Abstract
Foragers of several species of stingless bees deposit pheromone spots in the vegetation to guide recruited nestmates to a rich food source. Recent studies have shown that Trigona and Scaptotrigona workers secrete these pheromones from their labial glands. An earlier report stated that species within the genus Geotrigona use citral from their mandibular glands for scent marking. Since convincing experimental proof for this conjecture is lacking, we studied the glandular origin of the trail pheromone of Geotrigona mombuca. In field bioassays, newly recruited bees were diverted by artificial scent trails that branched off from the natural scent trail deposited by their nestmates only when they were baited with extracts from the foragers’ labial glands. Compounds extracted from the mandibular glands, however, did not release trail following behavior. This demonstrates that the trail pheromone of G. mombuca is produced in the labial glands, as in Trigona and Scaptotrigona. Furthermore, in chemical analyses citral was identified exclusively in the foragers’ mandibular glands, which disproves its supposed role as a trail pheromone. The labial glands contained a series of terpene- and wax type esters, with farnesyl butanoate as major constituent. We, therefore, postulate that the trail pheromone of G. mombuca is composed of a blend of esters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stingless bees, like other eusocial insects, developed communication systems in order to effectively allocate workers of the colony to the different tasks that need to be carried out (Wilson 1971). One important behavior in this connection is the recruitment of workers to rich food sources, which need to be exploited quickly and efficiently. The recruitment behavior of stingless bees is very diverse and ranges from a rather unspecific alerting of nest mates, without providing information about the spatial distribution of food sources, to a precise communication of the location of a particular resource by means of scent paths made of pheromone spots (Lindauer and Kerr 1958, 1960; Slaa et al. 2003b; Nieh 2004; Barth et al. 2008). The alerting of nestmates, which then leave the nest and search for food, is caused by foragers that run through the nest after their return from a successful search for food, jostle other bees and produce thorax vibrations, which are transmitted via direct body contact, via the substrate or as airborne sound (Lindauer and Kerr 1958, 1960; Hrncir et al. 2000, 2006a, b; Hrncir 2009). Recruiting foragers of the scent path laying species either deposit odor trails between the nest and a food source or lay short odor trails extending only a few meters from the food source towards the nest (Lindauer and Kerr 1958, 1960; Kerr et al. 1963; Nieh et al. 2003, 2004; for a detailed review see Jarau 2009).
Based on observations made by Lindauer and Kerr (1958, 1960), who reported that foragers rub their mandibles on the substrate while depositing a scent mark, it was assumed for a long time that the trail pheromones of stingless bees are produced in their mandibular glands. Therefore, all efforts to elucidate the chemical structures of trail pheromones of stingless bees had focused on the chemical analyses of mandibular gland secretions during the decades following Lindauer and Kerr’s observation. For example, Blum et al. (1970) claimed that the two stereoisomers of citral, geranial and neral, from workers’ mandibular glands are the main components of the trail pheromone of Geotrigona subterranea and suggested that this could be true for other Geotrigona species as well. However, Blum et al. (1970) did not conduct corresponding bioassays to test the effectiveness of either mandibular gland secretions or citral in releasing trail following behavior (see Discussion in Jarau et al. 2004; Jarau 2009). In fact, during experiments of Blum’s group, the bees showed aggressive behavior towards small citral impregnated wooden blocks that were presented to them rather than trail following behavior. Moreover, recent experiments have unequivocally shown that foragers of Trigona recursa (Jarau et al. 2004, 2006), T. spinipes (Schorkopf et al. 2007), T. corvina (Dambacher 2006; Dambacher et al. 2007), and Scaptotrigona pectoralis (Hemmeter 2008) produce trail pheromones in the labial glands and not in the mandibular glands. Newly recruited foragers of these species followed scent trails baited with labial gland extracts, whereas mandibular gland extracts did not trigger trail following behavior.
Recent advances in the understanding of stingless bee trail pheromone communication (see Jarau 2009) indicate that the involved volatiles in general originate from the labial glands of recruiting foragers. However, the possibility that different genera of stingless bees use secretions from different glands for scent trail marking can not be excluded without proper tests. In the present study we, therefore, examined the glandular origin of the trail pheromone of a species from the genus Geotrigona (G. mombuca) in order to test whether they indeed originate from the bees’ mandibular glands, as suggested by Blum et al. (1970). In addition, we analyzed the volatile compounds from foragers’ mandibular- and labial glands to re-evaluate the potential role of citral in the recruitment communication of this species.
Materials and methods
Study site and bee nests
All bioassays and preparations of gland extracts were done between October 2007 and March 2008 at the Ribeirão Preto Campus of the University of São Paulo, Brazil. For the experiments we used three colonies of Geotrigona mombuca (Smith, 1863), the nests of which are built in the ground and contain between 2000 and 3000 workers (Lindauer and Kerr 1960). The colonies were encountered on the University Campus and left at their natural sites. The species identity was confirmed by João M.F. Camargo.
Test substances for bioassays
We tested whether mandibular- and labial gland extracts or the pure solvent hexane (control) induce trail following behavior in recruited foragers of G. mombuca by means of bioassays applying artificial scent trails (see below).
For the preparation of gland extracts we used foragers collected at feeders containing sugar solution. The bees were freeze killed at −8°C, and their mandibular- and labial glands were carefully dissected in distilled water under a stereo microscope. To get neat extracts from a single gland type, all tissues other than the desired glands were carefully removed. We always prepared stock extracts made from five pairs of glands in 500 μl hexane, thus 100 μl contained the equivalent of the gland secretion from one bee. The extracts were prepared by leaving the glands in the solvent for 24 h at room temperature. Extracts from foragers of a certain nest were only used for bioassays with the same nest.
Bioassay set-up and experimental procedure
The general experimental procedure largely followed that of Schorkopf et al. (2007). Fifteen foragers (marked with acrylic paint on their thoraces) were trained to a training feeder, which contained unscented sugar solution in concentrations between 10% and 30%. The sugar concentration depended on the bees’ motivation to collect it, which varied considerably over the 6 months during which we conducted our experiments. The bees were trained from the nest entrance to the final distance (either 35, 45, 55, 65, 72, or 82 m away) by moving the feeder with foragers sitting on it in steps of a few meters in the desired direction (method after von Frisch 1967). Recruitment never occurred during the training phase. At its final location the training feeder was replaced by a clean recruitment feeder that contained a 50% sugar solution and always induced recruitment behavior in the trained bees.
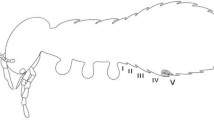
As soon as the first forager showed the conspicuous scent marking behavior that indicates the start of recruitment, we installed two artificial scent trails, which branched off from the bees’ natural scent trail 10 m before the final location of the recruitment feeder (henceforth termed branching point) at an angle of 60° (Fig. 1). Each experimental trail led to an experimental feeder, which was identical to the recruitment feeder in appearance and sugar solution concentration. The two trails consisted of five small twigs from a mango tree (Mangifera indica) put into the ground at intervals of 2 m. One of them was always baited with hexane, whereas the other one was baited with either labial- or mandibular gland extract. The sides of the control and extract trail were randomly exchanged on different days. The test substances were applied in an increasing concentration following the branching point (2, 4, 6, 8 and 10 μl on the twigs and 12 μl right on the feeder; with 10 μl corresponding to 0.1 bee equivalent). We applied higher concentrations of the test material at the feeders, because it is known from several species of stingless bees that the number of scent marks deposited by foragers is highest directly at the food source and decreases towards the nest (Kerr et al. 1963; Johnson 1987; Nieh et al. 2003, 2004). On the final 10 m of the natural scent trail, unbaited mango twigs were installed in order to provide an optically identical situation towards all feeders (Fig. 1). Each bioassay lasted for 30 min following the first application of hexane and a natural extract, which were renewed once after 15 min. Both gland extracts were tested against the control in seven independent trials each. We never carried out more than one experiment with a particular nest per day to allow for the recuperation of feeder naïve foragers. The entrances of the two nests not involved in a bioassay were always closed. The 15 trained foragers were allowed to collect sugar solution at the recruitment feeder and to recruit nest mates throughout the entire duration of an experiment. Newly recruited bees that alighted at the three feeders were immediately captured with a suction tube and kept imprisoned until the end of the respective trial. Trained foragers that reached one of the experimental feeders were also caught. In order to keep the number of recruiting bees constant they were replaced by a new bee, which was color marked and released at the recruitment feeder. After the end of an experiment all captured bees were color marked before they were released to allow their identification as experienced bees in subsequent experiments. For the analyses we only counted the unmarked recruits, which were inexperienced with regard to our bioassays, captured at the recruitment- and the two experimental feeders. Their distribution was determined by calculating the relative proportions at each food source (percentages from the total number of captured bees).
Set-up for the scent trail bioassays. Fifteen marked foragers collected sugar solution at the recruitment feeder (RF) and recruited additional bees from their nest. Two artificial scent trails, which led to experimental feeders (EF), branched off from the natural scent trail at the branching point (BP). One of these trails was always baited with the solvent hexane and the other one either with mandibular- or labial gland extract. The compounds were applied to short twigs put into the ground at intervals of 2 m (filled circles). To provide similar optical conditions towards all feeders, unbaited twigs were placed along the final 10 m towards RF (open circles). The distance from the nest to BP (X m) varied between the different experiments
Chemical analyses
For qualitative chemical analyses of the bees’ gland secretions we collected foragers from one nest at the feeding tables. The labial- and mandibular glands were dissected separately from 10 individuals as described above and left in 500 μl hexane for 24 h at room temperature. The compositions of the resulting extracts were compared by injecting 1 μl into a gas chromatograph (HP 5890, Hewlett Packard Series II, Palo Alto, CA, USA) equipped with a DB5 capillary column (30 m × 0.25 mm, 0.25 μm film thickness, J & W Scientific, Folson, CA, USA) and a FID (flame ionisation detector), with hydrogen as the carrier gas (2 ml/min constant linear flow rate). Injection was done in splitless mode with an initial temperature of 50°C, which was kept for 1 min. Subsequently, the splitter was opened, and the temperature was increased by 10°C per minute until the oven reached 310°C. The final temperature was kept for another 23 min.
Structure elucidation of the compounds was carried out by GC/MS analyses on a HP 6890 gas chromatograph (Hewlett Packard, Series, Palo Alto, CA) connected to a mass selective detector (GCMS, Agilent Quadrupol 5972). The GC parameters and column were the same as described above, with the difference that helium was used as the carrier gas (1.5 ml/min constant flow). To identify the compounds from the gland extracts, we compared their GC retention times and mass spectra with those of synthetic reference compounds from our stock collection under the same GC/MS conditions and with literature data (Bergström and Tengö 1974; Francke et al. 2000; NIST library).
Statistical analyses
All statistical analyses were done with the program SigmaStat 3.1 (Systat Software). Since not all of the bioassay data were normally distributed (Kolmogorov-Smirnov tests) and because of small sample sizes we generally applied nonparametric statistical analyses. A one-way ANOVA on ranks (Kruskal-Wallis test; Zar 1999) was used to test for statistically significant differences in the percentages of bees following their natural scent trails and the trails made of hexane or the gland extracts, respectively. Post-hoc comparisons between the groups were done with Student-Newman-Keuls tests. To compare the different percentages of bees reaching the recruitment feeders during the experiments testing labial- or mandibular gland extracts we conducted a Mann-Whitney test (Zar 1999).
Results
Bioassays
The majority of the recruited bees always followed the natural scent trails deposited by their nest mates. Interestingly, however, significantly less bees reached the recruitment feeder (RF) during the experiments when labial gland extracts were tested (74.0 ± 16.0% at RF; range: 53.5–96.2%) as compared to the tests involving mandibular gland extracts (90.7 ± 9.1% at RF; range: 76.5–100%) (Mann-Whitney test, N1 = N2 = 7, U = 7.0, P = 0.026; Fig. 2). Thus, more bees left the natural scent trails in favor of artificial trails baited with labial gland extract than with mandibular gland extract.
Comparison of the percentages of recruits captured at the recruitment feeder during the experiments testing mandibular- or labial gland extracts along one of the artificial scent trails. Bars give medians and whiskers the first and third quartile, respectively; N = number of individual recruits captured during all seven experiments conducted with each of the extracts; P-value calculated with a Mann-Whitney test
A comparison of the percentages of recruits that followed the artificial trails revealed a statistically significant difference in the effectiveness of labial gland extract, mandibular gland extract, and hexane in triggering trail following behavior (Kruskal-Wallis test, H2 = 8.044, P = 0.018). Significantly more bees followed the trails made of labial gland extracts (22.3 ± 15.7%; range: 3.5–41.9%) as compared to the solvent control trails (4.4 ± 4.2%; range: 0–11.4%) or to trails made of mandibular gland extracts (4.2 ± 3.3%; range: 0–8.8%) (Student-Newman-Keuls tests, q (labial-hexane) = 3.503, P < 0.05 and q (labial-mandibular) = 5.105, P < 0.05; Fig. 3). The number of bees that reached the experimental feeders at the end of trails made of mandibular gland extracts did not differ from the number of bees captured at the hexane feeders (Student-Newman-Keuls test, q (mandibular-hexane) = 0.0904, P > 0.05; Fig. 3).
Percentages of recruited bees that were captured at feeding tables at the end of artificial scent trails baited with the pure solvent hexane, mandibular gland extract, or labial gland extract. N = number of individual recruits captured during all seven experiments conducted with each of the extracts; P = significance levels for comparisons (Kruskal-Wallis and Student-Newman-Keuls tests)
Chemical analyses
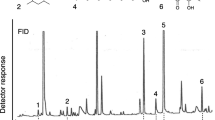
The mandibular gland extract was dominated by geranial and neral in a ratio of 3:1 and the aldehyde nonanal as the third main component (Fig. 4a). In contrast, the volatile bouquet of the labial glands was composed of a series of terpene- and wax type esters (Fig. 4b). The most abundant compound extracted from these glands was farnesyl butanoate, followed by neryl- and geranyl octanoate, octyl hexanoate, octyl octanoate, and octyl decanoate. The chemical structures of some minor compounds from both extracts remain to be elucidated.
Gas chromatograms of a mandibular gland extract (a) and a labial gland extract (b) prepared from Geotrigona mombuca foragers. The identified compounds are: 1—nonanal, 2—neral, 3—geranial, 4—octyl hexanoate, 5—decyl butanoate, 6—farnesol, 7—neryl hexanoate, 8—geranyl hexanoate, 9—octyl octanoate, 10—neryl octanoate, 11—geranyl octanoate, 12—octyl decanoate (+ an unidentified compound), 13—farnesyl butanoate, 14—neryl decanoate, 15—geranyl decanoate. Note that the two stereoisomers of citral, neral and geranial, are exclusively contained in the bees’ mandibular glands while corresponding esters are found in the labial glands
Importantly, the labial gland extract did not contain neral, geranial, or nonanal, whereas esters were completely absent in the mandibular gland extract, which results in highly specific compositions of the volatiles in the secretions of the two different glands (Fig. 4). Interestingly, esters of neral and geranial were found in the labial glands.
Discussion
During the bioassays, the majority of recruited bees always followed their natural scent trails to the recruitment feeder. This can be explained by a higher concentration of the bees’ pheromone near the food source visited by the trained bees, because we noted that they mainly deposited their scent marks directly at the food source and in its close proximity. This was also observed in G. subterranea by Blum et al. (1970). Scent marking in Geotrigona might rather lead to a strong signal that attracts the recruits around the food source rather than to a “scent trail” that guides them from the nest to the food. This situation probably was not imitated by the artificial scent trails in our experiments. It is also possible that the recruits were attracted to the recruitment feeders by cues provided by the 15 foragers that constantly visited them, i.e. by local enhancement (Slaa et al. 2003a), or that at least some of the recruited bees directly followed the experienced foragers to the recruitment feeders. Lindauer and Kerr (1958, 1960) already reported that newcomers of Geotrigona mombuca always appear at a food source in small groups that are guided by experienced foragers. We, therefore, assume that most of the newcomers that followed the natural scent-path in our experiments were piloted by recruiting foragers. A similar behavior was also observed in other scent trail laying stingless bees, such as Scaptotrigona postica (Lindauer and Kerr 1958, 1960), Trigona fulviventris (Johnson 1987) and T. corvina (Aguilar et al. 2005). Nevertheless, our bioassays clearly demonstrate that naïve recruits of Geotrigona mombuca can be attracted to short trails or feeders that bear labial gland extracts prepared from their nestmates, whereas compounds originating from the bees’ mandibular glands do not induce trail following behavior. We conclude from this finding that the trail pheromone of G. mombuca is produced in their labial glands, which is in accord with results of recent experiments conducted with Trigona recursa (Jarau et al. 2004, 2006), T. corvina (Dambacher 2006, Dambacher et al. 2007), T. spinipes (Schorkopf et al. 2007) and Scaptotrigona pectoralis (Hemmeter 2008). Therefore, it seems not to be premature to postulate that trail pheromones in stingless bees in general are produced in their labial glands. This is in contrast to earlier authors, who claimed that trail pheromones of stingless bees are produced in the mandibular glands, however, without providing convincing experimental proof (Lindauer and Kerr 1958, 1960; Kerr and da Costa Cruz 1961; Cruz-Landim and Ferreira 1968; Blum et al. 1970; Nieh et al. 2003).
Blum et al. (1970) suggested that their results with G. subterranea indicate that citral from the mandibular glands is used as trail pheromone by other Geotrigona species as well, but the glandular origin of the trail pheromone of G. mombuca was never investigated before. Although we did not test synthetic citral in our experiments we can definitely rule out that it acts as a trail pheromone in G. mombuca. Artificial trails baited with mandibular gland extracts, which are mainly composed of geranial and neral, did not induce trail following behavior in the recruits at all. The labial gland extracts, on the other hand, which were attractive in our bioassays, did not contain citral. Their secretions are dominated by esters, which is in accordance with known trail pheromone components of other trail laying stingless bees (Jarau et al. 2006; Dambacher 2006; Dambacher et al. 2007; Schorkopf et al. 2007; Hemmeter 2008; Jarau 2009). We did not yet carry out bioassays with single synthetic compounds and mixtures of synthetics to reveal which of the identified compounds make the trail pheromone of G. mombuca, but we postulate that it is composed of a blend of esters, as in T. recursa (Jarau et al. 2006) and T. corvina (Dambacher 2006; Dambacher et al. 2007). Alternatively, the major component from the labial glands of G. mombuca foragers, farnesyl butanoate, alone could be sufficient to guide recruits to a food source, as it was demonstrated for octyl octanoate in Trigona spinipes (Schorkopf et al. 2007). Farnesyl butanoate and related esters add a special facet to the picture, as these compounds represent a combination of a terpenoid with a fatty acid, in contrast to the hitherto known trail pheromones, which belong to the typical wax type.
A final interesting observation related to our chemical analyses worth to be mentioned here is that the volatile bouquet we detected in the gland extracts of G. mombuca largely differs from that reported for head extracts of this species by Francke et al. (2000). For example, these authors found octanal, whereas the mandibular gland extracts prepared in our study contained nonanal. The three most abundant compounds from our labial gland extracts, farnesyl butanoate, neryl octanoate and geranyl octanoate were not detected by Francke et al. (2000). Most likely, this apparent mismatch can be explained by the fact that “Geotrigona mombuca” comprises a species complex (João M.F. Camargo, pers. com.), and that the bees investigated by us and by Francke et al. (2000) in fact belonged to two different species. The composition of the volatile bouquets of cephalic glands, therefore, might be an useful chemotaxonomical character for the separation of species within the G. mombuca complex.
References
Aguilar I, Fonseca A, Biesmeijer JC (2005) Recruitment and communication of food source location in three species of stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 36:313–323
Barth FG, Hrncir M, Jarau S (2008) Signals and cues in the recruitment behavior of stingless bees (Meliponini). J Comp Physiol A 194:313–327
Bergström G, Tengö J (1974) Studies on natural odoriferous compounds, IX. Farnesyl- and geranyl esters as main volatile constituents of the secretion from Dufour’s gland in 6 species of Andrena (Hymenoptera, Apidae). Chemica Scripta 5:28–38
Blum MS, Crewe RM, Kerr WE, Keith LH, Garrison AW, Walker MM (1970) Citral in stingless bees: isolation and functions in trail-laying and robbing. J Insect Physiol 16:1637–1648
da Cruz-Landim C, Ferreira A (1968) Mandibular gland development and communication in field bees of Trigona (Scaptotrigona) postica. J Kans Entomol Soc 41:474–481
Dambacher J (2006) Nest specificity in the trail pheromone of a stingless bee, Trigona corvina (Apidae, Meliponini). Diplomarbeit, University of Ulm, Germany
Dambacher J, Jarau S, Twele R, Aguilar I, Francke W, Ayasse M (2007) Nest specific information in the trail pheromone of a stingless bee, Trigona corvina (Hymenoptera, Apidae, Meliponini). In: Proceedings of the 23rd Annual Meeting of International Society of Chemical Ecology, Jena, Germany, p 248
Francke W, Lübke G, Schröder W, Reckziegel A, Imperatriz-Fonseca V, Kleinert A, Engels E, Hartfelder K, Radtke R, Engels W (2000) Identification of oxygen containing volatiles in cephalic secretions of workers of Brazilian stingless bees. J Braz Chem Soc 11:562–571
Hemmeter K (2008) Wegpheromone und Futterrekrutierung bei der stachellosen Biene Scaptotrigona pectoralis (Apidae: Meliponini). Wissenschaftliche Arbeit für höheres Lehramt. University of Ulm, Germany
Hrncir M (2009) Mobilizing the foraging force—mechanical signals in stingless bee recruitment. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC Press, Taylor & Francis Group, Boca Raton, London, New York, in press
Hrncir M, Jarau S, Zucchi R, Barth FG (2000) Recruitment behavior in stingless bees, Melipona scutellaris and M. quadrifasciata. II. Possible mechanisms of communication. Apidologie 31:93–113
Hrncir M, Barth FG, Tautz J (2006a) Vibratory and airborne-sound signals in bee communication. In: Drosopoulos S, Claridge M (eds) Insect sounds and communication: physiology, behaviour, ecology, and evolution. CRC Press, Taylor & Francis Group, Boca Raton, London, New York, pp 421–436
Hrncir M, Schmidt VM, Schorkopf DLP, Jarau S, Zucchi R, Barth FG (2006b) Vibrating the food receivers: a direct way of signal transmission in bees (Melipona seminigra). J Comp Physiol A 192:879–887
Jarau S (2009) Chemical communication during food exploitation in stingless bees. In: Jarau S, Hrncir M (eds) Food exploitation by social insects. Ecological, behavioral, and theoretical approaches. CRC Press, Taylor & Francis Group, Boca Raton, London, New York, in press
Jarau S, Hrncir M, Zucchi R, Barth FG (2004) A stingless bee uses labial gland secretions for scent trail communication (Trigona recursa Smith 1863). J Comp Physiol A 190:233–239
Jarau S, Schulz CM, Hrncir M, Francke W, Zucchi R, Barth FG, Ayasse M (2006) Hexyl decanoate, the first trail pheromone compound identified in a stingless bee, Trigona recursa. J Chem Ecol 32:1555–1564
Johnson LK (1987) Communication of food source location by the stingless bee Trigona fulviventris. In: Eder J, Rembold H (eds) Chemistry and biology of social insects. Verlag Peperny, Munich, pp 698–699
Kerr WE, da Costa Cruz C (1961) Funções diferentes tomadas pela glândula mandibular na evolução das abelhas em general e em “Trigona (Oxytrigona) tataira” em especial. Rev Bras Biol 21:1–16
Kerr WE, Ferreira A, de Mattos NS (1963) Communication among stingless bees–additional data (Hymenoptera: Apidae). J NY Entomol Soc 71:80–90
Lindauer M, Kerr WE (1958) Die gegenseitige Verständigung bei den stachellosen Bienen. Z Vergl Physiol 41:405–434
Lindauer M, Kerr WE (1960) Communication between the workers of stingless bees. Bee World 41:29–41, 65–71
Nieh JC (2004) Recruitment communication in stingless bees (Hymenoptera, Apidae, Meliponini). Apidologie 35:159–182
Nieh JC, Contrera FAL, Nogueira-Neto P (2003) Pulsed mass recruitment by a stingless bee, Trigona hyalinata. Proc R Soc Lond B 270:2191–2196
Nieh JC, Contrera FAL, Yoon RR, Barreto LS, Imperatriz-Fonseca VL (2004) Polarized short odor-trail recruitment communication by a stingless bee, Trigona spinipes. Behav Ecol Sociobiol 56:435–448
Schorkopf DLP, Jarau S, Francke W, Twele R, Zucchi R, Hrncir M, Schmidt VM, Ayasse M, Barth FG (2007) Spitting out information: Trigona bees deposit saliva to signal resource locations. Proc R Soc B 274:895–898
Slaa EJ, Wassenberg J, Biesmeijer JC (2003a) The use of field-based social information in eusocial foragers: local enhancement among nestmates and heterospecifics in stingless bees. Ecol Entomol 28:369–379
Slaa EJ, Dijkstra MB, Biesmeijer JC, Sommeijer MJ (2003b) Social foraging in stingless bees: recruitment patterns and competitive dominance hierarchies. In: Slaa EJ (ed) Foraging ecology of stingless bees: from individual behavior to community ecology, pp 133–151. PhD Thesis, Utrecht University, The Netherlands
von Frisch K (1967) The dance language and orientation of bees, 2nd printing 1993. Belknap Press, Cambridge, MA
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge, MA
Zar JH (1999) Biostatistical analyses, 4th edn. Prentice-Hall, New Jersey
Acknowledgments
We are very grateful to Dirk Louis P. Schorkopf for his helpful comments on the procedure of the bioassays and to Sidnei Mateus for his great help with the experiments. We also thank João M.F. Camargo for the identification of the bees and Robert Twele for the preparation of synthetic compounds that were not commercially available, as well as for his advice concerning the GC/MS analysis. This study was supported by a grant from the German Academic Exchange Service (DAAD) to E.S.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stangler, E.S., Jarau, S., Hrncir, M. et al. Identification of trail pheromone compounds from the labial glands of the stingless bee Geotrigona mombuca . Chemoecology 19, 13–19 (2009). https://doi.org/10.1007/s00049-009-0003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-009-0003-0