Abstract
Foragers of several species of stingless bees (Hymenoptera, Apidae and Meliponini) deposit pheromone marks in the vegetation to guide nestmates to new food sources. These pheromones are produced in the labial glands and are nest and species specific. Thus, an important question is how recruited foragers recognize their nestmates’ pheromone in the field. We tested whether naïve workers learn a specific trail pheromone composition while being recruited by nestmates inside the hive in the species Scaptotrigona pectoralis. We installed artificial scent trails branching off from trails deposited by recruiting foragers and registered whether newly recruited bees follow these trails. The artificial trails were baited with trail pheromones of workers collected from foreign S. pectoralis colonies. When the same foreign trail pheromone was presented inside the experimental hives while recruitment took place a significant higher number of bees followed the artificial trails than in experiments without intranidal presentation. Our results demonstrate that recruits of S. pectoralis can learn the composition of specific trail pheromone bouquets inside the nest and subsequently follow this pheromone in the field. We, therefore, suggest that trail pheromone recognition in S. pectoralis is based on a flexible learning process rather than being a genetically fixed behaviour.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An efficient food collection by foragers of stingless bee colonies, which in some species can contain more than 100,000 adult workers (Michener 2000), is of major importance to guarantee the survival of all individuals within the nest. To achieve effectiveness in resource collection, many species have evolved communication systems allowing workers to recruit additional nestmates to collect at resources they have discovered (reviewed in Nieh 2004; Barth et al. 2008). Stingless bees of the genera Trigona, Scaptotrigona, Geotrigona, Cephalotrigona and Oxytrigona deposit pheromone marks at food sources and in the vegetation on their way back to the nest to communicate the exact location of valuable food sources (Lindauer and Kerr 1958, 1960; Kerr 1960, 1969, 1973; Kerr and Cruz 1961; Kerr et al. 1963, 1981; Hebling et al. 1964; Cruz-Landim and Ferreira 1968; Blum 1970; Johnson 1987; Noll 1997; Schmidt et al. 2003; Nieh et al. 2003, 2004; Jarau et al. 2004, 2006, 2010; Sanchez et al. 2004; Aguilar et al. 2005; Schorkopf et al. 2007). As a result of the quick and precise recruitment of nestmates, some species can even compete with the recruitment efficiency of honey bees (Lindauer and Kerr 1958, 1960). In contrast to the waggle dance, however, trail pheromones allow to communicate the location of a food source in three dimensions (Lindauer and Kerr 1958, 1960; Nieh et al. 2004), which appears advantageous in the tropical habitats of stingless bees, where food sources often are located high up in trees. On the other hand, foraging workers of other competing colonies could use the information of foreign scent trails to reduce the time spent for searching for food, a behaviour commonly known as eavesdropping (Nieh et al. 2004; Slaa and Hughes 2009).
Stingless bee foragers secrete trail pheromones from their labial glands (Jarau et al. 2004, 2006, 2010; Schorkopf et al. 2007; Stangler et al. 2009; reviewed in Jarau 2009). After food uptake, a forager briefly lands on vegetation or stones and rubs her extended proboscis over the substrate in order to deposit a scent mark before continuing her flight back to the nest (Jarau et al. 2004). Several such scent marks are deposited at the food source and partially along the way back to the nest (Lindauer and Kerr 1958, 1960; Kerr et al. 1963; Johnson 1987; Nieh et al. 2003, 2004). Experiments with Trigona corvina (Jarau et al. 2010) and Scaptotrigona pectoralis (Hemmeter 2008; Jarau 2009) demonstrated that recruited bees preferred the trail pheromone from their nestmates as compared to the pheromone from foreign, conspecific foragers. This behavioural preference is explained by nest-specific quantitative differences in the composition of the respective trail pheromone constituents (Hemmeter 2008; Jarau et al. 2010). Thus, foragers of both species are able to recognize their nest-specific trail pheromone and to distinguish it from the pheromones secreted by foragers from other conspecific nests, which only differ in the relative composition of the same compounds.
To explain mechanisms involved in recognition processes in the context of social encounters, several hypotheses have been considered (Crozier 1987). In insect societies, much interest has been devoted to the phenotype-matching hypothesis (reviewed in Michener and Smith 1987; van Zweden and d’Ettorre 2010), which requires that the discriminating individual compares a cue with a learned template. For example, recruited bees may learn their actual colony-specific pheromone composition within the nest, for example during the recruitment process.
Alternatively, scent trail recognition by the bees may result from an innate ability to recognize the pheromone blend of their foraging sisters (see, e.g. Waytt 2010). Pheromone recognition would then result in a rather fixed communication mechanism.
Social bees are well known for their ability to learn different cues, especially in regard to orientation and foraging (von Frisch 1965; Roubik 1989; Barth 1991; Biesmeijer and Slaa 2004). Workers of honey bees (von Frisch 1965; Reinhard and Srinivasan 2009; Farina and Grüter 2009), bumble bees (Dornhaus and Chittka 1999, 2004; Molet et al. 2009) and stingless bees (Lindauer 1956; Aguilar 2004; Reichle et al. 2010) are able to associate food with particular odours, which they learn inside the nest. After leaving the nest, the workers’ previous odour experience then influences their food preferences in the field (honey bees: Arenas et al. 2007; bumble bees: Dornhaus and Chittka 1999, 2004; Molet et al. 2009; stingless bees: Lindauer and Kerr 1960; Aguilar et al. 2005; Reichle et al. 2010). Thus, it is possible that stingless bee recruits not only learn food odours from their nestmates, but they may also learn the composition of the trail pheromone bouquet during recruitment.
To test this, we investigated whether workers of Scaptotrigona pectoralis can learn the trail pheromone bouquets of foragers from foreign, conspecific colonies when they perceive them during recruitment within the nest and subsequently follow these pheromones in the field when searching for food.
Materials and methods
Bee nests and study site
We used two nests of Scaptotrigona pectoralis (de Dalla Torre 1896) (Hymenoptera, Apidae, Meliponini) that were collected in the surroundings of Atenas, Alajuela Province, Costa Rica. The experiments with the first nest (nest 1) were carried out between September 2008 and January 2009 in the bee garden of the Centre for Tropical Bee Research (CINAT) of the National University in Heredia, Costa Rica (9°58.377′N, 84°07.754′W). The experiments with the second nest (nest 2) were carried out in Atenas (9°58.558′N, 84°25.640′W) between February and April 2009. Both colonies were kept in wooden nest boxes. A small wooden box (10 × 10 × 4 cm; henceforth named “recruitment box”) was installed in front of the entrance of each colony. The exchange of food between foraging bees and bees within the hive mainly took place in these boxes, where the foreign trail pheromones were presented during the experiments, too (see below). The experiments were always carried out between 9.00 and 12.00 am because in the afternoon recruitment behaviour of the bees usually decreased.
Head glands
Foraging bees were collected at sugar water feeders and killed by freezing at −15°C. Their labial glands containing the trail pheromone (see “Introduction”) were dissected from the heads in saline solution under a stereo microscope. All tissues other than the glands were carefully removed. The glands were then left in solvent (hexane) for 24 h at room temperature, and the extracts were subsequently stored in a freezer (−15°C) unless they were in use in experiments. For the bioassays, we extracted ten pairs of cephalic labial glands in 1 ml hexane. Thus, 100 μl of extract corresponded to the gland content of one individual bee. During the tests with each nest, we used the trail pheromones from foragers of two different foreign colonies (trail pheromones A and B), which were collected in the surroundings of Atenas, Alajuela Province, in addition to the two colonies used for the bioassays.
Training period
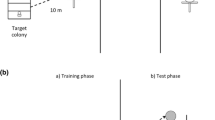
For each experiment, four bees were marked with colour (non-toxic on water basis) and trained to collect unscented 2 M sucrose solution at a training feeder (TF) at a distance of 20 m from the hive, widely following the training procedure described in Jarau et al. (2000). The feeders consisted of laboratory glass dishes (25 mm high, 40 mm in diameter) placed upside down on hexagonal plexiglass plates (75 mm in diameter) with 16 radially arranged grooves that allowed the bees to take up the sugar water. The training food source was presented on a wooden platform (10 × 7.5 × 2.5 cm) mounted on an iron rack (height: 62.5 cm). Importantly, recruitment never occurred during the training phase (due to the low-quality sugar solution).
Experimental procedure
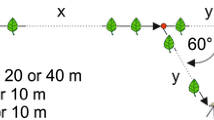
At its final location, the training feeder was replaced by a clean feeder (henceforth the recruitment feeder) containing an unscented 3 M sugar solution, at which free recruitment (Jarau et al. 2003) was allowed. As soon as the first forager began to scent-mark and to recruit nestmates from inside the nest, we installed an artificial scent trail between the nest and the recruitment feeder. The artificial trail branched off from the natural trail (bifurcation angle 60°) 10 m away from the nest and led to a test feeder that was identical to the recruitment feeder in appearance and food supply (see also Jarau et al. 2006 and Fig. 1 therein). The experimental trail consisted of ten short wooden sticks that were put into the ground at a distance of 1 m and baited with hexane (control experiments) or with the trail pheromone collected from foragers of a foreign, conspecific nest. We applied 10 μl of the solvent control or of a test substance (corresponding to 0.1 bee equivalent of labial gland extracts) to filter papers pinned on top of the wooden sticks and 30 μl to the test feeder itself. To test whether a specific trail pheromone blend can be learned by recruited bees within the nest, 50 μl labial gland extract containing the respective pheromone were presented on a filter paper inside the recruitment box simultaneously to the presentation of an artificial scent trail in the field. To prevent contamination, neither hexane (control) nor the foreign trail pheromone had direct contact with the wooden sticks of the artificial scent trail or with the wall of the recruitment box. Untreated wooden sticks were placed along the last 10 m towards the recruitment feeder (RF) in order to provide the same structural conditions as for the artificial trail.
Six experiments with nest 1 and five with nest 2 were carried out per setup (pheromones A and B, either with or without pheromone presentation in the nest) and with the hexane control treatment (making a total of 30 experiments with nest 1 and 25 with nest 2). On a particular day, a nest was used for no more than two experiments with a minimal recreation of 1 h between them. The order of the tested substances was randomized. Each trial lasted for 30 min, and the marks were renewed every 10 min. All bees arriving at the recruitment feeder for the first time were marked with colours (water based). The bees that followed the experimental trail and arrived at the test feeder were captured, colour marked, and only released after the end of the respective experiment. Only unmarked recruits, which had never visited a feeder during a preceding experiment (henceforth called newcomers), were considered for the statistical analyses. The percentage of bees that followed the experimental trail to the test feeder was calculated from the total number of newcomers that were counted at both feeders during an experiment (100% = sum of newcomers arriving at recruitment and test feeder). We excluded three experiments from the analysis, in which only 4, 7 and 8 newcomers were registered. With such small numbers the impact of single individuals on the result would be very high (25, 14.3 and 12.5%, respectively). In the remaining experiments, between 16 and 42 newcomers were captured.
Statistics
The data obtained in the experiments with a particular foreign trail pheromone differed between the two nests (2 × 2 χ2-analysis of contingency tables; all P values <0.05). Therefore, we separately analysed the data for the two nests. We applied non-parametric statistics because the data were not normally distributed. We compared the median percentage of redirected bees that arrived at the test feeders in the different experiments with Mann–Whitney U tests using SIGMASTAT 3.5 (Systat Software). Due to the multiple testing procedures, we applied a Benjamini–Hochberg correction to discover false positives.
Results
With the two nests, we tested a total of 1,304 individual bees in 55 experiments conducted. During the experiments, the new recruits followed the artificial scent trail in flight to finally reach the feeder without having direct contact with the pheromone marks. The newly recruited bees from both nests followed the artificial scent trail baited with a foreign trail pheromone significantly more often when the respective pheromone was simultaneously presented within the recruitment area of the nest as compared to experiments without pheromone presentation in the nest or to solvent control experiments, respectively (see Figs. 1, 2). This was true for both trail pheromones. The following results are given as medians with the first and third quartiles in brackets.
Percentage of newcomers from nest 1 that followed artificial scent trails baited with foreign trail pheromones, which were either presented in the nest atmosphere during the experiments or not. Boxes represent medians, whiskers the first and third quartiles, and n gives the total number of bees tested during the experiments under a given experimental condition (bees at RF + TF). The asterisks show significant differences to the solvent control experiments (P < 0.05). Significance levels (P value) calculated with Mann–Whitney U tests and Benjamini–Hochberg corrections
Percentage of newcomers from nest 2 that followed artificial scent trails baited with foreign trail pheromones, which were either presented in the nest atmosphere during the experiments or not. Boxes represent medians, whiskers the first and third quartiles, and n gives the total number of bees tested during the experiments under a given experimental condition (bees at RF + TF). The asterisks show significant differences to the solvent control experiments (P < 0.05). Significance levels (P value) calculated with Mann–Whitney U tests and Benjamini–Hochberg corrections
Nest 1
When the foreign trail pheromone A was presented within the recruitment box during the experiments, 58.0% (43.8/62.5) of the recruits followed the simultaneously installed artificial scent trail baited with the same pheromone. In contrast, without the presentation of the pheromone in the nest, only 5.3% (0.0/8.3) of the newcomers arrived at the test feeders. When trail pheromone B was used for the experiments, 24.3% (11.8/30.0) of the recruited bees were distracted by the artificial scent trail when the pheromone was presented in the nest, whereas only 1.6% (0.0/7.7) followed the pheromone trail when the respective extract was not presented in the nest. The percentage of redirected recruits was always significantly higher when the foreign trail pheromone used to bait the artificial trail was also presented in the recruitment box as compared to the experiments without presentation of the pheromone in the nest (pheromone A: U = 36, N 1 = N 2 = 6, P corr = 0.004; pheromone B: U = 32, N 1 = N 2 = 6, P corr = 0.039; Fig. 1). Furthermore, significantly more bees followed pheromone-baited trails when the pheromone was presented in the recruitment box as compared to solvent control trails (pheromone A: U = 36, N 1 = N 2 = 6, P corr = 0.004; pheromone B: U = 36, N 1 = N 2 = 6, P corr = 0.004; Fig. 1), whereas no significant differences were found between experiments without pheromone presentation and control experiments, respectively (pheromone A: U = 36, N 1 = N 2 = 6, P corr = 0.087; pheromone B: U = 25, N 1 = N 2 = 5, P corr = 0.180; Fig. 1).
Nest 2
Similar to nest 1, the simultaneous presentation of the pheromone used to bait the artificial scent trails within the nest had a significant effect on its attractiveness to newly recruited bees. Using trail pheromone A, 44.4% (43.6/52.0) of the recruits were distracted by the previously experienced pheromone, whereas 0.0% (0.0/8.6) followed the trails when the pheromone was not present within the recruitment box. The same was true for the trails with pheromone B, where more worker bees followed the artificial scent trails when the pheromone was presented in the recruitment box [with presentation 42.5% (41.0/47.6), without presentation 5.9% (0.0/6.7)]. For both pheromones, the differences between the percentages of redirected recruits during the experiments with and without their presentation within the recruitment box were statistically different (pheromone A: U = 25, N 1 = N 2 = 5, P corr = 0.016; pheromone B: U = 25, N 1 = N 2 = 5, P corr = 0.016; Fig. 2). Like in the experiments with nest 1, significantly more bees followed pheromone-baited trails when the pheromone was presented in the recruitment box as compared to solvent control trails (pheromone A: U = 36, N 1 = N 2 = 5, P corr = 0.016; pheromone B: U = 36, N 1 = N 2 = 5, P corr = 0.016; Fig. 2), whereas no significant differences were found between experiments without pheromone presentation and control experiments, respectively (pheromone A: U = 15, N 1 = N 2 = 5, P corr = 0.690; pheromone B: U = 17.5, N 1 = N 2 = 5, P corr = 0.413; Fig. 2).
Discussion
Previous studies have demonstrated that newly recruited workers of trail-laying stingless bees can be guided to a food source by an artificial scent trail baited with the trail pheromone (labial gland extracts) of their nestmate foragers (Jarau et al. 2006, 2010; Schorkopf et al. 2007; Stangler et al. 2009). Likewise, it was demonstrated that an artificial scent trail baited with the trail pheromone of a foreign, conspecific colony is not (Trigona corvina: Jarau et al. 2010) or only little (Scaptotrigona pectoralis: Hemmeter 2008) attractive to recruited bees when presented in similar experiments (see also Jarau 2009). Thus, foragers distinguish between the pheromone bouquets of workers from different nests. In our experiments, we could show that workers of Scaptotrigona pectoralis can learn a specific trail pheromone bouquet in their nest, even if it was taken from a foreign, conspecific colony. Later on, the bees use this information in order to find a food source marked with the respective pheromone in the field. The differences in the results obtained with nest 1 and 2 are interesting. There may be several explanations for this finding. The foreign trail pheromones may have been more similar to the pheromone of one of the two colonies, leading to different experiences with its compounds in the respective workers, or the individual bees tested varied in their learning capacities (for differences in learning capacities in bumble bees see, e.g. Raine and Chittka 2009). Furthermore, environmental conditions, like food availability at the two different sites, could have affected the experiments, too.
Similar to learning of flower odours in this species (Reichle et al. 2010), the presence of the foreign trail pheromone within the nest atmosphere is sufficient for learning. We, therefore, suggest that trail pheromone recognition is a flexible learning process rather than a genetically fixed trait. Despite the demonstration that S. pectoralis recruits can learn a foreign pheromone in their nest, more bees still followed their own trail pheromone in most of the experiments. Possibly, these bees perceived their nest-specific pheromone directly from a recruiting forager during trophallaxis and, thus, associated this pheromone bouquet with food. Unfortunately, the intranidal recruitment behaviour of trail-laying species of stingless bees remains largely unknown and certainly needs further observations. As a consequence, the questions of how, when, where and whether learning of the pheromone bouquet occurs during recruitment under natural circumstances remains elusive.
For food exploitation, in particular for a quick localization of new resources, it could be advantageous if the bees use the scent trail pheromones deposited by foragers from foreign colonies, or even of a different species, to minimize energetic costs otherwise spent with food searching. For example, workers of the aggressive stingless bee species, Trigona spinipes, can detect odour marks left at artificial sugar water feeders by another species, Melipona rufiventris (Nieh et al. 2004). In this case, the bees apparently profit from the information and save searching costs to reach new food sources by eavesdropping on the information of others (Nieh et al. 2004; Slaa and Hughes 2009). On the other hand, in unaggressive species that avoid competition, the ability to intercept odour messages from aggressive species could help to prevent serious attacks (Nieh et al. 2004). In their natural habitat, T. spinipes frequently takes over food sources from other species and aggressively drives them away, which classifies them as competition winners. In the present study, we have shown that Scaptotrigona pectoralis, which shows no aggression when collecting at food sources aside conspecific workers of other colonies (Johnson 1974; Biesmeijer and Slaa 2004), are able to learn and to use foreign trail pheromones. This makes sense because in this species, following the scent marks of a foreign colony is not associated with the risk of being killed in battles over food sources. However, the bees apparently do not generalize between their nestmates’ trail pheromone and that of foreign foragers. As our results show, they first have to learn a specific pheromone blend in order to use it as an indication for food sources. Trail pheromone learning is advantageous when the pheromone’s composition changes due to varying environmental conditions or to changes in the genetic structure of the nest population, e.g. due to the take over of the nest by a new queen.
In our experiments, the bees learned the foreign pheromone within their nest. Under natural conditions, they could learn it at food sources where they meet marking workers from other colonies. In that way, the bees could associate the scent marks from a foreign colony with food in the field, just as they do with floral odours inside the nest (honey bees: Gil and De Marco 2005; Arenas et al. 2007; Farina et al. 2007; bumble bees: Dornhaus and Chittka 1999, 2004; Molet et al. 2009; stingless bees: Lindauer and Kerr 1960; Aguilar et al. 2005; Reichle et al. 2010). The actual meaning of learnt odour information may then depend on positive or negative experience with the respective scent. A positive experience, such as undisturbed food collection at a rich food source, may animate a bee to follow the foreign scent marks (local enhancement), whereas a negative experience, such as aggressive attacks by the original discoverer of the food, may lead to subsequent avoidance of the respective odours (local inhibition). The influence of positive or negative experiences on a bee’s decision to visit or to avoid a food source, i.e. to lead to local enhancement or inhibition, has already been shown for scent marks deposited by foragers at food sources (Slaa and Hughes 2009). To fully understand the foraging ecology of stingless bee species that use pheromones for nestmate recruitment, learning processes at food sources have to be taken into account in future investigations. In particular, the way in which aggressive and non-aggressive species use pheromone markings from foreign, conspecific or heterospecific foragers could provide important insights into processes involved in resource partitioning and competitor avoidance in this important group of pollinators in tropical habitats.
References
Aguilar I (2004) Communication and recruitment for the collection of food in stingless bees: a behavioral approach. Dissertation, University of Utrecht
Aguilar I, Fonseca A, Biesmeijer JC (2005) Recruitment and communication of food source location in three species of stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 36:313–324
Arenas A, Fernández VM, Farina WM (2007) Floral odor learning within the hive affects honeybees’ foraging decisions. Naturwissenschaften 94:218–222
Barth FG (1991) Insects and flowers. The biology of a partnership. Princeton University Press, Princeton
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Physiol A 194:313–327
Biesmeijer JC, Slaa EJ (2004) Information flow and organization of stingless bee foraging. Apidologie 35:143–157
Blum MS (1970) The chemical basis of insect sociality. In: Beroza M (ed) Chemicals controlling insect behavior. Academic Press, New York, pp 61–94
Crozier RH (1987) Genetic aspects of kin recognition. In: Fletcher DJC, Michener CD (eds) Kin recognition in animals. Wiley, New York, pp 55–73
Cruz-Landim C, Ferreira A (1968) Mandibular gland development and communication in field bees of Trigona (Scaptotrigona) postica (Hymenoptera: Apidae). J Kansas Entomol Soc 41:474–481
de Dalla Torre CG (1896) Catalogus Hymenopterorum; Hucusque Descriptorum Systematicus et Synonymicus, vol 10. Engelmann, Leipzig, viii, pp 1–643
Dornhaus A, Chittka L (1999) Evolutionary origins of bee dances. Nature 401:38
Dornhaus A, Chittka L (2004) Information flow and regulation of foraging activity in bumble bees (Bombus spp.). Apidologie 35:183–192
Farina W, Grüter C (2009) Trophallaxis. A mechanism of information transfer. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC Press, Boca Raton, pp 183–197
Farina WM, Grüter C, Acosta L, Mc Cabe S (2007) Honeybees learn floral odors while receiving nectar from foragers within the hive. Naturwissenschaften 94:55–60
Gil M, De Marco RJ (2005) Olfactory learning by means of trophallaxis in Apis mellifera. J Exp Biol 208:671–680
Hebling NJ, Kerr WE, Kerr FS (1964) Divisao de trabalho entre operarias de Trigona (Scaptotrigona) xanthotricha Moure. Pap Avuls Dep Zool 16:115–127
Hemmeter K (2008) Wegpheromone und Futterrekrutierung bei der stachellosen Biene Scaptotrigona pectoralis (Apidae, Meliponini). Diploma thesis, University of Ulm, Ulm
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC Press, Boca Raton, pp 223–249
Jarau S, Hrncir M, Zucchi R, Barth FG (2000) Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata. I. Foraging at food sources differing in direction and distance. Apidologie 31:81–91
Jarau S, Hrncir M, Schmidt VM, Zucchi R, Barth FG (2003) Effectiveness of recruitment behavior in stingless bees (Apidae, Meliponini). Insect Soc 50:365–374
Jarau S, Hrncir M, Zucchi R, Barth FG (2004) A stingless bee uses labial gland secretions for scent trail communication (Trigona recursa Smith 1863). J Comp Physiol A 190:233–239
Jarau S, Schulz CM, Hrncir M, Francke W, Zucchi R, Barth FG, Ayasse M (2006) Hexyl decanoate, the first trail pheromone compound identified in a stingless bee, Trigona recursa. J Chem Ecol 32:1555–1564
Jarau S, Dambacher J, Twele R, Aguilar I, Francke W, Ayasse M (2010) The trail pheromone of a stingless bee, Trigona corvina (Hymenoptera, Apidae, Meliponini), varies between populations. Chem Senses 35(7):593–601
Johnson LK (1974) The role of agonistic behavior in the foraging strategies of Trigona bees. Doctoral thesis, University of California, Berkeley
Johnson LK (1987) Communication of food source location by the stingless bee Trigona fulviventris. In: Eder J, Rembold H (eds) Chemistry and biology of social insects. Verlag J. Peperny, Munich, pp 698–699
Kerr WE (1960) Evolution of communication in bees and its role in speciation. Evolution 14:386–387
Kerr WE (1969) Some aspects of the evolution of social bees (Apidae). Evol Biol 3:119–175
Kerr WE (1973) Sun compass orientation in the stingless bee Trigona (Trigona) spinipes (Fabricius, 1793) (Apidae). An Acad Bras Cienc 45:301–308
Kerr WE, Cruz CC (1961) Funções diferentes tomadas pela glândula mandibular na evolução das abelhas em geral e em “Trigona (Oxytrigona) tataira” em especial. Rev Bras Biol 21:1–16
Kerr WE, Ferreira A, de Mattos NS (1963) Communication among stingless bees—additional data (Hymenoptera: Apidae). J N Y Entomol Soc 71:80–90
Kerr WE, Blum M, Fales HM (1981) Communication of food source between workers of Trigona (Trigona) spinipes. Rev Brasil Biol 41:619–623
Lindauer M (1956) Über die Verständigung bei indischen Bienen. Z Vgl Physiol 38:521–557
Lindauer M, Kerr WE (1958) Die gegenseitige Verständigung bei den stachellosen Bienen. Z Vgl Physiol 41:405–434
Lindauer M, Kerr WE (1960) Communication between the workers of stingless bees. Bee World 41:29–41, 65–71
Michener CD (2000) The bees of the world. The Johns Hopkins University Press, Baltimore
Michener CD, Smith BH (1987) Kin recognition in primitively eusocial insects. In: Fletcher DJC, Michener CD (eds) Kin recognition in animals. Wiley, Chichester, pp 209–242
Molet M, Chittka L, Raine NE (2009) How floral odours are learned inside the bumblebee (Bombus terrestris) nest. Naturwissenschaften 96:213–219
Nieh JC (2004) Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35:159–182
Nieh JC, Contrera FAL, Nogueira-Neto P (2003) Pulsed mass recruitment by a stingless bee, Trigona hyalinata. Proc Biol Sci 270:2191–2196
Nieh JC, Contrera FAL, Yoon RR, Barreto LS, Imperatriz-Fonseca VL (2004) Polarized short odor-trail recruitment communication by a stingless bee, Trigona spinipes. Behav Ecol Sociobiol 56:435–448
Noll FB (1997) Foraging behavior on carcasses in the necrophagic bee Trigona hypogea (Hymenoptera: Apidae). J Insect Behav 10:463–467
Raine NE, Chittka L (2009) Measuring the adaptiveness of social insect foraging strategies. An empirical approach. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC Press, Boca Raton, pp 9–28
Reichle C, Jarau S, Aguilar I, Ayasse M (2010) Recruits of the stingless bee Scaptotrigona pectoralis learn food odors from the nest atmosphere. Naturwissenschaften 97:519–524
Reinhard J, Srinivasan MV (2009) The role of scents in honey bee foraging and recruitment. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC Press, Boca Raton, pp 165–182
Roubik DW (1989) Ecology and natural history of tropical bees. Cambridge University Press, Cambridge
Sanchez D, Nieh JC, Henaut Y, Cruz L, Vandame R (2004) High precision during food recruitment of experienced (reactivated) foragers in the stingless bee Scaptotrigona mexicana (Apidae, Meliponini). Naturwissenschaften 91:346–349
Schmidt VM, Zucchi R, Barth FG (2003) A stingless bee marks the feeding site in addition to the scent path (Scaptotrigona aff. depilis). Apidologie 34:237–248
Schorkopf DLP, Jarau S, Francke W, Twele R, Zucchi R, Hrncir M, Schmidt VM, Ayasse M, Barth FG (2007) Spitting out information: Trigona bees deposit saliva to signal resource locations. Proc Biol Sci 274:895–898
Slaa EJ, Hughes WHO (2009) Local enhancement, local inhibition, eavesdropping, and the parasitism of social insect communication. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC, Boca Raton, pp 147–164
Stangler ES, Jarau S, Hrncir M, Zucchi R, Ayasse M (2009) Identification of trail pheromone compounds from the labial glands of the stingless bee Geotrigona mombuca. Chemoecology 19:13–19
van Zweden JS, d’Ettorre P (2010) Nestmate recognition in social insects and the role of hydrocarbons. In: Blomquist GJ, Bagnères A-G (eds) Insect hydrocarbons. Biology, biochemistry, and chemical ecology. Cambridge University Press, New York, pp 222–243
von Frisch K (1965) Tanzsprache und Orientierung der Bienen. Springer, Berlin
Waytt T (2010) Pheromones and signature mixtures: defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J Comp Physiol A 196(10):685–700. doi:10.1007/s00359-010-0564-y
Acknowledgments
We would like to express our thanks to Javier Guevara from the Ministerio del Ambiente y Energia (MINAE) of Costa Rica, for his help in obtaining research permits (080-2006-SINAC, 133-2007-SINAC). This work was supported by a scholarship from the German Academic Exchange Service (DAAD) granted to CR. The present experiments comply with the “Principles of Animal Care” (publication No. 86-23, revised 1985) of the National Institutes of Health and the corresponding national current laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reichle, C., Aguilar, I., Ayasse, M. et al. Stingless bees (Scaptotrigona pectoralis) learn foreign trail pheromones and use them to find food. J Comp Physiol A 197, 243–249 (2011). https://doi.org/10.1007/s00359-010-0605-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-010-0605-6