heller HC, Musacchia XJ and Wang LCH (eds), Living in the cold. Elsevier, New York, pp 393–402
Geiser F (1988) Daily torpor and thermoregulation in Antechinus (Marsupialia): influence of body mass, season, development, reproduction, and sex. Oecologia 77:395–399
Geiser F (2003) Thermal biology and energetics of carnivorous marsupials. In: Jones M, Dickman C and Archer M (eds), Predators with pouches: the biology of carnivorous marsupials. CSIRO Publishers, Melbourne, pp 234–249
Geiser F, Baudinette RV (1987) Seasonality of torpor and thermoregulation in three dasyurid marsupials. J Comp Physiol B 157:335–344
Geiser F, Brigham RM (2000) Torpor, thermal biology, and energetics in Australian long-eared bats (Nyctophilus). J Comp Physiol B 170:153–162
Geiser F, Ruf T (1995) Hibernation versus daily torpor in mammals and birds: physiological variables and classification of torpor patterns. Physiol Zool 68:935–966
Geiser F, Matwiejczyk L, Baudinette RV (1986) From ectothermy to heterothermy: the energetics of the kowari, Dasyuroides byrnei (Marsupialia: Dasyuridae). Physiol Zool 59:220–229
Geiser F, Goodship N, Pavey CR (2002) Was basking important in the evolution of mammalian endothermy? Naturwissenschaften 89:412–414
Godfrey GK (1969) Reproduction in a laboratory colony of the marsupial mouse Sminthopsis larapinta (Marsupialia: Dasyuridae). Aust J Zool 17:637–654
Grigg GC, Beard LA, Augee ML (2004) The evolution of endothermy and its diversity in mammals and birds. Physiol Biochem Zool 77:982–997
Hiebert SM (1993) Seasonality of daily torpor in a migratory hummingbird. In: Carey C, Florant GL, Wunder BA and Horwitz B (eds), Life in the cold: ecological, physiological and molecular mechanisms. Westview, Boulder,Colorado, pp 25–32
Holloway JC, Geiser F (1996) Reproductive status and torpor of the marsupial Sminthopsis crassicaudata: effect of photoperiod. J Therm Biol 21:373–380
Holloway JC, Geiser F (2000) Development of thermoregulation in the sugar glider Petaurus breviceps (Marsupialia: Petauridae). J Zool 252:389–397
Lee AK and Cockburn A (1985) Evolutionary ecology of marsupials. Cambridge University Press, Cambridge
Lovegrove BG (1996) The low basal metabolic rates of marsupials: the influence of torpor and zoogeography. In: Geiser F, Hulbert AJ, Nicol SC (eds). Adaptations to the cold: tenth international hibernation symposium. University of New England Press, Armidale, pp 141–151
Lynch GR, White SE, Grundel R, Berger MS (1978) Effects of photoperid, melatonin administration and thyroid block on spontaneous daily torpor and temperature regulation in the white-footed mouse, Peromyscus leucopus. J Comp Physiol B 125:157–163
McNab BK (2002) The physiological ecology of vertebrates. Cornell University Press, Ithaca
Morrison PR (1960) Some interrelations between weight and hibernation function. In: Lyman CP, Dawe AR (eds) Mammalian Hibernation. Bull Mus Comp Zool, Harvard College, vol 24. Massachusetts, Cambridge, pp 75–91
Morrison PR, Petajan JH (1962) The development of temperature regulation in the opossum, Didelphis marsupialis virginiana. Physiol Zool 35:52–65
Nagel A (1977) Torpor in the European white-toothed shrews. Experientia 33:1454–1456
Nicol S, Pavlides D, Andersen NA (1997) Nonshivering thermogenesis in marsupials: absence of thermogenic response to ß3-adrenergic agonists. Comp Biochem Physiol 117A:399–405
Nuesslein-Hildesheim B, Imai-Matsumura K, Doering H, Schmidt I (1995) Pronounced juvenile circadian core temperature rhythms exist in several strains of rats but not in rabbits. J Comp Physiol B 165:13–18
Prinzinger R (1993) Life span in birds and ageing theory of absolute metabolic scope. Comp Biochem Physiol 105A:609–615
Prinzinger R, Siedle K (1988) Ontogeny of metabolism, thermoregulation and torpor in the house martin Delichon u. urbica (L.) and its ecological significance. Oecologia 76:307–312
Racey PA (1973) Environmental factors affecting the length of gestation in heterothermic bats. J Reprod Fert Suppl 19:175–189
Ruf T, Klingenspor M, Preis H, Heldmaier G (1991) Daily torpor in the Djungarian hamster (Phodopus sungorus): interactions with food intake, activity, and social behaviour. J Comp Physiol B 160:609–615
Schleucher E (1999) Energetics and body temperature regulation in two convergent dove species from extreme habitats. Ornis Fenn 76:199–210
Seymour RS, Bennett-Stamper CL, Johnston SD, Carrier DR, Grigg GC (2004) Evidence for endothermic ancestors of crocodiles at the stem of archosaur evolution. Physiol Biochem Zool 77:1051–1067
Smith FA, Lyons SK, Ernest SKM, Jones KE, Kaufman DM, Dayan T, Marquet PA, Brown JH, Haskell J (2003) Body mass of late quaternary mammals. Ecology 84:3403, Ecological Archives E084–094
Song X, Körtner G, Geiser F (1995) Reduction of metabolic rate and thermoregulation during daily torpor. J Comp Physiol B 165:291–297
Speakman JR, Król E, Johnson MS (2004) The functional significance of individual variation in basal metabolic rate. Physiol Biochem Zool 77: 900–915
Tyndale-Biscoe H, Renfree M (1987) Reproductive physiology of marsupials. Cambridge University Press, Cambridge
Wilkinson GS, South JM (2002) Life history, ecology and longevity in bats. Ageing Cell 1:124–131
Withers PC (1977) Measurements of V̇O2, V̇CO2, and evaporative water loss with a flow-through mask. J Appl Physiol 42:120–123
Zullinger EM, Ricklefs RE, Redford KH, Mace GM (1984) Fitting sigmoidal equations to mammalian growth curves. J Mammal 65:607–636
Acknowledgements
We thank Alison Goldzieher and Rebecca Drury for technical support, and Gerhard Körtner, Lisa Warnecke and four anonymous referees for comments on the manuscript. The work was supported by a grant from the Australian Research Council to FG and a grant from the Natural Sciences and Engineering Council, Canada to RMB.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume
Rights and permissions
About this article
Cite this article
Geiser, F., Westman, W., McAllan, B.M. et al. Development of thermoregulation and torpor in a marsupial: energetic and evolutionary implications. J Comp Physiol B 176, 107–116 (2006). https://doi.org/10.1007/s00360-005-0026-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-005-0026-y