Abstract
Ants are excellent navigators, using a combination of innate strategies and learnt information to guide habitual routes. The mechanisms underlying this behaviour are little understood though one avenue of investigation is to explore how innate sensori-motor routines are used to accomplish route navigation. For instance, Australian desert ant foragers are occasionally observed to cease translation and rotate on the spot. Here, we investigate this behaviour using high-speed videography and computational analysis. We find that scanning behaviour is saccadic with pauses separated by fast rotations. Further, we have identified four situations where scanning is typically displayed: (1) by naïve ants on their first departure from the nest; (2) by experienced ants departing from the nest for their first foraging trip of the day; (3) by experienced ants when the familiar visual surround was experimentally modified, in which case frequency and duration of scans were proportional to the degree of modification; (4) when the information from visual cues is at odds with the direction indicated by the ant’s path integration system. Taken together, we see a general relationship between scanning behaviours and periods of uncertainty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The characteristic robust navigational performance of the solitary desert ant forager is usually attributed to the co-ordinated action of multiple navigational systems (Collett et al. 2006; Cheng et al. 2009; Wehner 2009). Mechanisms requiring little learning such as path integration (PI) (Collett and Collett 2000; Wehner 2003) and systematic search (Wehner and Srinivasan 1981; Schultheiss and Cheng 2011) are complemented by strategies which rely on the learning and utilisation of cues from the environment. For many ants the primary sensory modality for such learnt navigation is vision (Collett et al. 2006; Cheng et al. 2009; Wehner 2009; Zeil 2012) which is evident when ants are shown to use information provided by the visual panorama to guide searches for their nest (Wehner and Räber 1979; Wehner et al. 1996; Narendra et al. 2007) or a food source (Durier et al. 2003) and guide habitual routes between the two (Collett et al. 1992; Kohler and Wehner 2005; Collett 2010; Wystrach et al. 2011b). Two outstanding questions regarding the implementation of visual navigation concern the fine details of the sensori-motor routines by which visual memories control direction and the way this process interacts with other navigational modalities.
The interaction of navigational modalities
The traditional view of how PI and information from terrestrial visual cues interact during navigation was that information provided by visual cues subsumes that provided by PI when ants are navigating in familiar, visually informative environments. If an experienced forager is allowed to take her habitual route home from a feeder and then captured close to the nest, she is described as a zero-vector (ZV) ant because her PI system no longer gives her a strong directional sense of which way is home. If released at the familiar feeder, this ZV ant will recapitulate her habitual route to the nest even though the direction indicated by the visual scene is in conflict with that indicated by PI, which because of the experimental displacement indicates that ‘home’ is towards the feeder (Wehner et al. 1996; Kohler and Wehner 2005; Mangan and Webb 2012). In an extreme version of this demonstration (Andel and Wehner 2004), ants were repeatedly displaced (up to 5 times) back to the feeder, each time recapitulating their habitual route. When these ants were subsequently released on a novel field, with no familiar visual cues to guide them, they were directed by PI and headed in the nest–feeder direction (i.e., away from home) for 5× the normal nest–feeder distance, showing that the PI system had remained active throughout the visually guided routes.
The problem with demonstrations of this type is that the two-directional signals (from terrestrial visual cues and PI) are often diametrically opposed [though see (Fukushi 2001; Narendra 2007)], meaning that the circular average will most of the time be identical to one or other of the inputs. Collett (2012) has shown that when PI and route memories are in a more subtle conflict, as might be expected in ants’ natural life, both systems contribute to the direction taken by ants (Collett 2012). Thus, the interaction between the outputs of different navigational modalities is likely more subtle than a simple hierarchy. For instance if ants encode uncertainty estimates along with directions, this would allow for Bayesian combination of information (Cheng et al. 2007). It is an open and intriguing question to ask whether small-brained navigators are capable of this, but we still lack the requisite detailed descriptions of how cue conflicts influence behaviour.
The fine details of visual navigation
The use of learnt visual scenes for navigation in insects (visual navigation in short) is usually characterised as a retinotopic matching process where remembered scenes are compared with the currently experienced visual scene to set a direction (Ants: Wehner and Räber 1979; Bees: Cartwright and Collett 1983; Hoverflies: Collett and Land 1975; Waterstriders: Junger 1991; Review: Collett et al. 2006). This sort of visual navigation is likely to utilise visual cues from across panoramic scenes (Collett et al. 2007; Graham and Cheng 2009a; Wystrach et al. 2011a; Lent et al. 2013; Narendra et al. 2013) with experiments suggesting that a significant part of the informational content of a panoramic scene lies in the skyline (Fourcassie 1991; Wehner et al. 1996; Fukushi 2001; Graham and Cheng 2009a; Reid et al. 2011). How these visual scenes are encoded by the ant’s visual system is still an open question (see Lent et al. 2013).
For insect visual navigation in general, certain case studies have given insight into the fine-grained sensori-motor processes involved. Wasps approaching a small feeder whose position has been learnt relative to a small cylinder will maintain a globally referenced orientation in the final stages (Zeil 1993; Collett 1995). This orientation matches the predominant orientations adopted by the wasp during the learning flights performed after previous feeder visits. A fixed orientation reduces the degrees of freedom in the wasp’s movements and simplifies the problem of view-based homing. Waterstriders accomplish a different task: staying still on flowing water as they wait for food to pass by (rather than moving through a static world). Intermittent pulses keep the insect in a relatively fixed location of the stream based on visual cues, by increasing pulse frequency when the retinal elevation of visual features decreases on the retina and vice versa (Junger 1991).
For ants, the problem is more complex; they can translate only in the direction of their body axis and, in the main, movement direction also defines their viewing direction. Experimentally, it has been difficult to discern the fine details of visual guidance mechanisms. One approach has been to observe the visually driven searches of ants (Wehner and Räber 1979; Nicholson et al. 1999; Durier et al. 2003; Graham et al. 2004; Narendra et al. 2008; Schultheiss et al. 2013), though a more productive method has been to study habitual routes where it is easy to record a high volume of data (Judd and Collett 1998; Graham and Collett 2002; Harris et al. 2007; Lent et al. 2009, 2010; Collett 2010) and the ‘correct’ behaviour at any point on a route is more apparent. Therefore, one can discern the rules which are used to control heading for a particular route location. Furthermore, a deeper understanding of visual navigation mechanisms has been gained by the application of high-resolution video technology. For example, Lent et al. (2010) described a fast saccadic behaviour by which wood ants correct errors in their visually defined headings, a behaviour that was previously overlooked because of the speed of the ants’ movements.
Scanning in Melophorus bagoti
In a similar spirit to the route experiments outlined above, we have undertaken a fine-grained analysis of a newly observed behaviour which seems to be part of the Australian desert ant’s navigational repertoire during route navigation. In previous experiments with this ant (e.g. Graham and Cheng 2009b; Wystrach et al. 2011b), we observed ants appearing to stop and scan the world by turning on the spot, before heading off in their chosen direction. To us, it appeared that scanning occurred more often in situations where the visual surround was unfamiliar to the ants, suggesting that scanning may be involved somehow in visual navigation. We present the results of experiments aimed at ascertaining under what conditions scanning occurs, thus we have encouraged ants to perform this scanning behaviour in the field of view of a high-speed camera.
Materials and methods
Species and study site
All experiments were performed with the Australian desert ant M. bagoti at a field station near Alice Springs, NT, Australia.
Experiment 1: scanning in response to visual scene changes
An active nest was selected and using a barrier, we constrained the area over which foragers could travel to an 8-m-long 2.5-m-wide corridor leading to a permanent feeder. The barrier was set in a groove so that it did not hinder the ants’ views of the surroundings (for detail see Wystrach et al. 2012). The feeder was dug into the ground such that ants could drop in from any direction. However, to exit the feeder, ants had to travel along a 1-m channel (Fig. 1). The exit of the channel was within the field of view of a high-speed camera. Five 1-m high poles were planted along a half circle 1.5 m away from the exit of the channel. Using black sheeting, we could erect a shield which obscured 90° of azimuth of the natural panorama as viewed from the exit of the channel (Fig. 3). There were two training conditions. In the first, the panorama was left natural as ants shuttled back and forth between feeder and their nest (No Shield training condition). In a second condition, during training the shield was permanently erected to the left of the direct line from channel exit to nest entrance (Left Shield training condition). Additionally in this condition, a 1-m high tarpaulin was erected parallel and along the left side of the route from the channel to the nest entrance. These two barriers together ensured that ants trained in this condition did not experience a clear view of the left-front portion of the panorama during training.
Feeder, channel and camera. a Side view and b top view. Ants’ collected food from a trap feeder which could only be exited along a channel which was 10 × 10 × 100 cm. The end of the channel was covered so that the visual scene changed markedly upon exit and the ants’ view of the world was not altered when the camera was in place. Ants’ exits from the channel were recorded with a high-speed camera with a field of view of approximately 30 × 20 cm. A removable gate in the channel allowed for control of the flow of ants
During training phases, the feeder was baited with biscuit crumbs and foragers arriving at the feeder were painted with day-specific colours. Thus, during testing we could ensure that we tested experienced foragers with at least 1 full day of foraging experience. For test runs, we placed a gate in the channel until we had three ants in the feeder holding biscuit crumbs. Those ants were then released and captured in individual pots as ZV ants just before they entered their nest. We then placed a barrier perpendicular to the route so that the nest was fully enclosed and no more ants could head to the feeder. For each training condition (No Shield and Left Shield) there were three tests: (1) No Shield; (2) Left Shield and (3) Right Shield. In the Right Shield condition, the shield of black cloth was placed on the right side of the route, while no shield was present at all on the left side of the route. Individual ants were then tested one by one by releasing them in the channel and recording their behaviour when exiting the channels for all three conditions, presented in a systematically varied order. After a test, ants were captured as soon as exiting the field of view of the camera, the shield changed to the next arrangement and the ant replaced into the channel for the next test. The multiple testing of ants had no appreciable effect on their motivation, and there was no influence of release order on the number of scanning bouts produced in the three conditions (no shield training: Kruskal–Wallis, p = 0.28; left shield training: p = 0.95).
Experiment 2: scanning along a route
A feeder was established 10 m from a Melophorus nest and ants captured at the feeder were marked with day-specific colours. The straight route was surrounded by large objects and thus provided a rich visual scene. Ants with 2 days of experience of foraging along the route were followed on their ordinary homeward route and their paths recorded onto gridded paper with a note made when ants performed a scanning bout. The first recorded trip of each ant corresponds to a full-vector (FV) trip, when their path integrator points towards the nest, and is in accordance with terrestrial visual cues. After their first trip, these ants were captured close to their nest, such that they no longer had a home vector from their PI system, and released from the feeder. Their second trip was recorded in the same way but this time the ants departed the feeder as ZV ants. ZV ants recapitulate their route by means of information from learnt visual cues only. In this situation, we do not expect ZV ants to have been disturbed by their capture and release as a repeated capture does not seem to influence scanning frequency (see above).
Experiment 3: scanning upon exiting the nest
In this experiment, we set a camera above a nest entrance and recorded ants while exiting their nest (recorded area 75 × 60 cm). A permanent feeder was set 5 m away and all ants were painted with a day-specific colour over 3 days. After 3 days of exhaustive painting, any unpainted ant exiting the nest is considered to be naïve, although we cannot be 100 % sure. Ants were recorded in three different conditions. Condition 1: naïve ants displaying their first trip. Any ants leaving the filming area on this trip were discarded as they are unlikely to be entirely naive. Condition 2: ants with experience of travelling to the feeder on Day 1 were tested in the afternoon of the subsequent day. Condition 3: the same cohort of experienced ants as in condition 2 but recorded in the following morning while displaying their first trip of the day.
Camera and tracking
Using a Casio Exilim Pro F1, we were able to record ants at 300 fps. The position and orientation of the ants were extracted from these videos using bespoke MATLAB programs. Processing of the videos was a two-stage process: firstly, the orientation and position are automatically extracted by fitting an ellipse to the image of the ant and then using the major axis and centre; secondly, the extracted data were checked by hand and orientation and positions corrected where necessary. Correction was usually required when there were significant shadows in the image or when the ant was ambitiously carrying a large piece of cookie. From the traces of ant speed and rate of change of body orientation, pauses were identified as segments of the path where linear and angular speed were simultaneously less than 5 cm/s and 80°/s, respectively. Multiple pauses were grouped into a scanning bout if there was <1 cm of translation between each pause. An example of a scanning bout can be seen at <youtu.be/u7LaPjMtmYM>. Inspection of a small set of higher-magnification videos shows that in M. bagoti there is some dissociation of head and body orientation which is not captured by our methods here. The head will often begin rotation before the body and at times their respective orientations may be up to 30° apart. However, our methods allow us to identify the frequency and duration of scanning bouts as well as the broad pattern of fixation directions.
Ants’ perspective views
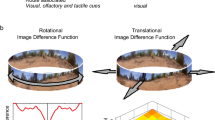
To approximate ant’s perspective views, we captured panoramic photographs from the centre of the high-speed camera’s field of view using a GoPano panoramic lens with a Canon G10. The panoramic images were then unwrapped with Photowarp© software. These images were processed to reflect the properties of insect vision by reducing resolution to approximately 4°/pixel, using a 270° field of view (Schwarz et al. 2011) and enhancing green contrast. Additionally, we homogenised the sky colour to reduce the influence of celestial gradients and accentuate the contrast between the sky and terrestrial objects. The images were subjected to a simple analysis to assess which directions in tests would result in the greatest similarity to the views experienced during training (Zeil et al. 2003). The reference images (panoramic views from the training set-up centred on the nest direction) are compared to ant’s perspective views from test conditions using the summed Root Mean Square (RMS) intensity difference between the reference and test image across all pixel positions. This gives an image difference score for a particular comparison, a process which is repeated for all possible orientations (1° resolution) of the test condition image. Plotting the image difference scores against the orientation of the test image gives a rotational image difference function (RIDF). The heading at which the minimum of the RIDF occurs is the orientation in the test condition which results in an ant’s perspective view which is most similar to the reference image (see also Zeil et al. (2014) for a similar analysis).
Results
Scanning in response to visual scene changes
The dynamics of scanning
As in their natural foraging behaviour, many ants ran smoothly and quickly from the end of the channel in the nestward direction. For these runs, the average speed of an ant is around 20 cm/s (e.g. Fig. 2a). During the paths of other ants, smooth and fast movement was broken by periods where the ant’s translational speed drops to zero. During these periods, ants either remain still with fixed orientation or exhibit a series of discrete fixations chained into a saccadic bout where there is no translation between fixations. We refer to this behaviour as scanning (e.g. Fig. 2b). Across all scans we see significant variation in the scan duration (i.e. number of fixations), the length of fixations and, to a lesser extent, inter-fixation angles (Fig. 2c–e). This variation suggests that scans are not simple predetermined motor patterns, which raises the possibility that the variability is due to scans being tuned to the current situation.
The dynamics of scanning. Example paths of ants in Experiment 1 are shown: a the ant passes through the camera’s field of view without pausing; b she stops and performs a scanning bout. The ball and stick diagrams show body position and orientation (every 15 frames or 50 ms) for paths from the end of the channel, with the ball representing the head. For the whole of the path in a and the scanning bout in b (highlighted with grey circle), we show (from top to bottom) speed profiles, body orientation and angular velocity. c–e Descriptive statistics from all scanning bouts from all ants in Experiment 1. c The number of fixations per scanning bout (median three fixations). d Duration of fixations (mean ± SD. 89 ± 74 ms; median 69 ms). e Angle between fixations during a bout (mean 24°)
The probability of scanning
To investigate if scanning bouts are related to the visual scene, we observed ants at the channel exit when the visual panorama had been altered by the addition, removal or shifting of a screen (“Methods”). We selected the orientation of the training channel so that the direct line towards the nest resulted in a scene with significantly more visual clutter on the right-hand side. This means that, without a shield, the outline of objects against the sky, a significant source of information for navigating ants (Graham and Cheng 2009a), has a high and low portion on the right and left, respectively (Fig. 3a). Thus, erecting the shield on the right of the direct nestward path changes the panorama only slightly. Erecting the shield on the left changes the panorama more significantly as the high skyline it creates replaces a portion of low skyline from the familiar training scene. Similarly, when ants are trained with the left shield in place, we get a significant change to the panorama by testing ants with the shield removed, and an even more significant change by moving the shield across to the right side of the direct nestward path (Fig. 3d). Thus for each training condition, the two test conditions alter the visual panorama by different amounts.
Scanning frequency as a consequence of visual change. a, d Ant’s perspective views from the channel exit in training and test conditions in Experiment 1. a No shield training condition. d Left shield training condition. b, e The number of scanning bouts for the three conditions in No Shield and Left Shield conditions, respectively. c, f The total number of fixations for the three conditions in No Shield and Left Shield conditions, respectively
Compared to control runs when the panorama appears as in training, tests produce significantly more scanning bouts (Fig. 3b, e; Mann–Whitney p <<0.001). More interestingly, ants trained with no shield scan most often during tests with the shield on the left (Fig. 3b; Kruskal–Wallis p = 0.004), whereas ants trained with a shield on the left scan most often with the shield on the right (Fig. 3e; Kruskal–Wallis p ≪ 0.001). Across all tests, the highest frequency of scanning occurred with the right shield for ants trained with the shield on the left where the visual scene is thus altered for 180° of azimuth. The same pattern was observed for the number of fixations per scanning bout, with 3.1 ± 2.8 (Mean ± SD) fixations per scanning bout during control runs rising to 5.2 ± 5.4 fixations per bout in tests (Fig. 3c, f). Overall, we see that scanning occurs most often in the test conditions with the largest changes to the panorama, thus meeting our prediction that unfamiliarity in the visual scene drives scanning.
Fixation directions and image statistics
Given that the frequency of scanning and the subsequent increase in fixations are modulated by the degree of unfamiliarity in the visual scene, we wanted to investigate how fixation directions depend on visual scene properties. Figure 4 shows the distribution of fixation directions for each condition. To assess how these distributions might relate to visual familiarity, we created RIDFs for each test condition. For each RIDF, the familiar view is a nest-oriented ant’s perspective view, as experienced from the end of the channel in training (see “Methods”). Views from test conditions can be systematically rotated and compared to the training view. The orientation of a test view that gives the least difference to the training view defines the direction that ants should orient if they are trying to recapture the scene ordinarily experienced at the channel exit. Figure 4 shows how the distribution of fixation directions does not seem to be centred on or biased by the minimum in RIDFs, thus suggesting that there is not a simple relationship between image similarity and the direction of fixations.
Relationship among visual panorama, heading and fixation directions. For each condition we show the “ant’s eye” panoramic scene from the channel exit in the training and test conditions (details of the visual processing are given in the “Methods” section); The headings of ant’s paths; the RIDF (see “Methods”) for the test panorama compared to the panorama experienced in training; the distribution of fixation directions from scanning bouts in that condition. a–c Ants trained with No Shield. d–f Ants trained in the Left Shield condition
An alternative probe into the relationship between the visual scene and fixations is via a more fine-grained analysis of the rare scans produced during control tests. During these scanning bouts, we can be confident about the direction of the most familiar view. Within this small dataset, we found no relationship between turn directions after fixations and the direction needed to reduce heading error; between final fixation directions and visual familiarity; or even between a scanning bout and a more accurate heading after the scanning bout. We must emphasise that this does not suggest that there is no such relationship. For that particular analysis a larger set of scans is required, preferably evoked in situations with more subtle changes to the visual scene. Such subtle changes can be produced by displacing ants’ small distances from their familiar routes leading to small variations in the familiar scene, whilst the scene will broadly retain the familiar visual features.
Scanning during an entire route
To investigate how other navigational modalities influence the production of scanning bouts we undertook a simple study. Ants were allowed to become familiar with a 10-m route, and then during target trials the number of scanning bouts was recorded during a FV homeward journey and a subsequent ZV trial performed immediately afterwards. In both situations, the visual scene is equally familiar throughout the route. Thus, if scanning is related only to guidance by familiar visual scenes we should see no difference in the production of scanning across the two conditions. However, ZV ants will experience a discrepancy between the directional information provided by visual cues––which points towards the nest––and that provided by PI, which on the ZV run points back to the feeder. Indeed the incidence of scanning is significantly higher for the ZV route than for the FV route (Fig. 5). This simple demonstration shows that scanning is not only related to the familiarity of the visual scene.
Scanning along full- and zero-vector routes. Ants were allowed to develop routes between their nest and a feeder. Target ants were then followed and the number of scanning bouts produced along their route recorded. These ants were then captured before they entered the nest and released again at the feeder (ZV ants). a The number of scanning bouts produced on FV runs. b The number of scanning bouts produced on ZV runs. Scanning is significantly more likely on ZV runs (Sign test; p <<0.001)
Scanning when leaving the nest
To investigate further the occurrence of scanning bouts, we recorded the frequency of scanning in either naïve or experienced foragers leaving their nest (Fig. 6). Naïve ants scan on 100 % of outward trajectories that ordinarily loop back to the nest (Fig. 6a). For experienced ants that were recorded in the afternoon of their first or second day of foraging, the probability of scanning drops markedly (Fig. 6b). However, at the start of each day frequency of scanning increases again; even for experienced ants (Fig. 6c), a pattern which mirrors the occurrence of learning flights in bees (Wei et al. 2002) and the production of return loops to a familiar feeder in ants (Graham and Collett 2006).
Scanning during outward paths. The early stages of the outward paths of three groups of ants were recorded and scanning bouts extracted. a Naïve ants; b experienced ants in the afternoon; c experienced ants in the morning. Morning ants are recorded on the day after afternoon ants and are therefore more experienced. Histograms show the distribution of fixation numbers as part of scanning bouts for ants in each of the three groups. The differences between fixation number in the three groups are statistically significant (Kruskal–Wallis p < 0.001; All post hoc comparisons p < 0.001)
Discussion
Here, we have described a new aspect of the navigational behaviour of the Australian desert ant M. bagoti. Although these ants spend much of their foraging lives travelling at high speed, occasionally they cease translation and maintain a fixed orientation for a period of ~30 ms. These fixations can be linked together by fast rotations (“saccadic scans”) into what we call a scanning bout. Our key finding is that both the probability and duration of these scanning bouts are increased as a function of how unfamiliar the visual scene is. That is, how much the visual panorama has been altered from the scene experienced by ants during training. Additionally, observing ants on their departure from their nest we see large amounts of scanning in naive ants and also from experienced ants each morning. By observing scanning along a route, we found it to be more common when the information from visual cues is at odds with the direction indicated by an ant’s path integration system.
The two most likely explanations for the impact of visual scene changes on the production of scanning bouts are (1) scanning is part of the visual navigation mechanisms that ordinarily control visually guided routes, or (2) the reduction in visual familiarity leads to a more general sense of spatial uncertainty, and uncertainty provokes scanning. We discuss these explanations in turn.
Is scanning a visual navigation mechanism?
It is well understood that remembered visual scenes can be used to drive behaviour via comparison with currently perceived scenes. A useful, and now almost traditional dichotomy, is to think of stored views being used as either an attractor (following Cartwright and Collett 1983) or to set a direction (Zeil et al. 2003; Philippides et al. 2011; Zeil 2012). A stored view can act as an attractor through detailed comparison of the location of visual features between stored and current views (Cartwright and Collett 1983). Alternatively, one can use gradient descent methods to minimise an image difference score from the comparison between current and stored views (Zeil et al. 2003). Via either of these methods the location from where a view was stored can be approached from any direction. A more parsimonious alternative is that stored visual scenes can be used simply to set a direction by acting as a terrestrial visual compass. Here, one’s heading during route recapitulation is determined by minimising the difference with those views stored when facing the correct direction during route learning, akin to finding the minimum in an RIDF, and then moving in this familiar direction. This type of visual route guidance mechanism has been suggested by behavioural experiments (Collett 2010; Wystrach et al. 2012; Zeil 2012). The form of the scanning behaviour fits well with the concept of terrestrial views being used as a visual compass, as scanning provides a sampling by which multiple possible directions can be directly evaluated before heading along the most familiar one.
If ants are using a stored view to set a direction we might have expected to see some tight correlation between fixation directions and RIDFs. We did not. Similarly, we might have expected to see an influence of familiar views within the fine details of those scans produced during control runs, where we can be sure that the world provides a good match with visual memories. Again, we did not. We did see an influence of the visual scene on the production of scans. Furthermore, fixations are aligned broadly with the homeward direction, even in tests, although there is not a precise relationship between fixation direction and visual familiarity. Of course, we cannot discount that scanning plays some role in visual route guidance via visual compass mechanisms, though we cannot divine the details of that role from this data set. The majority of fixations do occur within the range of most familiar views, and variation in the views experienced during training might result in a wider familiar ‘envelope’. Within that broadly familiar range of directions it may be a good strategy to sample a range of possible directions since the ant has already ceased translation, and the saccades are quick to perform.
In contrast to visual compass methods, it is less straightforward to make predictions about the form that scanning would take if it were being used to drive visual homing via an attractor process. Möller (2012) provides a computational model whereby multiple snapshots stored at different orientations at a goal location can drive homing. In this scheme, stored views are used as attractors and a scanning behaviour is used to sample the world during recapitulation. However, it is difficult to provide evidence for views being used as “snapshot attractors” because the relationship between experience (where views are stored) and recapitulation (where views are used) is much more complex. Suffice it to say that behavioural experiments have posited separate roles for both “visual compass” and “attractor” mechanisms (Collett 2010; Wystrach et al. 2012) with on-route guidance mechanisms attributed mostly to the use of a terrestrial visual compass and off-route mechanisms attributed mostly to the use of snapshot attractors (see also Baddeley et al. 2012). The key objective data required to separate these strategies are a precise record of exactly which locations (and crucially at what orientations) ants have experienced the world and how this relates to subsequent performance. Recent experiments (e.g. Narendra et al. 2013) suggest how this might be achieved.
Is scanning a response to uncertainty?
Of course a rotational sampling behaviour, such as scanning, is useful for evaluating directional information from any modality. The general value of scanning is that spatial computation is outsourced to a directional sampling behaviour (c.f. Möller 2012; Wystrach et al. 2013). We see such scanning behaviours in a range of invertebrate orientation tasks, such as the path planning of jumping spiders (Tarsitano and Andrew 1999), the reorientation of disturbed dung beetles (Baird et al. 2012), scanning in the desert ant (Cataglyphis bombycina) which has been attributed to compass calibration (Wehner et al. 1992), and scanning during the learning walks of another desert ant (Ocymyrmex robustior) (Muller and Wehner 2010).
Given the general value of a scanning behaviour for evaluating information from different orientations, we consider a second putative role for scanning: a more general response to navigational uncertainty. In our first experiment all ants were ZV and the major variation between test conditions came from changes to the visual scene, with scanning more likely for more unfamiliar visual scenes. We also examined scanning when the information from visual scenes was familiar but conflicted with PI. Here, we see an increase in scanning when the information provided by learnt visual cues is at odds with that provided by PI (Fig. 5). It was traditionally assumed that information from familiar visual scenes would override PI information (Wehner 2009). However, Collett (2012) has shown how navigational modalities can remain co-engaged and simultaneously influence motor output. It is plausible that scanning somehow reflects that integration when there is a conflict between the directions provided by learnt visual scenes and PI.
An additional source of uncertainty that might explain some properties of scanning, especially from Experiment 2, has recently been described (Collett, submitted). Here, ants learn a simple route from a feeder back to their nest. When they are captured at the nest and returned to the feeder they demonstrate some disturbance in their path. This effect is shown to be a consequence of repeating portions of a familiar route at short notice rather than a conflict between visual navigation and PI. This suggests that in some way visual memories are tagged by their use and that short-notice exploitation of the same visual memories provokes uncertainty.
In general, uncertain situations provoke behaviours conducive to taking in information. These behaviours might include the well-known orientation reactions to novel stimuli such as sounds and smells or exploratory behaviours in a new environment. In our study species, even directed paths are more meandering when the situation is less than fully familiar (Wystrach et al. 2011b), with meandering increasing in a dose-dependent fashion based on the extent of unfamiliarity. Scanning behaviours may be part and parcel of information gathering and learning under navigational uncertainty.
Conclusion
The role of terrestrial visual scenes in insect navigation is well established. Here, we have added to the known suite of navigational behaviours with a description of a scanning behaviour. The production of these scans can be reliably triggered by changes to the familiar visual scene. However, scans are not simply a visual phenomenon as they are also produced at times when overall spatial uncertainty is high, for instance when the information from terrestrial visual cues is at odds with that of PI, or during ‘learning walks’. It is possible that a scanning behaviour has dual function: being at the service of learning as well as retrieval of the correct direction. Parsimoniously, both these putative functions of scanning can be served if scanning is triggered by general spatial uncertainty. The intricacies of how navigational systems encode uncertainty through behaviour are still largely unresolved. These results, however, give hope that some resolution will come from studying navigational behaviour in fine-grained detail.
References
Andel D, Wehner R (2004) Path integration in desert ants, Cataglyphis: how to make a homing ant run away from home. Proc R Soc B 271(1547):1485–1489
Baddeley B, Graham P, Husbands P, Philippides A (2012) A model of ant route navigation driven by scene familiarity. Plos Comp Biol 8(1):e1002336
Baird E, Byrne MJ, Smolka J, Warrant EJ, Dacke M (2012) The dung beetle dance: an orientation behaviour? PLoS One 7(1):e30211
Cartwright BA, Collett TS (1983) Landmark learning in bees: experiments and models. J Comp Physiol 151(4):521–543
Cheng K, Shettleworth SJ, Huttenlocher J, Rieser JJ (2007) Bayesian integration of spatial information. Psychol Bull 133(4):625–637
Cheng K, Narendra A, Sommer S, Wehner R (2009) Travelling in clutter: navigation in the Central Australian desert ant M. bagoti. Behav Process 80(3):261–268
Collett TS (1995) Making learning easy––the acquisition of visual information during the orientation flights of social wasps. J Comp Physiol A 177(6):737–747
Collett M (2010) How desert ants use a visual landmark for guidance along a habitual route. Proc Natl Acad Sci USA 107(25):11638–11643
Collett M (2012) How navigational guidance systems are combined in a desert ant. Curr Biol 22(10):927–932
Collett M, Collett TS (2000) How do insects use path integration for their navigation? Biol Cyber 83(3):245–259
Collett TS, Land MF (1975) Visual spatial memory in a hoverfly. J Comp Physiol 100:59–84
Collett TS, Dillmann E, Giger A, Wehner R (1992) Visual landmarks and route following in desert ants. J Comp Physiol A 170(4):435–442
Collett TS, Graham P, Harris RA, Hempel-De-Ibarra N (2006) Navigational memories in ants and bees: memory retrieval when selecting and following routes. Adv Stud Behav 36:123–172
Collett TS, Graham P, Harris RA (2007) Novel landmark-guided routes in ants. J Exp Biol 210(12):2025–2032
Durier V, Graham P, Collett TS (2003) Snapshot memories and landmark guidance in wood ants. Curr Biol 13(18):1614–1618
Fourcassie V (1991) Landmark orientation in natural situations in the Red Wood Ant Formica-Lugubris-Zett (Hymenoptera, Formicidae). Ethol Ecol Evol 3(2):89–99
Fukushi T (2001) Homing in wood ants, Formica japonica: use of the skyline panorama. J Exp Biol 204(12):2063–2072
Graham P, Cheng K (2009a) Ants use the panoramic skyline as a visual cue during navigation. Curr Biol 19(20):R935–R937
Graham P, Cheng K (2009b) Which portion of the natural panorama is used for view-based navigation in the Australian desert ant? J Comp Physiol A 195(7):681–689
Graham P, Collett TS (2002) View-based navigation in insects: how wood ants (Formica rufa L.) look at and are guided by extended landmarks. J Exp Biol 205(16):2499–2509
Graham P, Collett TS (2006) Bi-directional route learning in wood ants. J Exp Biol 209(18):3677–3684
Graham P, Durier V, Collett TS (2004) The binding and recall of snapshot memories in wood ants (Formica rufa L.). J Exp Biol 207(3):393–398
Harris RA, Graham P, Collett TS (2007) Visual cues for the retrieval of landmark memories by navigating wood ants. Curr Biol 17(2):93–102
Judd SPD, Collett TS (1998) Multiple stored views and landmark guidance in ants. Nature 392(6677):710–714
Junger W (1991) Waterstriders (Gerris paludum F) compensate for drift with a discontinuously working visual position servo. J Comp Physiol A 169(5):633–639
Kohler M, Wehner R (2005) Idiosyncratic route-based memories in desert ants, M. bagoti: how do they interact with path-integration vectors? Neurobiol Learn Mem 83(1):1–12
Lent DD, Graham P, Collett TS (2009) A motor component to the memories of habitual foraging routes in wood ants. Curr Biol 19(2):115–121
Lent DD, Graham P, Collett TS (2010) Image-matching during ant navigation occurs through saccade-like body turns controlled by learned visual features. Proc Natl Acad Sci USA 107(37):16348–16353
Lent DD, Graham P, Collett TS (2013) Visual scene perception in navigating wood ants. Curr Biol 23(8):684–690
Mangan M, Webb B (2012) Spontaneous formation of multiple routes in individual desert ants (Cataglyphis velox). Behav Ecol 23(5):944–954
Möller R (2012) A model of ant navigation based on visual prediction. J Theor Biol 305:118–130
Müller M, Wehner R (2010) Path integration provides a scaffold for landmark learning in desert ants. Curr Biol 20(15):1368–1371
Narendra A (2007) Homing strategies of the Australian desert ant M. bagoti. II. Interaction of the path integrator with visual cue information. J Exp Biol 210(12):2212
Narendra A, Si A, Sulikowski D, Cheng K (2007) Learning, retention and coding of nest-associated visual cues by the Australian desert ant, M. bagoti. Behav Ecol Sociobiol 61(10):1543–1553
Narendra A, Cheng K, Sulikowski D, Wehner R (2008) Search strategies of ants in landmark-rich habitats. J Comp Physiol A 194(11):929–938
Narendra A, Gourmaud S, Zeil J (2013) Mapping the navigational knowledge of individually foraging ants, Myrmecia croslandi. Proc R Soc B 280:20130683
Nicholson DJ, Judd SPD, Cartwright BA, Collett TS (1999) Learning walks and landmark guidance in wood ants (F. rufa). J Exp Biol 202(13):1831–1838
Philippides A, Baddeley B, Cheng K, Graham P (2011) How might ants use panoramic views for route navigation? J Exp Biol 214(3):445–451
Reid SF, Narendra A, Hemmi JM, Zeil J (2011) Polarised skylight and the landmark panorama provide night-active bull ants with compass information during route following. J Exp Biol 214(3):363–370
Schultheiss P, Cheng K (2011) Finding the nest: inbound searching behaviour in the Australian desert ant, M. bagoti. Anim Behav 81(5):1031–1038
Schultheiss P, Wystrach A, Legge ELG, Cheng K (2013) Information content of visual scenes influences systematic search of desert ants. J Exp Biol 216(4):742–749
Schwarz S, Narendra A, Zeil J (2011) The properties of the visual system in the Australian desert ant M. bagoti. Arthropod Struct Dev 40(2):128–134
Tarsitano MS, Andrew R (1999) Scanning and route selection in the jumping spider Portia labiata. Anim Behav 58:255–265
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189(8):579–588
Wehner R (2009) The architecture of the desert ant’s navigational toolkit (Hymenoptera: Formicidae). Myrmecol News 12:85–96
Wehner R, Räber F (1979) Visual Spatial memory in desert ants, Cataglyphis bicolor. Experientia 35:1569–1571
Wehner R, Srinivasan MV (1981) Searching behavior of desert ants, genus Cataglyphis (Formicidae, Hymenoptera). J Comp Physiol 142(3):315–338
Wehner R, Fukushi T, Wehner S (1992) Rotatory components of movement in high speed desert ants, Cataglyphis bombycina. In: Paper presented at the 20th Göttingen Neurobiology Conference, Göttingen
Wehner R, Michel B, Antonsen P (1996) Visual navigation in insects: coupling of egocentric and geocentric information. J Exp Biol 199:129–140
Wei CA, Rafalko SL, Dyer FC (2002) Deciding to learn: modulation of learning flights in honeybees, Apis mellifera. J Comp Physiol A 188(9):725–737
Wystrach A, Beugnon G, Cheng K (2011a) Landmarks or panoramas: what do navigating ants attend to for guidance? Front Zool 8:21
Wystrach A, Schwarz S, Schultheiss P, Beugnon G, Cheng K (2011b) Views, landmarks, and routes: how do desert ants negotiate an obstacle course? J Comp Physiol A 197(2):167–179
Wystrach A, Beugnon G, Cheng K (2012) Ants might use different view-matching strategies on and off the route. J Exp Biol 215(1):44–55
Wystrach A, Mangan M, Philippides A, Graham P (2013) Snapshots in ants? New interpretations of paradigmatic experiments. J Exp Biol 216(10):1766–1770
Zeil J (1993) Orientation flights of solitary wasps (Cerceris, Sphecidae, Hymenoptera) 2: similarities between orientation and return flights and the use of motion parallax. J Comp Physiol A 172(2):207–222
Zeil J (2012) Visual homing: an insect perspective. Curr Opin Neurobiol 22(2):285–293
Zeil J, Hofmann MI, Chahl JS (2003) The catchment areas of panoramic snapshots in outdoor scenes. J Opt Soc Am A 20:450–469
Zeil J, Narendra A, Stürzl W (2014) Looking and homing: how displaced ants decide where to go. Phil Trans R Soc 369(1636):20130034
Acknowledgments
The experiments detailed here comply with the ethical standards of the countries where they were undertaken and analysed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wystrach, A., Philippides, A., Aurejac, A. et al. Visual scanning behaviours and their role in the navigation of the Australian desert ant Melophorus bagoti . J Comp Physiol A 200, 615–626 (2014). https://doi.org/10.1007/s00359-014-0900-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-014-0900-8