Abstract
Marsupials have a slow rate of development and this allows a detailed examination of thermoregulatory developmental changes and stages. We quantified the cooling rates of marsupial dunnarts (Sminthopsis crassicaudata) at 40–56 days (d) old, and torpor and basking behaviour in animals given the option to bask in four age groups from 60 to 150 d. The development of thermoregulation was a continuum, but was characterised by three major thermoregulatory stages: (1) at 40 d, animals were unable to maintain a constant high body temperature during short-term cold exposure; (2) at 60 d, animals could maintain a high T b for the first part of the night at an ambient temperature of 15.0 ± 0.7 °C; later in the night, they entered an apparent torpor bout but could only rewarm passively when basking under a heat lamp; (3) from ~90 d, they expressed prolonged torpor bouts and were able to rewarm endogenously. Young newly weaned 60 d animals were able to avoid hypothermia by basking. In this case, basking was not an optional behavioural method of reducing the cost of rewarming from torpor, but was essential for thermoregulation independent of the nest temperature. Results from our study suggest that basking is a crucial behavioural trait that permits young marsupials and perhaps other juvenile altricial mammals to overcome the developmental stage between poikilothermy early in development and full endothermy later in life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most mammals and birds are altricial at birth with little or no insulation, little coordination, and a low metabolic rate (MR), and are, therefore, unable to maintain a high and constant body temperature (T b) independently (Hill 1976). Endothermic thermoregulation develops gradually with growth, increased insulation, and neural control (Morrison and Petajan 1962; Hill 1976). Marsupials are extreme examples of mammalian altricial development; they are born at <1% of the maternal body mass (Tyndale-Biscoe and Renfree 1987), have an extremely low MR (Hulbert 1988) and essentially non-functional lungs, but they can satisfy the required oxygen uptake via their skin (Mortola et al. 1999). As the rate of growth in marsupials is approximately half that of placental mammals, which show similar, but brief developmental stages, marsupials permit a detailed sequential examination of the morphological and physiological changes during development (Janssens et al. 1997).
For a large part of marsupial development, the low MR is of little thermoregulatory consequence, because the T b of the developing young, in most species, is buffered by the mother’s pouch, which has a temperature similar to that of the mother’s core T b (Bartholomew 1956). However, near the time of pouch exit, the young must be able to regulate their T b and it has been shown that increased size, insulation, and increased heat production permit them to do so (Janssens et al. 1997). The problem that the young of small marsupials must overcome when they leave the pouch at approximately 10–40% of the mother’s size (Russell 1982; Tyndale-Biscoe and Renfree 1987) is their relatively large heat loss due to their large surface area to volume ratio and high thermal conductance (Geiser and Brigham 2012). In small adult mammals, such energetic challenges are commonly dealt with by entering torpor and temporarily reducing MR and T b to conserve energy (Ruf and Geiser 2015). This is also the case in some growing juveniles, which use torpor during development to reduce energetic costs and enhance growth on limited resources (Giroud et al. 2014). However, although torpor saves large quantities of energy, the enormous increase in heat production required during the arousal process at the end of a torpor bout (Lyman et al. 1982) is not only costly, but also physiologically challenging (Currie et al. 2015). While this increase in heat production at a low T b and the associated energetic costs may be possible for adults, it is not likely the case for all endothermic developmental stages of juveniles with a large relative surface area and a limited capacity to increase MR. One possible solution would be to use passive rewarming or basking permitting arousal with a relatively low MR (Geiser et al. 2004; Currie et al. 2015). Behavioural thermoregulation in the form of basking to reduce thermoregulatory costs in mammals has been well documented, however, basking during rewarming from torpor or for normothermic thermoregulation in mammals has only been observed in the adults of a few species (Schradin et al. 2007; Warnecke et al. 2008; Signer et al. 2011; Rojas et al. 2012; Thompson et al. 2015; Geiser et al. 2016). Although huddling and passive rewarming via raised nest temperatures in juvenile fully endothermic mammals have been observed (Sealander 1952; Arnold 1988; Schradin et al. 2006; Gilbert et al. 2007; Terrien et al. 2011), there are no data on whether and at what developmental stage juveniles use behavioural thermoregulation when offered radiant heat to rewarm from torpor and when normothermic.
The aim of our study was to provide detailed measurements on the developmental thermoregulatory stages in a marsupial. To achieve this, we measured developmental changes in body mass, thermoregulation, thermal energetics, basking behaviour, and insulation in the fat-tailed dunnart (Sminthopsis crassicaudata, adult body mass 12–18 g, and hereafter ‘dunnart’). This arid-zone marsupial weighs approximately 10 mg at birth, is weaned at ~60–65 days (d) of age, and the females are sexually mature at ~5 months (Godfrey and Crowcroft 1971). Dunnarts use torpor in both the laboratory, and the field and adults are known to bask during rewarming from torpor in the wild (Warnecke et al. 2008). However, the expression of torpor and the use of basking at different developmental stages have not previously been investigated in this or any other species. To some extent, this has been due to the problem of measuring T b continuously in very small animals, but with the development of tiny 0.1 g temperature-sensitive transponders, small body size has become less of a constraint (Wacker et al. 2012). We predicted that the slow developmental rate of marsupial dunnarts would allow us to determine intermediate developmental stages that have not been previously identified.
Materials and methods
Animal care
Four dunnart breeding pairs produced four litters (litter sizes of eight, six, six, and five young). Animals were housed in cages (55 × 38 × 22 cm) with wood shaving substrate and nesting boxes filled with shredded paper. Animals were fed a mixture of “Whiskas” cat biscuits (26.0% crude protein and 9.5% crude fat) soaked in water overnight and tins of “Whiskas” tinned cat food (7.0% crude protein and 5.5% crude fat) 7 days a week. Water and food were always available if not otherwise specified. Animals were kept under the natural Summer photoperiod (30.50°S, 151.65°E) from LD 14:10 to 13:11 and at an ambient temperature (T a) of 18.0 ± 2.0 °C.
Development of endothermy (experiment one)
Four pouch young from one litter (Table 1) at 40 d (age group one) were removed from the nest at the same time and placed individually in 1 L glass jars at T a 18.0 ± 0.3 °C. A fine, lubricated (Sylk natural personal lubricant, Geneva Marketing PTY LTD, Surry Hills, Australia) and pliable thermocouple was used for rectal temperature (T rec) measurements (calibrated to the nearest 0.1 °C between 10.0 and 40.0 °C in ~5.0 °C increments in a water bath with a precision thermometer traceable to a national standard). The thermocouple was inserted only 5 mm rectally, because the young animals are so small (crown-rump length ~20 mm, body mass 3–4 g at the beginning of measurements), and no damage was caused by this insertion. Rectal temperature measurements were taken within 10 s of the animal being removed from the mother/nest and then every 10 min for 60 min.
To quantify the changes in hair length, and as a proxy for insulation (Steudel et al. 1994; Wacker et al. 2016), four individual hairs taken from the mid-dorsal area of each animal were straightened using 100% ethanol. Prior to measurements, a small clump of hair (approximately 10–15 hairs) had been soaked in ethanol for 24 h to verify that ethanol did not damage the hair structure. Photographs of each hair were taken using a digital camera (IC80 HD Integrated Stand Alone Digital Camera for Stereo Microscopes, Leica Heerbrugg Switzerland) attached to the microscope (M80 Modular Routine Stereo Microscope with 8:1 Zoom, Leica Heerbrugg, Switzerland) and the length of the hair was measured. At the end of the measurements, the animals were weighed, four hairs removed for measurement of total hair length and morphometrics taken (pes length, head length, tail length, and crown-rump length). All measurements were repeated at 48 d (age group two) and 56 d (age group three).
For comparison, cooling rates were also determined for three deceased dunnarts (Petajan and Morrison 1962); one juvenile (45 d, 3.8 g), one small adult (273 d, 10.2 g), and one large adult (760 d, 14.1 g) were warmed to 37.0 °C (animals placed in water proof bags in a water bath) and a calibrated thermocouple inserted rectally. Rectal temperature measurements (T rec) were taken at T a 18.3 ± 0.7 °C every 10 min for 60 min after removal from the water bath and a cooling constant calculated (Petajan and Morrison 1962).
Thermoregulation, basking, and torpor (experiment two)
Eleven juvenile (~60 d, age group four) fat-tailed dunnarts were implanted with calibrated (as for thermocouples) transponders (IPTT-300, 0.13 g, 14 mm × 2 mm, Bio Medic Data Systems, Seaford, Delaware, USA) to measure subcutaneous temperature (T sub, Wacker et al. 2012).
Over approximately 21 h open-flow respirometry was used to measure MR as the rate of oxygen consumption. Outside air was dried (Silica gel) and pumped to the respirometry chamber (300 ml volume Perspex cylinder, 10 cm long, sealed with rubber stoppers containing an air inlet on one side and outlet on the other) and then through a mass flow meter (Omega FMA-5606, Stamford, CT, USA) at a rate of ~350 ml min−1. This resulted in 99% equilibrium times of <4 min. A sub-sample of 150 ml min−1 was analysed for O2 content (FX301-01R, Sable System International, Henderson, Nevada, USA). The oxygen analyser was calibrated prior to measurements against high-purity compressed Nitrogen (BOC GASES) and a calibration gas (O2 = 19.9 ± 0.03% in Nitrogen, BOC GASES). The flow meter was calibrated using a custom-made bubble meter. Oxygen consumption was calculated using Eq. 3a of Withers (1977) assuming an RQ of 0.85.
The respirometry chamber was large enough to allow for free movement by the animals and was placed within a temperature-controlled cabinet maintained at 15.0 ± 0.7 °C; the artificial photoperiod was similar to natural (LD 14:10, 30.50°S, 151.65°E). The T a within the respirometry chamber was measured with a calibrated thermocouple (HH-71T, Omega Stamford, CT, USA). A 2 × 2 cm piece of paper towel was secured to the base of the chamber to allow for the absorption of urine and faeces, but the animal was unable to burrow underneath. A cardboard roll (3 cm in length) was provided for refuge. Oxygen concentration was measured in sequence; respirometry chamber once/min for 12 min followed by a reference (outside air) measurement once/min for 3 min. The transponder signal was read with a DAS-7006/7R/S Handheld Reader (Bio Medic Data Systems, Seaford, Delaware, USA). The reader was modified and connected to a computer and read automatically via a program written in Microsoft Visual Basic V6. Transponder readings were taken at the same time as oxygen consumption readings.
Animals were measured once a week for 2 weeks (1 week without radiant heat access and the following week with access to radiant heat, and individuals were randomised) at each age group (Table 1); when the animals were 60–70 d (age group four), 90–100 d (age group five), 120–130 d (age group six), and 150–160 d (age group seven). The heat lamp (SunGlo basking spot lamp, 50 W tight beam Colour Rendering Index of 98, 6700 K colour temperature, ExoTerra, Montreal, Canada) was placed at a 45° angle and 20 cm away from the ceiling of the chamber tube. By giving the animals the cardboard tube refuge, they were able to choose whether or not to bask. It is known from the previous work that torpid dunnarts at a T b of ~20 °C can move at ~0.3 m s−1 (Rojas et al. 2012). Animals were placed in the respirometry chamber between 1530 and 1630 (AEST) overnight, without food and water to induce torpor. Animals were weighed immediately before being placed into and immediately after being removed from the chamber and a linear mass loss over the measurement period was assumed. The chamber was maintained at T a of 15.0 ± 0.7 °C even with the basking lamp on and a natural photoperiod was used. If the animal was torpid the following morning, and once its T sub was between 19.5 and 20.0 °C, the heat lamp was switched on to encourage basking and aid rewarming. A camera (Swann Secura View, Security Monitoring Kit, Melbourne Australia) in the chamber was used to observe whether the animal was resting, active, basking, or not basking.
Data analysis
Development of thermoregulation (experiment one)
Numeric values are presented as means ± SD for ‘n’ the number of individuals. Linear mixed effects models were used to determine the effect of age on, body mass, and length of the hair on cooling rate in 40, 48, and 56 d dunnarts. Conventional least-square linear regression analyses were also used for comparison. Cooling rates are the maximum rate of cooling over 10 min and were calculated over the first 10 min, the animals were removed from their mother (since this is when cooling rate was steepest and changed the most with age). The cooling constants over the entire cooling process were calculated from the slope of log10 (T rec − T a) over time (Petajan and Morrison 1962).
Thermoregulation, basking, and torpor (experiment two)
Numeric values are presented as means ± SD for ‘n’ the number of individuals. Torpor was defined as a T sub below the threshold of 30.0 °C (Barclay et al. 2001) and torpor bout duration (TBD) was defined as the time, the animal’s T sub was below 30.0 °C. The oxygen consumption during resting periods of normothermic individuals was calculated using the average of three 5-min intervals of the lowest, stable, non-torpid values. Cooling rates were calculated from the maximum rate of cooling over 10 min and rewarming rates from the maximum rate of rewarming over 10 min. Normothermic and basking apparent thermal conductance (C) was calculated by dividing total RMR by the T sub − T a differential and converted to J h−1 °C−1 using a conversion factor of 20.1 (Schmidt-Nielsen 1997). Mass-specific conductance (J g−1 h−1 °C−1) was also calculated for comparison with other studies. Linear mixed effects models were used to determine the effect of body mass on basking and non-basking RMR, TBD, rate of cooling during torpor entry and rate of active rewarming, maximum rewarm rate, total and mass-specific thermal conductance, and head length. The conventional least-square linear regression analyses were also used for comparison. Repeated-measures ANOVAs were used to determine differences between means. Regressions for maximum rewarm rate as a function of body mass and RMR as a function of body mass in basking and non-basking animals were compared using ANCOVAs. All statistical analyses were performed with R Studio version 0.99.489.
Results
Development of endothermy (experiment one)
At 40 d, dunnarts (n = 4, body mass 3.3 ± 0.1 g) that had been removed from their nest, cooled rapidly, and almost to T a (T a = 18.0 ± 0.3 °C) over 30 min (Fig. 1a). Within the first 10 min, the animals had reduced T rec by 6.5 ± 1.1 °C; by 40 min, T rec was only 0.6 ± 0.2 °C above T a; and at 60 min, T rec was 18.6 ± 0.2 °C. Animals at 48 d (n = 4, body mass 4.8 ± 0.1 g, T a = 18.0 ± 0.1 °C) cooled soon after being removed from their nest, but only by 2.1 ± 0.4 °C, during the 60-min period. Between 20 and 30 min, all animals rewarmed slightly (by 0.5 ± 0.5 °C) despite T a remaining constant, and then cooled again. At 56 d (n = 4, body mass 6.7 ± 0.3 g, T a 18.0 ± 0.2 °C), T rec was almost constant (starting T rec of 33.1 ± 0.6 °C, ending T rec of 33.4 ± 0.2 °C). The maximum rate of cooling over 10 min decreased with age; mean cooling rate at 40 d was 0.65 ± 0.11 °C min−1, 0.14 ± 0.05 °C min−1 at 48 d, and 0.03 ± 0.02 °C min−1 at 56 d. Cooling constants (in log10 °C min−1) also decreased with age (0.0188 ± 0.0012 at 40 d, 0.0012 ± 0.0003 at 48 d, and 0.0001 ± 0.0001 °C at 56 d). Cooling constants were larger in living dunnarts than in deceased animals, as expected (Petajan and Morrison 1962).
Development of endothermy in the fat-tailed dunnart. The decrease in T rec (a) over 60 min at a T a (dotted line) of 18.0 ± 0.3 °C for four individuals at 40 (circles), 48 (triangles), and 56 (diamonds) days of age. Correlation between body mass and the rate of cooling during the first 10 min after being removed from the nest (b, r 2 = 0.85, p < 0.001, log10 y = −0.86x + 1.60) and the length of the animal’s hair and the rate of cooling (c, r 2 = 0.81, p < 0.001, log10 y = −0.84x + 1.54). T a = 18.0 ± 0.3 °C
The maximum rate of cooling over 10 min was correlated with both body mass (Fig. 1b, r 2 = 0.85, p < 0.001, df = 7, t = 8.67, log10 y = −0.86x + 1.60), and the total length of the individual animal’s hair (Fig. 1c, r 2 = 0.81, p < 0.001, df = 7, t = 9.67, log10 y = −0.84x + 1.54). Hair length increased with age: 3.1 ± 0.1 mm at 40 d, 4.8 ± 0.6 mm at 48 d, and 6.2 ± 0.8 mm at 56 d, and was strongly correlated with body mass (r 2 = 0.89, p < 0.001, df = 7, t = 16.58, y = 0.85x + 0.52).
Thermoregulation, basking, and torpor (experiment two)
The resting metabolic rate (RMR) at T a 15.0 ± 0.7 °C increased linearly with body mass in non-basking (r 2 = 0.29, p = 0.048, y = 2.02 x + 42.41) but decreased linearly in basking (r 2 = 0.21, p = 0.047, y = −2.35x + 69.82) dunnarts (Fig. 2). The RMR when animals were basking was on average 54% that of non-basking individuals and the two regressions differed significantly (ANCOVA F = 115.10; p < 0.001).
Total thermal conductance (C) in normothermic non-basking dunnarts increased with age; 53.91 ± 2.89 J h−1 °C−1 (mass-specific 5.72 ± 0.01 J g−1 h−1 °C−1) in age group four, 63.16 ± 6.42 J h−1 °C−1 (mass-specific 5.23 ± 0.31 J g−1 h−1 °C−1) in age group five, 73.47 ± 3.97 J h−1 °C−1 (mass-specific 5.36 ± 0.24 J g−1 h−1 °C−1) in age group six, and 77.2 ± 6.40 J h−1 °C−1 (mass-specific 5.09 ± 0.32 J g−1 h−1 °C−1) in age group seven. Both total C (r 2 = 0.87, p < 0.001, df = 11, t = 7.74, y = 4.22x + 13.70) and mass-specific C (r 2 = 0.34, p < 0.001, df = 11, t = 13.49, y = −0.09x + 6.49) were correlated with body mass.
In basking dunnarts, total thermal C (r 2 = 0.72, p < 0.001, df = 11, t = 6.33, y = −0.42x + 8.50) and mass-specific thermal C (r 2 = 0.37, p < 0.001, df = 11, t = 47.96, y = −2.59x + 71.65) were also correlated with body mass, and both were substantially lower than in non-basking animals (both total C and mass-specific C, p < 0.001). Basking dunnarts had a mean total C of 46.29 ± 6.18 J h−1 °C−1 (mass-specific 4.11 ± 0.28 J g−1 h−1 °C−1) in age group 4, 43.19 ± 2.27 J h−1 °C−1 (mass-specific 3.60 ± 0.47 J g−1 h−1 °C−1) in age group five, 34.79 ± 10.00 J h−1 °C−1 (mass-specific 2.56 ± 0.78 J g−1 h−1 °C−1) in age group six, and 31.55 ± 9.94 J h−1 °C−1 (mass-specific 2.08 ± 0.79 J g−1 h−1 °C−1) in age group seven.
Torpor use and expression changed substantially with age. At 60–70 d (<9 g), all animals (large enough to implant with transponders, n = 8) were normothermic for ~7 h and then showed an apparent torpor entry (at ~0300 h in the example in Fig. 3a), with an initial rapid reduction of MR and a little change in T sub, followed by a slow reduction in T sub (Fig. 3b). These individuals were unable to rewarm despite attempts at increasing MR and T sub (two such attempts after 0940 in Fig. 3a) and were, therefore, deemed to be hypothermic (Geiser et al. 2014). All of the eight animals in this age group showed this pattern. However, when they were given access to radiant heat, all animals moved under the heat lamp and were able to rewarm from a low T sub (example in Fig. 3c). When the heat lamp was switched on at 0800, MR gradually increased to values well above the phase of torpor/hypothermia as T sub was raised partially passively from ~15 to 19 °C. Only after T sub exceeded 20 °C at 0900 did MR further increase, peaking at 0930, before returning to resting MR. T sub increased immediately in all animals when the heat lamp was switched on, but MR increased immediately in only six animals (Fig. 3d), whereas in two, it remained rather low until T sub reached ~25 °C.
Development of torpor in juvenile fat-tailed dunnarts with oxygen consumption and T sub traces on the left-hand side and their corresponding plots of oxygen consumption as a function of T sub on the right-hand side for a, b a dunnart in age group four (8.6 g) and unable to rewarm, c, d a dunnart in age group four (8.9 g), able to rewarm with the aid of a basking lamp, e, f a dunnart in age group five (9.8 g), a long, deep torpor bout with endogenous rewarming, g, h a dunnart in age group seven (14.6 g) and no torpor entry. Dark bars represent scotophase. Cross hatched bar in Fig. 3b represents the time, the basking lamp was on. Cooling data are in blue, rewarming in red. (Color figure online)
At 90–100 d (body mass 11.9 g, example in Fig. 3e), 9 of the 11 juvenile dunnarts expressed a long, deep torpor bout. At this age, 5 of the 11 animals were able to rewarm actively and spontaneously, whereas the other six were unable to rewarm at T a 15 °C (but could rewarm at higher Tas when removed from the respirometer). All animals capable of active rewarming at low T a showed a similar pattern to that in Fig. 3f, with T sub decreasing gradually with MR during cooling, with a much higher MR during the rewarming phase. At 120–130 d (body mass >13.0 g, Fig. 3g, h), seven of the animals no longer entered torpor regularly and four individuals expressed shallow and brief torpor bouts.
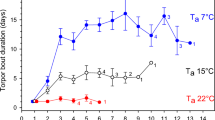
During torpor entry, smaller animals cooled more quickly than larger animals and the rate of cooling was inversely correlated with body mass (Fig. 4a, r 2 = 0.49, p < 0.001, df = 7, y = −0.01x + 0.24). Torpor bout duration of animals capable of actively rewarming was inversely correlated with body mass (Fig. 4b, n = 11, Fig. 4 r 2 = 0.57, p < 0.001, df = 7, y = −41.25x + 853.08). On average, at 90–100 d dunnarts had very long torpor bouts (6.6 ± 0.9 h), which decreased to 5.0 ± 1 h at 120–130 d and lasted only 4.3 ± 1.0 h at >150 d with 64% of animals in the last age group not using torpor at all. Both the maximum rewarming rate over 10 min during basking and the maximum active (non-basking) rewarming rate increased steeply (Fig. 4c, basking: r 2 = 0.87, p < 0.001, y = 0.06x − 0.41, non-basking: r 2 = 0.81, p < 0.001, y = 0.07x − 0.58) with body mass and the two regressions differed significantly (ANCOVA F = 21.14, p < 0.001). The animals’ body mass did not change significantly between runs with and without a basking lamp (p < 0.157).
Maximum rate of cooling during torpor entry over 10 min (a, r 2 = 0.49, p < 0.001, y = −0.01x + 0.24), torpor bout duration (b, r 2 = 0.57, p < 0.001, y = −41.25x + 853.08), and the maximum rewarming rate over 10 min during radiant heat-assisted (c, open circles, r 2 = 0.87, p < 0.001, y = 0.06x − 0.41) and active rewarming (c, filled circles, r 2 = 0.81, p < 0.001, y = 0.07x − 0.58) as a function of body mass in dunnarts. Data for animals in age group four were excluded; as at this age, the animals were unable to rewarm without the aid of a basking lamp
When offered a heat lamp during bouts of torpor, 100% of dunnarts basked by moving out from under the cardboard tube refuge and into the direct path of the radiant heat, but juveniles and adults differed in their basking behaviour. All young animals at 60–70 d basked with their heads directed towards the heat lamp. At 90–100 d, nine of 11 animals also basked in this manner. All the older animals (>120 d) basked by turning their backs to the heat lamp. Head length in the youngest animals (60–70, 90–100 d), which all basked by exposing their heads to the radiant heat, was positively correlated with the rate of passive rewarming (r 2 = 0.71, p < 0.001, df = 7, t = 178681.9, y = 0.71x − 15.74). No correlation between head length and passive rewarming rate was observed in older animals (>120 d), likely because these always basked with their back to the heat lamp rather than with their head.
Discussion
Our data show that the development of marsupial endothermy involves three major steps. Initially, when individual heat loss was high and heat production was low, animals were poikilothermic when removed from the nest and incapable of physiological thermoregulation, and so were metabolically similar, but not identical, to ectotherms (Hill 1976; Geiser et al. 2006). Second, when heat production increased with growth and when insulation was improved, the animals were able to maintain a constant high T b for several hours during cold exposure, but eventually entered an apparent torpid stage. However, since they were unable to rewarm, most likely because of the low T b limiting heat production, by definition, these animals were hypothermic (Hill 1976; Geiser et al. 2006). Importantly, during this stage, when animals were offered the opportunity to bask, they were able to rewarm despite their low capacity for increasing MR, similar to behavioural thermoregulation in reptiles. Third, when animals were subadults and adults, physiological thermoregulation was fully developed, torpor including endogenous arousal was expressed and normothermy could be maintained even under prolonged cold exposure.
The poikilothermic stage early in the thermal development of dunnarts has been observed in many endothermic species (Dawson and Evans 1960; Morrison and Petajan 1962; Westman et al. 2002; Geiser et al. 2006; Gilbert et al. 2010). These individuals are unable to maintain a high T b during cold exposure as MR cannot fully compensate for heat loss due to a high relative surface area, limited insulation, and large total thermal conductance, and leading to a reduction in T b. However, very young small marsupials (up to ~40 d) typically rely on pouch or nest temperature to maintain T b, and without this shared heat, they become hypothermic (Geiser et al. 2006).
In contrast, at 60 d, dunnarts were partially endothermic and could maintain T sub for some time independently of the T a and could also enter an apparent state of torpor. Dunnarts at this age cooled more quickly than dead individuals due to high thermal conductance (Bradley and Deavers 1980) and were unable to produce enough heat to rewarm from torpor at a T a of 15 °C, and, therefore, by definition were hypothermic. Importantly, the decline of T b and MR showed the pattern expressed during entry into daily torpor and hibernation (i.e., a physiologically controlled process characterised by an initial rapid reduction in MR followed by a reduction in T sub that further reduced MR), which differs from the pattern during entry into cold-induced hypothermia where animals try to maintain a high T b via a high MR, but fail to do so, because heat loss exceeds heat production (Geiser et al. 2014). The rapid decline of MR and T b during the entry phase strongly suggests that it was not caused by energy depletion, which is characterised by a slow reduction in MR and T b, but instead was an ‘intended’ and regulated torpor entry. While these dunnarts were unable to rewarm endogenously, they could move and position their body under a basking lamp and use the external heat source to rewarm. This suggests that juveniles in the wild, even before being fully endothermic, may also experience a reduction of T b in the second part of the night or early morning and then bask to rewarm.
Although field studies in the Australian arid zone have been conducted for decades, basking during rewarming, which usually occurs in the late morning, has only recently been observed in adults in the wild (Geiser et al. 2002; Warnecke et al. 2008). Since juveniles are small and hard to see and perhaps more cautious than adults, it is possible that juveniles that are not yet fully endothermic bask as they always do (when normothermic or torpid) when either given the opportunity to bask in captivity. It is this transitional stage in the development of thermoregulation that has not been observed or recognised before: torpor entry without the capacity of endogenous rewarming but with the ability to use passive rewarming to raise T b to normothermic values. Our data further support the hypothesis that the ability to enter torpor develops before mammals are fully endothermic (Geiser et al. 2002; Geiser 2008). However, the development of the intermediate heterothermic stage occurs at such a young age that animals are unable to rewarm without the aid of passive rewarming, for example via basking in the sun.
Basking is not restricted to juveniles; no matter what age, sex, or body mass, when a radiant heat source was available all animals basked when rewarming from torpor. Interestingly, basking dunnarts leave the refuge head-first to warm their anterior before the posterior but only expose half of the body. All juvenile fat-tailed dunnarts in our study basked leading with their head, likely because, at this age, the head is proportionally very large and this increase in surface area allows them to rewarm more quickly. In the youngest of these dunnarts, basking is not an optional method for reducing energy thermoregulatory expenditure as in adults (Bartholomew and Rainy 1971; Warnecke et al. 2010; Wacker et al. 2016), but rather is an essential part of being able to rewarm from an otherwise hypothermic condition. Non-torpid animals also chose to bask when a heat lamp was present, but, in these cases, radiant heat mainly reduced the energetic costs of thermoregulation. Our observations of juvenile fat-tailed dunnarts and data from studies on related species show that torpid animals both in the wild and the laboratory with a T b of ~15 °C are able to move from their nest and actively seek a radiant heat source and, at the same time, are also able to respond to and avoid approaching predators (Geiser et al. 2002; Warnecke et al. 2008; Rojas et al. 2012).
Our data indicate that heterothermy is an intermediate stage between poikilothermy and endothermy in the development of thermoregulation in marsupials and perhaps other altricial mammals, and suggest that basking is a crucial step in the development of heterothermy in this species. This heterothermic step requires that animals have access to an external heat source, not as an option to reduce the energetic costs of rewarming, but as an essential requirement for the ability to rewarm from torpor. Basking behaviour in such young marsupials has not been observed before and our data suggest that juvenile dunnarts at the time of weaning, when heat loss is high, may need a radiant heat source to rewarm, so they can reach or maintain a high T b independently of nest temperature.
References
Arnold W (1988) Social thermoregulation during hibernation in alpine marmots (Marmota marmota). J Comp Physiol B 158:151–156. doi:10.1007/BF01075828
Barclay RMR, Lausen CL, Hollis L (2001) What’s hot and what’s not: defining torpor in free-ranging birds and mammals. Can J Zool 79:1885–1890. doi:10.1139/z01-138
Bartholomew GA (1956) Temperature regulation in the macropod marsupial, Setonix brachyurus. Physiol Zool 29:26–40
Bartholomew GA, Rainy M (1971) Regulation of body temperature in the rock hyrax, Heterohyrax brucei. J Mammal 52:81–95. doi:10.2307/1378434
Bradley SR, Deavers DR (1980) A re-examination of the relationship between thermal conductance and body weight in mammals. Comp Biochem Physiol A 65A:465–476. doi:10.1016/0300-9629(80)90060-2
Currie SE, Noy K, Geiser F (2015) Passive rewarming from torpor in hibernating bats: minimizing metabolic costs and cardiac demands. Am J Physiol 308:R34–R41. doi:10.1152/ajpregu.00341.2014
Dawson WR, Evans FC (1960) Relation of growth and development to temperature regulation in nestling vesper sparrows. Condor 62:329–340. doi:10.2307/1365163
Geiser F (2008) Ontogeny and phylogeny of endothermy and torpor in mammals and birds. Comp Biochem Physiol A 150:176–180. doi:10.1016/j.cbpa.2007.02.041
Geiser F, Brigham RM (2012) The other functions of torpor. In: Ruf T, Bieber C, Arnold W, Millesi E (eds) Living in a seasonal world. Springer, Berlin, Heidelberg, New York, pp 109–121. doi:10.1007/978-3-642-28678-0_10
Geiser F, Goodship N, Pavey CR (2002) Was basking important in the evolution of mammalian endothermy? Naturwissenschaften 89:412–414. doi:10.1007/s00114-002-0349-4
Geiser F, Drury RL, Körtner G, Turbill C, Pavey C, Brigham RM (2004) Passive rewarming from torpor in mammals and birds: energetic, ecological and evolutionary implications. In: Barnes BM, Carey HV (eds) Life in the cold: 12th international hibernation symposium. Institute of Arctic Biology, University of Alaska, Fairbanks, pp 51–62
Geiser F, Westman W, McAllan BM, Brigham RM (2006) Development of thermoregulation and torpor in a marsupial: energetic and evolutionary implications. J Comp Physiol B 176:107–116. doi:10.1007/s00360-005-0026-y
Geiser F, Currie SE, O’Shea KA, Hiebert SM (2014) Torpor and hypothermia: reversed hysteresis of metabolic rate and body temperature. Am J Physiol 307:R1324–R1329. doi:10.1152/ajpregu.00214.2014
Geiser F, Gasch K, Bieber C, Stalder GL, Gerritsmann H, Ruf T (2016) Basking hamsters reduce resting metabolism, body temperature and energy costs during rewarming from torpor. J Exp Biol 219:2166–2172. doi:10.1242/jeb.137828
Gilbert C, Blanc S, Trabalon SG, Maho YL, Perret M, Ancel A (2007) Role of huddling on the energetic of growth in a newborn altricial mammal. Am J Physiol Regul Integr Comp Physiol 293:R867–R976. doi:10.1152/ajpregu.00081.2007
Gilbert C, McCafferty D, Maho YL, Martrette J, Giroud S, Blanc S, Ancel A (2010) One for all and all for one: the energetic benefits of huddling in endotherms. Biol Rev Camb Philos Soc 85:545–569. doi:10.1111/j.1469-185X.2009.00115.x
Giroud S, Zahn S, Criscuolo F, Chery I, Blanc S, Turbill C, Ruf T (2014) Late-born intermittently fasted juvenile garden dormice use torpor to grow and fatten prior to hibernation: consequences for ageing processes. Proc Roy Soc Lond B Biol 281:1–9. doi:10.1098/rspb.2014.1131
Godfrey GK, Crowcroft P (1971) Breeding the fat-tailed marsupial mouse Sminthopsis crassicaudata. Int Zoo Yearb 11:33–38. doi:10.1111/j.1748-1090.1971.tb01839.x
Hill RW (1976) The ontogeny of homeothermy in neonatal Peromyscus leucopus. Physiol Zool 49:292–306
Hulbert AJ (1988) Metabolism and the development of endothermy. In: Tyndale-Biscoe CH, Janssens PA (eds) The developing marsupial: models for biomedical research. Springer, Berlin, pp 148–161. doi:10.1007/978-3-642-88402-3_11
Janssens PA, Hulbert AJ, Baudinette RV (1997) Development of pouch young from birth to pouch vacation. In: Saunders N, Hinds L (eds) Marsupial biology: recent research, new perspectives. University of New South Wales Press LTD, Sydney, pp 71–89
Lyman CP, Willis JS, Malan A, Wang LCH (1982) Hibernation and torpor in mammals and birds. Academic, New York
Morrison P, Petajan JH (1962) The development of temperature regulation in the opossum, Didelphis marsupialis virginiana. Physiol Zool 35:52–65
Mortola JP, Frappell PB, Woolley PA (1999) Breathing through skin in a newborn mammal. Nature 397:660–661. doi:10.1038/17713
Petajan JH, Morrison P (1962) Physical and physiological factors modifying the development of temperature regulation in the opossum. J Exp Biol 149:45–57. doi:10.1002/jez.1401490106
Rojas AD, Körtner G, Geiser F (2012) Cool running: locomotor performance at low body temperature in mammals. Biol Lett 8:868–870. doi:10.1098/rsbl.2012.0269
Ruf T, Geiser F (2015) Daily torpor and hibernaton in birds and mammals. Biol Rev 90:891–926. doi:10.1111/brv.12137
Russell EM (1982) Patterns of parental care and parental investment in marsupials. Biol Rev 57:423–486. doi:10.1111/j.1469-185X.1982.tb00704.x
Schmidt-Nielsen K (1997) Animal physiology: adaptation and environment. 5th edition. Cambridge University Press, Cambridge
Schradin C, Schubert M, Pillay N (2006) Winter huddling groups in the striped mouse. Can J Zool 84:693–698. doi:10.1139/z06-048
Schradin C, Krackow S, Schubert M, Keller C, Schradin B, Pillay N (2007) Regulation of activity in desert-living striped mice: the importance of basking. Ethology 113:606–614. doi:10.1111/j.1439-0310.2007.01361.x
Sealander JA (1952) The relationship of nest protection and huddling to survival of Peromyscus at low temperature. Ecology 33:63–71
Signer C, Ruf T, Arnold W (2011) Hypometabolism and basking: the strategies of Alpine ibex to endure harsh over-wintering conditions. Funct Ecol 25:537–547. doi:10.1111/j.1365-2435.2010.01806.x
Steudel K, Porter WP, Sher D (1994) The biophysics of Bergmann’s rule: a comparison of the effects of pelage and body size variation on metabolic rate. Can J Zool 72:70–77. doi:10.1139/z94-010
Terrien J, Perret M, Aujard F (2011) Behavioral thermoregulation in mammals: a review. Front Biosci 16:1428–1444. doi:10.2741/3797
Thompson ML, Mzilikazi N, Bennett NCM, McKechnie AE (2015) Solar radiation during rewarming from torpor in Elephant Shrews: supplementation or substitution of endogenous heat production? PLoS One. doi:10.1371/journal.pone.0120442
Tyndale-Biscoe CH, Renfree M (1987) Reproductive physiology of marsupials. Cambridge University Press, Cambridge
Wacker CB, Rojas AD, Geiser F (2012) The use of small subcutaneous transponders for quantifying thermal biology and torpor in small mammals. J Therm Biol 37:250–254. doi:10.1016/j.jtherbio.2011.11.007
Wacker CB, McAllan BM, Körtner G, Geiser F (2016) The functional requirements of mammalian hair: a compromise between crypsis and thermoregulation? The Science of Nature 103:1–9. doi:10.1007/s00114-016-1376-x
Warnecke L, Turner J, Geiser F (2008) Torpor and basking in a small arid zone marsupial. Naturwissenschaften 95:73–78. doi:10.1007/s00114-007-0293-4
Warnecke L, Schleucher E, Geiser F (2010) Basking behaviour in relation to energy use and food availability in one of the smallest marsupials. Physiol Behav 101:389–393. doi:10.1016/j.physbeh.2010.07.003
Westman W, Körtner G, Geiser F (2002) Developmental thermoenergetics of the dasyurid marsupial, Antechinus stuartii. J Mammal 83:81–90. doi:10.1644/1545-1542(2002)083<0081:DTOTDM>2.0.CO;2
Withers PC (1977) Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J Appl Physiol 42:120–123
Acknowledgements
This project was funded by an Australian Postgraduate Award to CW and an Australian Research Council Grant (DP130101506) to FG.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Communicated by I. D. Hume.
Rights and permissions
About this article
Cite this article
Wacker, C.B., McAllan, B.M., Körtner, G. et al. The role of basking in the development of endothermy and torpor in a marsupial. J Comp Physiol B 187, 1029–1038 (2017). https://doi.org/10.1007/s00360-017-1060-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1060-2