Abstract
This essay presents and discusses the state of the art in studies of desert ant (Cataglyphis) navigation. In dealing with behavioural performances, neural mechanisms, and ecological functions these studies ultimately aim at an evolutionary understanding of the insect's navigational toolkit: its skylight (polarization) compass, its path integrator, its view-dependent ways of recognizing places and following landmark routes, and its strategies of flexibly interlinking these modes of navigation to generate amazingly rich behavioural outputs. The general message is that Cataglyphis uses path integration as an egocentric guideline to acquire continually updated spatial information about places and routes. Hence, it relies on procedural knowledge, and largely context-dependent retrieval of such knowledge, rather than on all-embracing geocentred representations of space.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the Saharan desert, Cataglyphis ants travel thousands of times their own body length to finally arrive at a pin-point goal. How does the 0.1-mg brain (Fig. 1) housed in the cockpit of this 10-mg ant accomplish such feats of navigation? This is the question my students and I have worked towards answering since I was first startled by Cataglyphis—its elegant appearance and superb navigational performances (Wehner 1968). Hence, what I now want to present in this survey is neither impersonal nor encyclopaedic, but a rather idiosyncratic reflection of the major lessons Cataglyphis has taught us about its behavioural stratagems employed in navigation—or, in other words, about the architecture of its navigational toolkit.
Cataglyphis brain. Three-dimensional reconstruction of the corpora pedunculata (orange) superimposed on a frontal section through the brain (grey) of C. bicolor. The 3-D reconstruction is based on confocal microscopical sections through a brain stained immunohistochemically with a monoclonal antibody against Synorf-1 and further processed by the Amira software. Preparation by S. Bühlmann
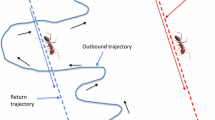
A time-honoured example in ant navigation is path integration, the ant's lifeline on any of its foraging journeys (Fig. 2A). This mode of navigation has perplexed generations of biologists and aroused their curiosity. Pieron (1904) argued that an ant while homing would retrace the steps made during its outward journey (loi du contre-pied; Reynaud 1898) by employing certain proprioceptive means (sens musculaire). When Cornetz (1910) remarked that the ants in question integrated rather than retraced their outbound paths, Pieron (1912) retreated, at least partly, from his argument, and controversial discussions about the basic strategies of ant navigation continued throughout the next decades (see, e.g., Santschi 1913). Even though it might be hard for us today to grasp the mind-set of the early 1900s, it seems as if most arguments had revolved around the question of what an ant should be able to perceive and compute in meeting its navigational demands, and how it should be able to do so in a most general and versatile way, rather than what task it needed—and what it did not need—to accomplish within the constraints of its ecological setting. These disturbing questions, which were to reverberate throughout the remainder of the century and well into the next, bring us right to the core of present discussions in the cognitive neurosciences (compare, for example, Gallistel 2000 and Menzel and Giurfa 2001).
Path integration (vector navigation) in Cataglyphis fortis. A An ant's tortuous outward (foraging) and straight homeward path recorded in a featureless salt pan. B Straight outward paths indicated by the dotted line and multi-leg homeward paths caused by experimental barriers (grey bars), which the ant could pass on its way out (from N to F) but not on its way in. Nine successive runs of one ant. F feeding site, N nest. A from Wehner and Wehner (1990), B from D. Andel and R. Wehner (unpublished observations)
In the following I shall outline some of our lessons from Cataglyphis. I shall especially focus on the notion that the ant's navigational machinery has evolved to solve particular problems encountered by Cataglyphis during its foraging lifetime. Certainly, natural selection does not allow a minuscule brain to provide space and energy for computational and storage resources that are not of immediate use to the navigator, particularly because in energetic terms the brain is by far the most costly organ of any animal (Martin 1981; Allman 1999). Next comes the question of how particular navigational modules are interlinked so as to orchestrate the ant's navigational performances. To make my point succinctly, and to leave room for discussion and disagreement, I shall start each section of this essay with a somewhat provocative statement, in the hope that future research will refine or even refute my conjectures.
Skylight compass
The ant's polarization (e-vector) sensitive system does not encode skylight information about individual e-vectors per se, but just about compass directions
Ever since von Frisch's (1949) epochal discovery that bees can use the polarized light in the sky (Fig. 3A) as an effective compass cue, the provocative question has been raised: what information do the insect's eyes and brain actually derive from the celestial polarization (e-vector) patterns? In the 1970s, when there was a worldwide resurgence of interest in this question, it was generally assumed that the insects were able to determine the e-vector orientation in isolated patches of skylight (under conditions first clearly outlined by Kirschfeld 1972), and that they then would use certain kinds of detailed knowledge about the geometry of the global e-vector pattern to derive, say, the solar meridian (the azimuthal position of the sun) even from isolated patches of polarized skylight. For example, on the basis of Brines' (1978) data, Gould et al. (1985) argued that bees would accurately interpret celestial e-vector patterns, i.e. that they would know the exact positions where individual e-vectors occurred in the sky (at least in the highly polarized anti-solar half of the sky). Most probably von Frisch (1949, 1965) had adhered to the same hypothesis, but in his unpublished note-books he already expressed some scepticism by referring to a number of observations in which the bees had exhibited ambiguous and bimodal orientation performances when presented with individual e-vectors. Such "navigational errors" were later reported, and systematically studied, by Rossel and Wehner (1984) and Wehner and Rossel (1985). They were found in Cataglyphis as well (Fent 1985; Müller 1989). In addition, however, in the cataglyphid ants it was possible to show that the navigational errors occurred only when the animals were trained under a restricted skylight pattern and tested under the full sky, or vice versa. They disappeared completely when the same restricted skylight pattern was present in both the training and the test situation (Wehner 1991). These behavioural analyses show that desert ants—as well as honey bees—are not informed exactly about where, within a sun-based system of coordinates, any particular e-vector occurs at any particular time of day. Nevertheless, their compass system can successfully deal with any partial skylight pattern, as long as the foraging insect can experience the same partial pattern during its entire round-trip.
A Skylight polarization measured on 26 August 1999 in the Chott-el-Djerid, Tunisia. Elevation of sun: 41°. Degree (left) and angle (right) of polarization as recorded by full-sky (180°) imaging polarimetry in the blue (450 nm) range of the spectrum. The white dot marks the position of the sun. The line passing through the sun represents the image of a wire bar holding a small disk that screened off the sun (ov. overexposure). B Zenith projection of the fan array of the polarization analysers of the right eye of Cataglyphis bicolor. The black bars indicate the e-vector tuning axes of the (contralaterally looking) polarization analysers. 0° horizon, C caudal, F frontal, L and R left and right visual hemisphere. Modified from A Pomozi et al. (2001) and B Wehner (1982)
Neurobiological analyses might well tell a similar story. First, in both bees (Wehner and Strasser 1985) and ants (Fent 1985) the polarization analysers are restricted to a small dorsal part of the insect's eye and brain (Fig. 3B). This dorsal rim (POL) area contains sets of specialized ultraviolet receptors, which act as polarization analysers by sharing a suite of functional properties (for reviews see Wehner 1994, Labhart and Meyer 1999). However, the deeper we move into the brain, the more we have to face a conceptual chimera problem; namely, that of arguing on the basis of behavioural data obtained from bees and ants and of neuronal characteristics studied in crickets and locusts. In the latter insects, polarization-sensitive interneurons have been found in the medulla (Gryllus: Labhart 1988; Petzold 2001; Labhart et al. 2001; Fig. 4; Schistocerca: Homberg and Würden 1997), the central complex and the anterior optic tubercle (Schistocerca: Vitzthum et al. 2002; Pfeiffer and Homberg 2002). All these POL neurons, be they located in the visual system or in the more central neuropiles, exhibit polarization-opponent properties insofar as their spike frequencies are sinusoidal functions of e-vector orientation with their maxima (tuning axes) and minima arranged in mutually perpendicular ways (Fig. 4). Outside the orthopteran insects, it is only in the cockroach Leucophaea (Loesel and Homberg 2001) and, fortunately enough, in the ant Cataglyphis (Labhart 2000) that such (medullar) POL neurons have also been described.
Polarization-sensitive interneuron of the cricket medulla. A Camera lucida drawing of a neurobiotin-stained cell with its ipsilateral (left) and contralateral (right) dendritic arborizations (m* accessory medulla). B Recordings from the ipsilateral (left) and contralateral (right) part of the neuron, while the e-vector orientation (Φ) of the light stimulus is rotated through 360°. In the ipsilateral recording (left) the baseline undulates as a result of the summation of EPSPs (see arrow). In the contralateral recording (right) the white triangle marks the onset of the visual stimulation, i.e. the onset of the modulation of the spontaneous firing rate. The ipsilateral and contralateral recordings are from different neurons of the same type of neuron. Combined and modified from Petzold (2001)
With the above-mentioned chimera problem in mind, and treating it as cautiously as possible, we can outline the following hypothesis (Fig. 5). First, responses of particular populations of retinal analysers are pooled by a small number of (probably only three) large-field integrators, each characterized by a particular e-vector tuning axis (medullar POL interneurons, lit.cit.). The response pattern of this set of integrators would vary systematically as the animal rotates about its dorsoventral body axis: the larger these variations, the more accurate the compass readings. Hence, over evolutionary time there must have been a strong selection pressure to adapt the contralaterally looking fan-array of retinal analysers (Fig. 3B) to the celestial e-vector pattern (Fig. 3A) such that the response modulations resulting from the ant's angular movements were maximized. Furthermore, it is a plausible hypothesis that the integrator neurons feed their outputs into an array of "compass neurons" (sensu Hartmann and Wehner 1995), each of which fires maximally when the animal is oriented in a particular compass direction. (For a model simulation see Labhart and Lambrinos 2001.) At first glance, it might be tempting to assume that the polarization-sensitive interneurons reported from the central complex of locusts (lit.cit.) could serve the function of such compass neurons, although certain properties of these interneurons cannot yet easily be reconciled with this assumption. Nevertheless, the various neuropiles of the central complex, with their arrays of 2×8 columnar segments ("glomeruli": Strausfeld 1976, p 85) and their involvement in azimuthal orientation (Drosophila: Strauss 2002), render this area of the brain a hot spot for studies of oriented locomotor behaviour.
Hypothesis about way stations of the insect's polarization compass: analysers (array of polarization-sensitive photoreceptors, see Fig. 3B), integrators (wide-field polarization-sensitive medullar interneurons, see Fig. 4), and hypothetical compass neurons each responding maximally when the animal is oriented in a particular compass direction. The number of compass neurons (n=24) is chosen arbitrarily
In any event, however, the large-field integration of analyser responses destroys information about individual e-vectors in the sky. Let us further assume that—as is most likely the case in Cataglyphis—the large-field integrators sample the outputs of analyser populations looking at different areas of the celestial hemisphere. The array of compass neurons would then encode particular headings of the ant relative to a celestial system of reference rather than particular e-vectors. Seen in this light, there would be no stage along the polarization-vision pathway at which information about the orientation of individual e-vectors is represented.
The spatial low-pass characteristics of the large-field integrators help to attenuate response variations caused by atmospheric disturbances or canopy obstructions (Labhart 1999; Pomozi et al. 2001). However, non-random disturbances of the celestial e-vector patterns such as those caused, for instance, by clouds or experimental interference will lead to systematic alterations of the integrator response profiles. These alterations might result in the ants' navigational errors observed under particular experimental paradigms (see above). However, these errors disappear as soon as the ants are presented with the altered skylight patterns during their entire round-trip journey rather than only during short "experimental time windows", i.e. as long as there are consistent readings of the integrator/compass-neuron network. Only if the ant, under different skylight conditions,set out to leave the nest in a particular (e.g., the previous) foraging direction, would it have to recalibrate its compass against an earthbound system of reference. Do the rotatory movements performed by the ants at the start of their foraging journeys (Wehner et al. 1992) assist in calibrating the compass?
In conclusion, celestial e-vector patterns are characterized by relatively simple geometrical rules, which the insect navigator exploits in straightforward and robust ways. One should never underestimate the functional economy of nervous systems: once they have been adapted, over evolutionary time, to the principal physical properties of a predictable environment, they can employ comparatively simple neural strategies to solve quite sophisticated computational tasks. (For a striking example from the visual world of fiddler crabs see Hemmi and Zeil 2003.)
Path integration
Path-integration vectors are used as an egocentric guideline in acquiring spatial information rather than as a means for geocentred (cartographic) triangulation.
Information from both the skylight compass and the odometer, i.e. information about the angular and linear components of movement, is simultaneously fed into an integrator. At any one time, this integrator informs the ant about its current position relative to its point of departure. In the following I side-step the proximate issue of how the insect's odometer and integrator might work (for a review see Wehner and Srinivasan 2003). Instead, I focus on the ultimate question of what use Cataglyphis might make of path integration within its overall navigational context.
When C. fortis forages within the vast and essentially featureless terrains of the Saharan salt pans, path integration is its only means of acquiring positional information. In cluttered environments, however, as they occur at the fringes of the salt pans or in the habitats of other Cataglyphis species, navigation by landmarks can override the path-integration system (Wehner 1968; Sassi and Wehner 1997; Bisch and Wehner 1998; Collett et al. 1998). But even then the integrator keeps running continually (Sassi and Wehner 1997). For example, when Cataglyphis is trained to return home along a familiar landmark route—say, along a linear array of black cylinders—and later subjected to an experimental paradigm in which the ant's global home vector and the direction defined by the array of landmarks do not coincide, about half of the ants select the vector rather than the landmark course (Fig. 6). They keep their path integrator running until—at the nest entrance—the current state of the home vector matches the stored one. The same holds true for those ants that have initially followed the landmark route (Fig. 6). They continue to update their path integrator and, upon leaving the landmark route, choose the course leading directly to the nest. In fact, these ants behave similarly to those presented with the forced-detour paradigm (Fig. 2B). The major difference is that in the present case the ants have selected the landmark-based detour voluntarily rather than compulsorily.
Continuous updating of the ant's path integrator. Ants trained to associate their homeward (global) vector with a landmark route (a "corridor", grey, consisting of 6 differently sized black cylinders) are subjected to an experimental paradigm, in which the landmark route deviates from the vector course. Some ants choose the latter from the very start (black trajectories). Others first follow the landmark corridor, but nevertheless keep their integrator running. Upon leaving the corridor, they head directly for the goal (green and red trajectories). N 1 and N 2 positions of the nest as indicated by the ant's most recently acquired global vector and the landmark route, respectively. For the slight deviations of the global vector courses from the direction leading to N 1 see Müller and Wehner (1988). R point of release. Modified from Sassi and Wehner (1997)
This is only a cursory glance at the diverse sets of experiments available to demonstrate that vector navigation provides Cataglyphis with a lifeline used and updated during its entire foraging career. Wherever it goes, the state of its path integrator connects the ant with its home or a place within its foraging area. The vector pointing home is always the inverse of the one pointing towards the feeding site. In open-jaw experiments, in which on return the point of departure differed from the point of arrival, we have never been able to train Cataglyphis to outward and homeward vectors that were not 180° reversals of each other (Wehner and Flatt 1972; Collett et al. 1999; Wehner et al. 2002). It is merely the sign of the vector that is reversed once the ant has arrived at the nest or the feeder.
This experimental finding is in accord with the ant's foraging ecology. By remaining faithful to directions taken during previous foraging trips, Cataglyphis ants exhibit pronounced sector fidelity. At the beginning of their foraging lives, they keep rotating their foraging directions whenever they have returned from an unsuccessful trip, but head out along the direction of the previous trip if this trip has been successful. Moreover, as the number of successful runs increases during an ant's foraging life, the ants maintain their sector fidelity even if they have returned from an unsuccessful foray (Wehner 1987). Consequently, sector fidelity increases during an ant's lifetime; and foraging distance does as well (Fig. 7A). The latter results from the ant's common strategy of passing the previously visited feeding site—the site of an ephemeral (non-renewable) food source, an arthropod corpse—and heading out over greater distances from the nest. Similarly, if honey bees return to a previously visited but now exhausted nectar source, and are even held captive there for several hours, they subsequently fly farther outward from the hive along the same hive-feeder direction (Dyer et al. 2002).
A Sector fidelity and increasing search distance during the lifetime of an ant, Cataglyphis fortis, foraging within a salt-pan environment. Runs nos. 3–7 (left) and nos. 26–31 (right) of the same specimen. B Route fidelity of Melophorus bagoti foraging within a cluttered environment. Path density plots of 5 successive outbound (left) and 5 corresponding inbound (right) runs of the same ant. In the right figure the area covered by the outbound runs is shown in grey. F feeder, N nest entrance, grey polygons grass tussocks, Cenchrus ciliaris. Recordings by A K. Selchow and B M. Kohler
In functional terms "vector fidelity" assists the ant in acquiring and using landmark information, since the same landmark route is encountered time and again on successive foraging trips (Fig. 7B). It is certainly for that very reason that vector fidelity evolved in the first place. This raises the question whether, and to what extent, Cataglyphis is able to link particular landmark memories to particular states of the path integrator. We know that ants while homing expect landmark views acquired at the nesting site only when the integrator is close to its zero state (Wehner et al. 1996). Otherwise, however, the possible attachment of vector states to landmark views is still a little-explored topic. In principle, such an ability would provide Cataglyphis with a set of site-specific co-ordinates, which in turn could be used for triangulation and hence for computing novel routes between familiar sites (Biegler 2000). However, there is not yet the least bit of evidence that Cataglyphis would be able to accomplish this kind of cartographic task. Instead, considerable evidence has been amassed that Cataglyphis is bound to the limited environmental information captured en route within its egocentric system of spatial representation.
Landmark-based navigation
Places are recognized by view-matching systems
Even though Cataglyphis relies on path integration as its predominant navigational means, it makes intensive use of landmark information. It defines a place not only in terms of a vector leading to it from another place, e.g. the nesting site, but also in terms of visual landmarks labelling that place (e.g. Fig. 8). This labelling occurs by view-dependent learning of visual scenes from particular vantage points (see for example Judd and Collett 1998 for Formica ants). On return to such places, the ants employ some kind of retinotopically organized image matching (Fig. 9; Wehner et al. 1996; for the algorithm and simulation of this process within the ant robot Sahabot II, see Lambrinos et al. 2000). The ants can define their viewing directions at the vantage points in terms of an allocentric system of reference, e.g. celestial co-ordinates, but can also decouple them from such a system (Fig. 10). Furthermore, they can associate familiar landmark scenes with local vectors (Collett et al. 1998) and motor commands (Collett et al. 2001; Bisch-Knaden and Wehner 2001), which enable them to proceed directly from one place to the visual catchment area of the next. In this way, they can follow fixed routes by learning landmarks distributed along the "visual corridors" defined by these routes. The path integrator guides the ant to the part of the landmark route that is close to the goal (Müller and Wehner 1988; Sommer and Wehner 2003). The same ant can learn different routes for the outward and homeward journeys (Wehner et al. 1983) and for journeys leading to different feeding sites (R. Wehner, unpublished data). In navigating its visually guided routes, Cataglyphis must remember and recall a sequence of memories. Most probably, these memories are internally linked to each other and/or to the state of the global vector. In addition, external linkages might play a major role: as the ant proceeds along its route, familiar landmarks appear one after another in the same sequence in which they have previously been experienced. (See, in a broader sense, the concept of "stigmergy" in Grassé 1959.)
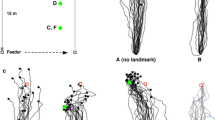
Search density distributions (upper row) of ants trained to the centre of a 4-cylinder landmark array and tested A within the training array and B, C within altered arrays. In B the retinal image perceived at the goal is identical with the one in the training situation, but in C it is not. The middle and lower row depict 3-D and 2-D representations, respectively, of the landmark arrays. The open arrows point at the positions at which a particular matching-to-memory algorithm yields the best possible fit between current and stored images. Modified from Åkesson and Wehner (1997)
Search density distributions of ants, which have been trained to a goal (white dot) located asymmetrically within a 4-cylinder array (compare the 3-cylinder array in Fig. 8). The landmarks are positioned at the corners of the diagrams. NW, NE, SE, SW indicate the directions from which the ants approach the landmark array. The search density peaks in the south-east are due to the fact that at this location the stored and the current image are in register with the celestial system of reference. In addition, the ants search at the position closest to the direction of approach, although at this place stored and current landmark images are out of register with the skylight reference. Adapted from Åkesson and Wehner (2002)
In spite of this vast amount of landmark-based information, with which Cataglyphis becomes acquainted during its foraging life, there is as yet no indication that the ants might use this information to acquire a "general landscape memory" as proposed for honey bees (Menzel et al. 2000), i.e. a geocentric representation in which the spatial relations between multiple places are defined in cartographic terms. The fact that this is not the case at least in Cataglyphis is perhaps most strikingly evident from the often convoluted and idiosyncratic layouts of the ants' route-based trajectories. These idiosyncrasies reflect the history of how an ant has acquired route information over time rather than the most efficient way for the ant to get from one place to another.
Orchestration of navigational modules
Procedural rather than unitary spatial representations: information acquired and used in navigation is bound together in procedural and largely context-dependent ways.
Let me now return to the more general question raised at the beginning about the operational structure of the ant's navigational toolkit. As we have seen, Cataglyphis displays a rich repertoire of spatial behaviours mediated by a suite of domain-specific processing modules and memory stores.
At this juncture, it is worth digressing somewhat for the sake of a broader perspective by raising the question whether different Cataglyphis species inhabiting differently structured desert environments vary in their spatial capabilities. Given the high costs of operating neural tissues, such variations are to be expected. For example, in food-storing parid and corvid birds those species which store more and for longer periods of time exhibit greater capabilities for spatial localization and storage (McGregor and Healy 1999; Biegler et al. 2001). They also exhibit larger volumes of the hippocampus—a brain area involved in the processing of spatial information—than congeneric species exhibiting inferior storing capacities (Brodbeck 1994; Healy and Krebs 1996; Basil et al. 1996). Similarly, male microtine rodents, which cover more ground in searching for females, solve laboratory maze problems more efficiently (Gaulin and Fitzgerald 1989) and have larger hippocampal volumes (Jacobs et al. 1990) than males of less far-ranging species.
A related question, of course, is whether species of desert ants that are phylogenetically distant from each other but occupy similar ecological niches (Fig. 11) have arrived at similar solutions to their spatial problems, and whether they have done so through independent selective processes rather than through shared though distant ancestry. These are intriguing questions for comparative evolutionary studies (Harvey and Pagel 1991) that might reveal further characteristics of processing modules and memory stores involved in navigation.
In praise of comparing differences. Three species of desert ants belonging to different genera, taxonomic tribes and even subfamilies, but occupying the same ecological niche of a thermophilic scavenger. Left: the northern African Cataglyphis bicolor (Formicinae: Formicini; location 34.6°N, 10.5°E); middle: the southern African Ocymyrmex velox (Myrmicinae; location 24.0°S, 15.6°E); right: the central Australian Melophorus bagoti (Formicinae: Melophorini; location 23.4°S, 133.4°E)
Let us now return from this digression to the main story. What are the ways by which the different navigational tools discussed in this essay are knitted together to produce coherent motor outputs? In the following, I shall argue that it is the contextual consistency of the environment and the ant's previous responses to it that form the major integrative bond.
In recent years, a considerable body of evidence for contextual learning has accumulated for bees and ants. First, the visual scenery experienced at a particular place, or a visual signpost leading to this place, can serve as a contextual cue. For example, honey bees (Menzel et al. 2001) and bumble bees (Colborn et al. 1999; Fauria et al. 2002) can simultaneously learn to choose different visual stimuli at two different places, even if the rewarded stimulus at one place is the unrewarded one at the other. In these cases, it is the landmark panorama that defines the place-specific contextual cue. Secondly, and more importantly in the present context, ants (Collett et al. 1998) and bees (small-scale laboratory experiments: Collett et al. 1993; Collett and Baron 1995; large-scale field experiments: Wehner et al. 1990; Menzel et al. 1998) can attach movement vectors to particular places. These vectors enable the animals to efficiently follow familiar landmark routes, along which one landmark memory can be retrieved after another. A further cue for such retrievals is the state of the ant's path integrator. Only after the homeward-directed global vector has been run off is the nest-site landmark memory activated. Furthermore, movement vectors are learned and recalled separately along the ant's outbound and inbound routes (Bisch-Knaden and Wehner 2003a) and, at the same place, during the ant's outbound and inbound motivational states (Bisch and Wehner 1999). Finally, memories of the same landmark configuration are more robust against extinction and/or suppression when they define a nesting site rather than part of a landmark route (Bisch-Knaden and Wehner 2003b). All of this makes sound ecological sense.
Taken together, these results lend support to the concept that the ants use landmarks as contextual cues to recall particular steering commands, i.e., to decide when to do what—or, more generally, that the ant's spatial knowledge is structured in a procedural way rather than in geocentred all-embracing spatial representations. The latter have been proposed for honey bees (Menzel et al. 2000, Menzel and Giurfa 2001). With these flying hymenopterans, multiple-feeder training paradigms (Wehner et al. 1990; Greggers and Mauelshagen 1997; Menzel et al. 1998) and hive-displacement experiments (Robinson and Dyer 1993) have shown that bees can simultaneously memorize a number of places and reach them from a number of other places. Digger wasps can do so as well (Baerends 1941). However, as intriguing as these spatial behaviours might be, any sound interpretation in terms of the questions raised in the present context has to await detailed recordings of the bees' flight trajectories (e.g. by using harmonic radar tracking: Riley and Smith 2002).
In conclusion, even the small-brained navigator Cataglyphis exhibits considerable flexibility in coupling and decoupling different modules and referential systems to and from each other. Hence, one might wonder how far the ants are able to combine vectors and places in novel and versatile ways. At present, however, I doubt that any such versatility would result in a cartographic representation of geometrical relations between places, in short: in a map in the ant's mind. From an evolutionary perspective it is exciting to see how these lessons from Cataglyphis and other insects are beginning to affect even the ways in which psychologists think about human spatial representations (Gallistel 2000, Wang and Spelke 2002).
References
Åkesson S, Wehner R (1997) Visual snapshot memory of desert ants, Cataglyphis fortis. Proc Neurobiol Conf Göttingen 25:482
Åkesson S, Wehner R (2002) Visual navigation in desert ants Cataglyphis fortis: are snapshots coupled to a celestial system of reference? J Exp Biol 205:1971–1978
Allman JM (1999) Evolving brains. Scientific American Library, New York
Baerends GP (1941) Fortpflanzungsverhalten und Orientierung der Grabwespe Ammophila campestris. Tijdschr Entomol 84:68–275
Basil JA, Kamil AC, Balda RP, Fite KV (1996) Differences in hippocampal volume among food storing corvids. Brain Behav Evol 47:156–164
Biegler R (2000) Possible use of path integration in animal navigation. Anim Learn Behav 28:257–277
Biegler R, McGregor A, Krebs JR, Healy SD (2001) A larger hippocampus is associated with longer-lasting spatial memory. Proc Natl Acad Sci USA 98:6941–6944
Bisch S, Wehner R (1998) Visual navigation in ants: evidence for site-based vectors. Proc Neurobiol Conf Göttingen 26:417
Bisch S, Wehner R (1999) Context-dependent retrieval of local vectors in desert ants. Proc Neurobiol Conf Göttingen 27:429
Bisch-Knaden S, Wehner R (2001) Egocentric information helps desert ants to navigate around familiar obstacles. J Exp Biol 204:4177–4184
Bisch-Knaden S, Wehner R (2003a) Local vectors in desert ants: context-dependent learning during outbound and inbound runs. J Comp Physiol A 189:181–187
Bisch-Knaden S, Wehner R (2003b) Landmark memories are more robust when acquired at the nest site than en route. Experiments in desert ants. Naturwissenschaften 90:127–130
Brines ML (1978) Skylight polarization patterns as cues for honeybee orientation: physical measurements and behavioural experiments. PhD thesis, Rockefeller University, New York
Brodbeck DR (1994) Memory for spatial and local cues: a comparison of a storing and a nonstoring species. Anim Learn Behav 22:119–133
Colborn M, Ahmad-Annuar A, Fauria K, Collett TS (1999) Contextual modulation of visuomotor associations in bumble-bees (Bombus terrestris). Proc R Soc Lond Ser B 266:2413–2418
Collett M, Collett TS, Bisch S, Wehner R (1998) Local and global vectors in desert ant navigation. Nature 394:269–272
Collett M, Collett TS, Wehner R (1999) Calibration of vector navigation in desert ants. Curr Biol 9:1031–1034
Collett TS, Baron J (1995) Learnt sensory-motor mappings in honeybees: interpolation and its possible relevance to navigation. J Comp Physiol A 177:287–298
Collett TS, Fry SN, Wehner R (1993) Sequence learning by honeybees. J Comp Physiol A 172:693–706
Collett TS, Collett M, Wehner R (2001) The guidance of desert ants by extended landmarks. J Exp Biol 204:1635–1639
Cornetz V (1910) Trajets de Fourmis et Retours au Nid. Paris: Institut Général Psychologique
Dyer FC, Gill M, Sharbowski J (2002) Motivation and vector navigation in honey bees. Naturwissenschaften 89:262–264
Fauria K, Dale K, Colborn M, Collett TS (2002) Learning speed and contextual isolation in bumblebees. J Exp Biol 205:1009–1018
Fent K (1985) Himmelsorientierung bei der Wüstenameise Cataglyphis bicolor: Bedeutung von Komplexaugen und Ocellen. PhD thesis, University of Zürich
Frisch K von (1949) Die Polarisation des Himmelslichts als orientierender Faktor bei den Tänzen der Bienen. Experientia 5:142–148
Frisch K von (1965) Tanzsprache und Orientierung der Bienen. Springer, Berlin Heidelberg New York
Gallistel CR (2000) The replacement of general-purpose learning models with adaptively specialized learning modules. In: Gazzaniga MS (ed) The new cognitive neurosciences. MIT Press, Cambridge, MA, pp 1179–1191
Gaulin SJC, FitzGerald RW (1989) Sexual selection for spatial learning ability. Anim Behav 37:322–331
Gould JL, Dyer FC, Towne WF (1985) Recent progress in the study of the dance language. Fortschr Zool 31:141–161
Grassé PP (1959) La reconstruction du nid et les coordinations interindividuelles chez Bellicositermes natalensis et Cubitermes sp. La théorie de la stigmergie: essai d'interprétation du comportement des termites constructeurs. Insectes Soc 6:41–83
Greggers U, Mauelshagen J (1997) Matching behavior of honeybees in a multiple-choice situation: the differential effect of environmental stimuli on the choice process. Anim Learn Behav 25:458–472
Hartmann G, Wehner R (1995) The ant's path integration system: a neural architecture. Biol Cybern 73:483–497
Harvey PH, Pagel MD (1991) The comparative method in evolutionary biology. Oxford University Press, Oxford
Healy SD, Krebs JR (1996) Food storing and the hippocampus in Paridae. Brain Behav Evol 47:195–199
Hemmi JM, Zeil J (2003) Robust judgement of inter-object distance by an arthropod. Nature 421:160–163
Homberg U, Würden S (1997) Movement-sensitive, polarization-sensitive and light-sensitive neurons of the medulla and accessory medulla of the locust, Schistocerca gregaria. J Comp Neurol 386:329–346
Jacobs LF, Gaulin SJC, Sherry DF, Hoffman GE (1990) Evolution of spatial cognition: sex-specific patterns of spatial behavior predict hippocampal size. Proc Natl Acad Sci USA 87:6349–6352
Judd SPD, Collett TS (1998) Multiple stored views and landmark guidance in ants. Nature 392:710–714
Kirschfeld K (1972) Die notwendige Anzahl von Rezeptoren zur Bestimmung der Richtung des elektrischen Vektors linear polarisierten Lichtes. Z Naturforsch 27C:578–579
Labhart T (1988) Polarization-opponent interneurons in the insect visual system. Nature 331:435–437
Labhart T (1999) How polarization-sensitive interneurones of crickets see the polarization pattern of the sky: a field study with an opto-electronic model neurone. J Exp Biol 202:757–770
Labhart T (2000) Polarization-sensitive interneurons in the optic lobe of the desert ant Cataglyphis bicolor. Naturwissenschaften 87:133–136
Labhart T, Lambrinos D (2001) How does the insect nervous system code celestial e-vector information? A neural model for "compass neurons". Proc Int Conf Invert Vis, p 66
Labhart T, Meyer EP (1999) Detectors for polarized skylight in insects: a survey of ommatidial specializations in the dorsal rim area of the compound eye. Microsc Res Tech 47:368–379
Labhart T, Petzold J, Helbling H (2001) Spatial integration in polarization-sensitive interneurons of crickets: a survey of evidence, mechanisms and benefits. J Exp Biol 204:2423–2430
Lambrinos D, Möller R, Labhart T, Pfeifer R, Wehner R (2000) A mobile robot employing insect strategies for navigation. Robot Autonom Syst 30:39–64
Loesel R, Homberg U (2001) Anatomy and physiology of neurons with processes in the accessory medulla of the cockroach Leucophaea madurae. J Comp Neurol 439:193–207
Martin RD (1981) Relative brain size and metabolic rate in terrestrial vertebrates. Nature 293:57–60
McGregor A, Healy SD (1999) Spatial accuracy in food-storing and nonstoring birds. Anim Behav 58:727–734
Menzel R, Giurfa M (2001) Cognitive architecture of a mini-brain: the honeybee. Trends Cogn Sci 5:62–71
Menzel R, Geiger U, Müller J, Joerges J, Chittka L (1998) Bees travel novel homeward routes by integrating separately acquired vector memories. Anim Behav 55:139–152
Menzel R, Brandt R, Gumbert A, Komischke B (2000) Two spatial memories for honeybee navigation. Proc R Soc Lond Ser B 267:961–968
Menzel R, Giurfa M, Gerber B, Hellstern F (2001) Cognition in insects: the honeybee as a study case. In: Roth G, Wullimann M (eds) Brain, evolution and cognition. Wiley, New York, pp 333–366
Müller M (1989) Mechanismus der Wegintegration bei Cataglyphis fortis (Hymenoptera, Insecta). PhD thesis, University of Zürich
Müller M, Wehner R (1988) Path integration in desert ants, Cataglyphis fortis. Proc Natl Acad Sci USA 85:5287–5290
Petzold J (2001) Polarisationsempfindliche Neuronen im Sehsystem der Feldgrille Gryllus campestris: Elektrophysiologie, Anatomie und Modellrechnungen. PhD thesis, University of Zürich
Pfeiffer K, Homberg U (2002) Visual signal processing in neurons of the anterior optic tubercle of the locust, Schistocerca gregaria. Zoology 105 [Suppl 5]:45
Pieron H (1904) Du rôle du sens musculaire dans l'orientation des fourmis. Bull Inst Gen Psychol Paris 4:168–186
Pieron H (1912) Le problème de l'orientation envisagé chez les fourmis. Scientia 12:217–243
Pomozi I, Horvath G, Wehner R (2001) How the clear-sky angle of polarization pattern continues underneath clouds: full-sky measurements and implications for animal orientation. J Exp Biol 204:2933–2942
Reynaud G (1898) Les lois de l'orientation chez les animaux. Rev Mondes 148:380–402
Riley JR, Smith AD (2002) Design considerations for an harmonic radar to investigate the flight of insects at low altitude. Comput Electron Agric 35:151–169
Robinson GE, Dyer FC (1993) Plasticity of spatial memory in honey-bees: reorientation following colony fission. Anim Behav 46:311–320
Rossel S, Wehner R (1984) How bees analyse the polarization patterns in the sky. Experiments and model. J Comp Physiol A 154:607–615
Santschi F (1913) Comment s'orientent les fourmis. Rev Suisse Zool 21:347–426
Sassi S, Wehner R (1997) Dead reckoning in desert ants, Cataglyphis fortis: can homeward-bound vectors be reactivated by familiar landmark configurations? Proc Neurobiol Conf Göttingen 25:484
Sommer S, Wehner R (2003) How does the precision of the ant's odometer depend on the distances travelled? Proc Neurobiol Conf Göttingen 29:364–365
Strausfeld NJ (1976) Atlas of an insect brain. Springer, Berlin Heidelberg New York
Strauss R (2002) The central complex and the genetic dissection of locomotor behaviour. Curr Opin Neurobiol 12:633–638
Vitzthum H, Müller M, Homberg U (2002) Neurons of the central complex of the locust Schistocerca gregaria are sensitive to polarized light. J Neurosci 22:1114–1125
Wang RF, Spelke ES (2002) Human spatial representation: insights from animals. Trends Cogn Sci 6:376–382
Wehner R (1968) Optische Orientierungsmechanismen im Heimkehrverhalten von Cataglyphis bicolor (Formicidae, Hymenoptera). Rev Suisse Zool 75:1076–1085
Wehner R (1982) Himmelsnavigation bei Insekten. Neurophysiologie und Verhalten. Neujahrsbl Naturforsch Ges Zürich 184:1-132
Wehner R (1987) Spatial organization of foraging behavior in individually searching desert ants, Cataglyphis (Sahara Desert) and Ocymyrmex (Namib Desert). In: JM Pasteels, J-L Deneubourg (eds) From individual to collective behavior in social insects. Birkhäuser, Basel, pp 15–42
Wehner R (1991) Visuelle Navigation: Kleinstgehirn-Strategien. Verh Dtsch Zool Ges 84:89–104
Wehner R (1994) The polarization-vision project: championing organismic biology. In: Schildberger K, Elsner N (eds) Neural basis of behavioural adaptation. Fischer, Stuttgart, pp 103–143
Wehner R, Flatt I (1972) The visual orientation of desert ants, Cataglyphis bicolor, by means of terrestrial cues. In: Wehner R (ed) Information processing in the visual systems of arthropods. Springer, Berlin Heidelberg New York, pp 295–302
Wehner R, Rossel S (1985) The bee's celestial compass—a case study in behavioural neurobiology. Fortschr Zool 31:11–53
Wehner R, Srinivasan MV (2003) Path integration in insects. In: Jeffery K (ed) Biological basis of navigation. Oxford University Press, Oxford (in press)
Wehner R, Strasser S (1985) The POL area of the honey bee's eye: behavioural evidence. Physiol Entomol 10:337–349
Wehner R, Wehner S (1990) Insect navigation: use of maps or Ariadne's thread? Ethol Ecol Evol 2:27–48
Wehner R, Harkness RD, Schmid-Hempel P (1983) Foraging strategies in individually searching ants, Cataglyphis bicolor (Hymenoptera: Formicidae). Akad Wiss Lit Mainz, Abh Math Naturwiss Kl. Fischer, Stuttgart
Wehner R, Bleuler S, Nievergelt C, Shah D (1990) Bees navigate by using vectors and routes rather than maps. Naturwissenschaften 77:479–482
Wehner R, Fukushi T, Wehner S (1992) Rotatory components of movement in high speed desert ants, Cataglyphis bombycina. Proc Neurobiol Conf Göttingen 20:303
Wehner R, Michel B, Antonsen P (1996) Visual navigation in insects: coupling of egocentric and geocentric information. J Exp Biol 199:129–140
Wehner R, Gallizzi K, Frei C, Vesely M (2002) Calibration processes in desert ant navigation: vector courses and systematic search. J Comp Physiol A 188:683–693
Acknowledgements
I am very grateful indeed to my graduate students Simone Bühlmann, David Andel, Katja Selchow and Martin Kohler for providing the data shown in Figs. 1B, 2, 7A and B, respectively, and to Dr. Ursula Menzi for her skillful cooperation in designing the figures. Financial support came from the Swiss National Science Foundation, the Human Frontier Science Program (HFSP), and the G. and A. Claraz Foundation. All experiments complied with current Swiss legislation concerning animal care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wehner, R. Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol A 189, 579–588 (2003). https://doi.org/10.1007/s00359-003-0431-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-003-0431-1