Abstract
Purpose
Patients with localised renal cell carcinoma (RCC) receiving curative surgery, either radical or partial nephrectomy, have been shown in contemporary studies to develop recurrence within 5 years in 20–30% of case. Therefore, post-operative follow-up (FU) imaging plays a crucial role in detecting recurrent or metastatic disease. A number of prognostic scores have been developed to predict risk of recurrence. This review summarises the current knowledge on established FU protocols and their limitations.
Methods
A non-systematic literature search was conducted using Medline. Furthermore, major guidelines [European Association of Urology (EAU), American Urological Association (AUA) and National Comprehensive Cancer Network (NCCN)] were reviewed and assessed.
Results
The EAU, AUA and NCCN post-operative follow-up guidelines differ in the frequency and type of imaging modalities recommended. The optimal duration of follow-up remains to be elucidated as does the impact of follow-up protocols on patient outcomes and quality of life. Established follow-up protocols do not take non-RCC-related factors, such as patient age and performance status into account. However, in the future individualised duration of FU based on competing risks of cancer recurrence and non-RCC death may be optimised, maximising resources and patient quality of life.
Conclusion
There is a clear need to establish evidence-based follow-up protocols and to assess the impact of follow-up protocols on individual patients and society.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with localised renal cell carcinoma (RCC) receiving curative surgery, either radical nephrectomy (RN) or partial nephrectomy (PN), have been shown in contemporary studies to develop recurrence within 5 years in 20–30% of cases [1,2,3,4,5,6,7]. For these patients, a potential cure or prolonged survival is dependent on the possibility of resection of the local recurrence or metastases [8, 9]. Post-operative follow-up (FU) imaging, therefore, plays a crucial role in detecting recurrent or metastatic disease. To date, no superior strategy has been found to address optimal post-operative surveillance of these patients. Major guidelines such as the European Association of Urology (EAU), American Urological Association (AUA) and National Comprehensive Cancer Network (NCCN) use risk-stratified approaches for their FU recommendations and have been compared in several publications [10,11,12]. These guidelines all have 5 year FU recommendations, but differ in number and type of imaging procedures, and none take non-RCC-related factors such as patient age and performance status into account [13,14,15]. Furthermore, the 5-year FU time frames in each guideline have been based on the cumulative incidence of recurrence shown in previous studies [16,17,18]. In this review, we focus on the oncological aspects of FU and discuss different known FU protocols and their limitations.
Methods
A non-systematic literature search was conducted using Medline. The reference lists of selected manuscripts were checked manually for eligible articles. Furthermore, major guidelines (EAU, AUA, NCCN) were reviewed and assessed.
Results
Prediction of recurrence
For predicting risk of RCC recurrence, several contemporary risk stratification models based on both tumour node metastasis (TNM) classification and histopathological factors have been developed. Capogrosso et al. summarised the available models and analysed their utility for follow-up [19]. The University of California-Los Angeles Integrated Staging System (UISS) incorporates TNM stage, Fuhrman Grade, and Eastern Cooperative Oncology Group (ECOG) status to stratify patients according to their probability of RCC recurrence and survival [7]. Kattan et al. (Memorial Sloan-Kettering Cancer Centre; MSKCC) have developed a 5-year recurrence-free survival predictive nomogram based on symptoms at presentation, histologic subtype, tumour size and pT stage [20]. According to Cindolo et al. who compared both the Kattan and UISS model together with two other preoperative models in 2404 patients, the Kattan model was the most accurate risk assessment tool [21]. The Kattan model has later been modified with microvascular invasion as an additional parameter, but stratifies patients with clear cell RCC only [22]. Finally, the Leibovich score developed at the Mayo Clinic predicts 1-, 3-, 5-, 7- and 10-year risk of recurrence in patients treated for localised RCC [23]. It is based on TNM stage, Fuhrman Grade and presence of tumour necrosis albeit only aimed at clear cell RCC subtypes. A variant of the Leibovich risk score is the tumour stage, size, grade and necrosis (SSIGN score) estimating cancer-specific survival rather than the risk of recurrence. In addition, the Sorbelini nomogram has been developed making use of similar risk factors. Nevertheless, the Kattan nomogram, UISS score and Leibovich risk model are most frequently used. In addition, these three models have been externally validated having a concordance index (C-index) of 74–84% [24].
Follow-up protocols
Prior to combining both TNM and histopathological factors in predictive models, some FU protocols based on TNM alone have previously been proposed although these studies have a low level of evidence [25, 26]. More contemporary recurrence risk models, such the UISS, MSKCC and Leibovich score, have led to newer risk-stratified FU protocols.
Lam et al. used the UISS model to stratify 559 patients surgically treated for localised RCC into four risk groups and suggest a FU protocol based on time to recurrence (TTR) and recurrence sites [7]. They suggested low-risk patients to be followed for 5 years with annual chest computed tomography (CT) and abdominal CT at 2 and 4 years after primary surgery. This was based on low-risk patients having recurrence most frequently in the lungs with one-third of these within the first year, while abdominal recurrence developed later (median TTR 32 months). For intermediate-risk patients with a higher recurrence rate (32%), especially within the first year, recurrences in the lung and abdomen were found in 74.4 and 58.1%, respectively. Therefore, chest CT every 6 months for 3 years and annually thereafter was proposed together with abdominal CT at 1 year after surgery and every other year thereafter. For high-risk patients, recurrence rates were the highest, recurrences occurred frequently within 6 months and were more aggressive. As such, chest CT every 6 months for the first 3 years and annually thereafter was suggested with the option of alternating CT and plain chest X-ray after 3 years. For high-risk patients, abdominal CT was recommended every 6 months, the first 2 years and annually thereafter. Finally, a forth risk group consisting of patients with positive lymph nodes at primary surgery (i.e. TanyN1-2M0) was defined. These patients presented with more aggressive and early recurrence lead the authors to suggest a vigorous surveillance with both chest and abdominal CT at 3, 6, 12, 18, and 24 months followed by annual CTs thereafter.
Also, based on the UISS, a long-term FU protocol by Antonelli et al. in 2007 is taking into account the risk of late recurrence [27]. In the study, 814 patients were stratified based on UISS into three risk groups: low, intermediate and high risk and followed for a mean time of 6.3 (range 0.33–24.6) years. Based on recurrence sites and TTR, they concluded a more simplified FU imaging protocol for low, intermediate and high-risk patients. For low-risk patients, recommendation was chest CT every 30 months and annual abdominal CT the first 5 years followed by chest and abdominal CT every 30 months until the tenth year and only abdominal CT every 5 years thereafter. For intermediate-risk patients, the recommendation was chest and abdominal CT every 6 months the first 5 years, chest CT annually and abdominal CT every 30 months the following 5 years and finally abdominal CT every 5 years thereafter. For high-risk patients, chest and abdominal CT was suggested every 6 months for the first 5 years, annually the following 5 years and only abdominal CT thereafter.
Siddiqui et al. proposed tailored FU protocols for clear cell, papillary and chromophobe RCC subtypes based on 2339 patients treated for localised RCC at the Mayo Clinic [28]. Using a Cox multiple regression model, they identified independent pathological features (surgical margin, tumour stage and size, lymph node status, Fuhrman grade and tumour necrosis) determining recurrence risk in the abdomen and thorax, respectively. Based on the severity of these features, patients were scored and stratified into low, intermediate and high-risk groups. For clear cell RCC, if receiving 0 points in the thoracic score algorithm, a minimal risk subgroup was available. For papillary and chromophobe RCC, only tumour stage and grade stood out as independent prognostic factors. Finally for each subtype, a tailored and detailed imaging surveillance algorithm for chest and/or abdominal imaging was recommended depending on patient’s risk score. Imaging frequency was between 3 and 18 month intervals for the first 3 years depending on risk score and subtype.
More recently, a publication by Stewart-Merrill et al. highlighted a novel approach to FU by recognising age and co-morbidity, in a competing risk analysis model, as important co-factors in determining length and necessity of FU [29]. In their retrospective analysis, 2511 patients with localised RCC treated with curative intent were stratified based on TNM stage (T1Nx-0M0, T2Nx-0M0, T3/4Nx-0M0 and TanyN1M0), age (< 50, 50–59, 60–69, 70–79, ≥ 80 years), Charlson co-morbidity index (CCI ≤ 1 or ≥ 2) and site of recurrence (chest, abdomen, bone, other). A Weibull distribution model, which contrary to Cox’s proportional hazard regression model, allows for both estimation of hazard ratio and relative increase or decrease of risk of event over time, was used to determine risk of recurrence but also risk of non-RCC death. Recurrence rates for each TNM stage and site were in accordance with previous published data [1]. They concluded that when age and co-morbidity are accounted for in determining length of FU, risk of non-RCC death exceeds that of risk for RCC recurrence at different time points, suggesting shorter durations of surveillance the older and the higher CCI the patient has. Based on the above, the authors suggest a novel approach for individualising duration of FU and they argue that for some patients (e.g. T2N0 patients with CCI ≥ 2 and over 80 years old) the risk of non-RCC death exceeds risk of recurrence as early as 30 days after primary surgery making any FU redundant.
Follow-up protocols endorsed by major guidelines
The FU protocols of the EAU, AUA and NCCN are been summarised in Table 1 [10]. The EAU guideline FU protocol recommends risk stratification of patients based on prognostication scoring tools such as the UISS to stratify patients into low, intermediate and high-risk score groups. The NCCN and AUA FU recommendations are based solely on TNM stage. The most recent and comprehensive assessment of these guidelines has been done by Lobo et al. [11]. Using a simulation model, they evaluated cost, radiation exposure and recurrence detection accuracy of each guideline protocol and concluded that for local recurrence and distant metastases, when adhering to AUA or NCCN recommendations on FU, 59 and 63% of low-risk patients’ local and distant recurrence, respectively, would be missed due to FU duration less than 3 years. In the EAU guidelines, adherence to protocol would render 95% detection of low-risk recurrences. For high-risk patients, the detection rate for all types of recurrence was > 92% for all three guideline FU recommendations.
Duration of FU is discussed within each guideline, and a minimum of at least 5-year duration based on recurrence risks is recommended [17, 25]. Although most recurrences occur within this time frame, several studies have shown late RCC recurrences [18, 30, 31]. Stewart et al. have assessed the detection of recurrence based on either the AUA or NCCN recommendations and found that discharging patients from surveillance after 5 years or earlier may result in a rate of up to 33% of non-detected recurrences depending on the risk profile [32].
As another example, Kim et al. showed that between 5 and 15% of patients had late recurrence detected 10 years after the initial 5-year FU [18]. It has been indicated that these patients may have a less aggressive tumour and more likely are asymptomatic [33]. Therefore, detection of these patients is still perhaps warranted as some studies indicate that metastasectomy of late recurrences may prolong the overall survival or even provide cure [8].
The optimal duration of follow-up remains to be elucidated. As Stewart-Merrill et al. point out in their Weibull model, there is a strong need to not only address FU based on TNM and histopathological parameters but also address patient age and co-morbidities [29]. Indeed, life expectancy should play a major role in determining the frequency and duration of a FU protocols, and also is a determinant in reducing health care costs in countries where health services are an integral part of the state system. In addition, certain patient populations among low-risk groups may have an extremely low likelihood of recurrence. Further investigation is warranted, comparing guideline recommendations to a more evidence-based approach with age and co-morbidity standardised protocols.
Choice of follow-up imaging modality
Regarding type of imaging performed, chest and abdominal CT is today’s gold standard, especially for thoracic imaging as cross-sectional topography allows for more detailed and earlier detection of metastases compared to conventional X-ray [34]. Ultrasound (US) and magnetic resonance imaging (MRI) are also recommended in all three guidelines, but play an auxiliary role to reduce radiation or are used when CT with intravenous contrast is not suitable. Brain and bone metastases are symptomatic in most cases, and FU imaging should be tailored if symptoms arise but is not recommended on a routine basis [6]. Fluorodeoxyglucose positron emission tomography (FDG-PET) combined with CT is showing some promise in the detection of RCC recurrence as both spatial and functional aspects of tumours are visualised. However, in a recent meta-analysis of FDG-PET for detection of RCC malignancy, the pooled sensitivity and specificity were 79 and 90%, respectively [35]. FDG-PET has been shown to have limitations in detecting lesions smaller than two centimetres but has a higher potential for detecting more aggressive tumours [36, 37]. Novel approaches with immuno-PET are showing promise with the girentuximab chimeric antibody to carbonic anhydrase IX in detecting localised clear cell RCC, occult regional lymph node metastases and distant metastases [38,39,40].
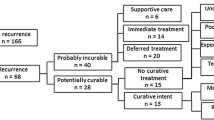
EAU guidelines advise cross-sectional imaging (CT or MRI) as the imaging modality of choice for follow-up of RCC and discourage the use of conventional X-ray. Beisland et al. [41] recently analysed the pattern of follow-up imaging in 1612 patients and explored if more frequent use of cross-sectional imaging improves survival. The patients were from the RECUR database setup to develop comparators between pattern of recurrence and type of FU applied for the EAU RCC guidelines [42]. A particular feature of the protocol used was to distinguish recurrences according to how they were managed. Potentially, curable recurrences were defined as single-, local- or oligo-recurrences at a single site amenable to local therapies such as metastasectomy or radiation, whereas probably incurable recurrences were multiple and at multiple sites treated by systemic therapy or observation. Of the 17,333 FU imaging procedures performed, 7953 were CT (46%), 6540 (38%) were conventional X-rays, 183 (1%) were MRI and 2651 (15%) were US. In general, cross-sectional imaging was more frequently used in high-risk patients. However, there was no significant difference in the overall survival between patients with potentially curable or probably incurable recurrences based on type of imaging with which the recurrence was detected.
Impact of follow-up on patient outcomes
The rationale for surveillance of patients curatively treated for localised RCC is to detect recurrence within a window of opportunity in which surgery, radiotherapy or ablative techniques are possible either to cure or prolong the patient’s life. Current studies, which have been reviewed above, have not yet shown that stratification into risk groups and follow-up based on risk-adapted protocols help us find more potentially curable patients or prolong survival. A major reason for this lack of data is the fact that most studies used detection of metastasis or recurrence as their final objective to investigate the quality of the respective follow-up protocols. However, without investigating if further management or intervention after detection changes the natural course of the disease this question cannot be answered. To this effect, the EAU RCC guideline panel has set up the RECUR protocol to retrospectively capture data and develop comparators to guide future FU recommendations. A first analysis confirms that FU should be tailored to risk of recurrence and age [42]. Another way of addressing this issue would be to stipulate evidence-based FU protocols that could then be compared to each other in terms of overall- or cancer-specific survival or delay of systemic therapy in a randomised controlled trial (RCT) setting. Alternatively, the link between optimal FU protocol and association of any potential survival benefit could be addressed by cohort studies which allow comparisons of patterns and time of recurrence stratified by risk groups in patients with different imaging frequencies, recurrence burden and intended recurrence treatment.
Although cross-sectional imaging improves recurrence detection compared to conventional X-ray, there may be a subgroup of patients who would benefit from avoiding CT imaging during their FU. Age, kidney function, RCC risk group and co-morbidities perhaps render cross-sectional imaging redundant in a subgroup of patients. Without data supporting a change of the natural course of the disease by cross-sectional imaging, we cannot rule out that better resolution imaging simply leads to earlier diagnosis without changing prognosis and survival. Also for patients with risk of late recurrence, perhaps a post 5-year FU period clinical appraisal (including physical examination and laboratory blood tests) instead of perfunctory annual imaging is more suitable to avoid excessive radiation exposure and costs. Retrospective comparative (between risk groups, age groups, co-morbidity levels etc.) analysis of prognostic risk factors associated with benefit of avoiding cross-sectional imaging may be performed alternatively; the same could be setup in a RCT setting where physician discretion (control) could be compared to a risk-factor-based algorithm where patient is randomised either to cross-sectional imaging, conventional imaging or to clinical follow-up. In addition, patients’ preference for attributes of FU schedules and quality of life can be explored in the trials.
References
Dabestani S, Thorstenson A, Lindblad P, Harmenberg U, Ljungberg B, Lundstam S (2016) Renal cell carcinoma recurrences and metastases in primary non-metastatic patients: a population-based study. World J Urol 34:1081–1086
Kuijpers YA, Meijer RP, Jonges GN, de Jong J, Bosch JL, Horenblas S, Bex A (2016) Potentially curable recurrent disease after surgically managed non-metastatic renal cell carcinoma in low-, intermediate- and high-risk patients. World J Urol 34:1073–1079
Mouracade P, Kara O, Dagenais J, Maurice MJ, Nelson RJ, Malkoc E, Kaouk JH (2017) Perioperative morbidity, oncological outcomes and predictors of pT3a upstaging for patients undergoing partial nephrectomy for cT1 tumors. World J Urol 35:1425–1433
O’Malley RL, Hayn MH, Brewer KA, Underwood W 3rd, Hellenthal NJ, Kim HL, Sorokin I, Schwaab T (2015) Cancer control of partial nephrectomy for high-risk localized renal cell carcinoma: population-based and single-institutional analysis. World J Urol 33:1807–1814
Simone G, Tuderti G, Anceschi U, Papalia R, Ferriero M, Misuraca L, Minisola F, Mastroianni R, Costantini M, Guaglianone S, Sentinelli S, Gallucci M (2017) Oncological outcomes of minimally invasive partial versus minimally invasive radical nephrectomy for cT1-2/N0/M0 clear cell renal cell carcinoma: a propensity score-matched analysis. World J Urol 35:789–794
Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Leibovich BC, Zincke H (2003) A multifactorial postoperative surveillance model for patients with surgically treated clear cell renal cell carcinoma. J Urol 170:2225–2232
Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS (2005) Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol 174:466–472
Dabestani S, Marconi L, Hofmann F, Stewart F, Lam TBL, Canfield SE, Staehler M, Powles T, Ljungberg B, Bex A (2014) Local treatments for metastases of renal-cell carcinoma: a systematic review. Lancet Oncol 15:e549–e561
Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML (2011) Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 117:2873–2882
Marconi L, Gorin MA, Allaf ME (2015) Post partial nephrectomy surveillance imaging: an evidence-based approach. Curr Urol Rep 16:23
Lobo JM, Nelson M, Nandanan N, Krupski TL (2016) Comparison of renal cell carcinoma surveillance guidelines: competing trade-offs. J Urol 195:1664–1670
Beisland C, Guethbrandsdottir G, Reisaeter LA, Bostad L, Hjelle KM (2016) A prospective risk-stratified follow-up programme for radically treated renal cell carcinoma patients: evaluation after eight years of clinical use. World J Urol 34:1087–1099
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, Volpe A, Bex A (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913–924
Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, Choueiri TK, Figlin RA, Fishman M, Hancock SL, Hudes GR, Jonasch E, Kessinger A, Kuzel TM, Lange PH, Levine EG, Margolin KA, Michaelson MD, Olencki T, Pili R, Redman BG, Robertson CN, Schwartz LH, Sheinfeld J, Wang J (2009) NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Cancer Netw 7:618–630
Donat SM, Diaz M, Bishoff JT, Coleman JA, Dahm P, Derweesh IH, Herrell SD 3rd, Hilton S, Jonasch E, Lin DW, Reuter VE, Chang SS (2013) Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 190:407–416
Klatte T, Lam JS, Shuch B, Belldegrun AS, Pantuck AJ (2008) Surveillance for renal cell carcinoma: why and how? When and how often? Urol Oncol 26:550–554
Ljungberg B, Alamdari FI, Rasmuson T, Roos G (1999) Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int 84:405–411
Kim SP, Weight CJ, Leibovich BC, Thompson RH, Costello BA, Cheville JC, Lohse CM, Boorjian SA (2011) Outcomes and clinicopathologic variables associated with late recurrence after nephrectomy for localized renal cell carcinoma. Urology 78:1101–1106
Capogrosso P, Capitanio U, La Croce G, Nini A, Salonia A, Montorsi F, Bertini R (2016) Follow-up after treatment for renal cell carcinoma: the evidence beyond the guidelines. Eur Urol Focus 1:272–281
Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P (2001) A postoperative prognostic nomogram for renal cell carcinoma. J Urol 166:63–67
Cindolo L, Patard JJ, Chiodini P, Schips L, Ficarra V, Tostain J, de La Taille A, Altieri V, Lobel B, Zigeuner RE, Artibani W, Guille F, Abbou CC, Salzano L, Gallo C (2005) Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer 104:1362–1371
Sorbellini M, Kattan MW, Snyder ME, Reuter V, Motzer R, Goetzl M, McKiernan J, Russo P (2005) A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol 173:48–51
Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, Weaver AL, Parker AS, Zincke H (2003) Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer 97:1663–1671
Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, Pantuck AJ, Zigeuner R, Karakiewicz PI (2011) Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 60:644–661
Sandock DS, Seftel AD, Resnick MI (1995) A new protocol for the followup of renal cell carcinoma based on pathological stage. J Urol 154:28–31
Hafez KS, Novick AC, Campbell SC (1997) Patterns of tumor recurrence and guidelines for followup after nephron sparing surgery for sporadic renal cell carcinoma. J Urol 157:2067–2070
Antonelli A, Cozzoli A, Zani D, Zanotelli T, Nicolai M, Cunico SC, Simeone C (2007) The follow-up management of non-metastatic renal cell carcinoma: definition of a surveillance protocol. BJU Int 99:296–300
Siddiqui SA, Frank I, Cheville JC, Lohse CM, Leibovich BC, Blute ML (2009) Postoperative surveillance for renal cell carcinoma: a multifactorial histological subtype specific protocol. BJU Int 104:778–785
Stewart-Merrill SB, Thompson RH, Boorjian SA, Psutka SP, Lohse CM, Cheville JC, Leibovich BC, Frank I (2015) Oncologic surveillance after surgical resection for renal cell carcinoma: a novel risk-based approach. J Clin Oncol 33:4151–4157
Lam JS, Shvarts O, Leppert JT, Figlin RA, Belldegrun AS (2005) Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol 173:1853–1862
Capitanio U, Cloutier V, Zini L, Isbarn H, Jeldres C, Shariat SF, Perrotte P, Antebi E, Patard JJ, Montorsi F, Karakiewicz PI (2009) A critical assessment of the prognostic value of clear cell, papillary and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int 103:1496–1500
Stewart SB, Thompson RH, Psutka SP, Cheville JC, Lohse CM, Boorjian SA, Leibovich BC (2014) Evaluation of the National Comprehensive Cancer Network and American Urological Association renal cell carcinoma surveillance guidelines. J Clin Oncol 32:4059–4065
Adamy A, Chong KT, Chade D, Costaras J, Russo G, Kaag MG, Bernstein M, Motzer RJ, Russo P (2011) Clinical characteristics and outcomes of patients with recurrence 5 years after nephrectomy for localized renal cell carcinoma. J Urol 185:433–438
Doornweerd BH, de Jong IJ, Bergman LM, Ananias HJ (2014) Chest X-ray in the follow-up of renal cell carcinoma. World J Urol 32:1015–1019
Wang HY, Ding HJ, Chen JH, Chao CH, Lu YY, Lin WY, Kao CH (2012) Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging 12:464–474
Majhail NS, Urbain JL, Albani JM, Kanvinde MH, Rice TW, Novick AC, Mekhail TM, Olencki TE, Elson P, Bukowski RM (2003) F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol 21:3995–4000
Kumar R, Shandal V, Shamim SA, Jeph S, Singh H, Malhotra A (2010) Role of FDG PET-CT in recurrent renal cell carcinoma. Nucl Med Commun 31:844–850
Divgi CR, Uzzo RG, Gatsonis C, Bartz R, Treutner S, Yu JQ, Chen D, Carrasquillo JA, Larson S, Bevan P, Russo P (2013) Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: results from the REDECT trial. J Clin Oncol 31:187–194
Povoski SP, Hall NC, Murrey DA Jr, Sharp DS, Hitchcock CL, Mojzisik CM, Bahnson EE, Knopp MV, Martin EW Jr, Bahnson RR (2013) Multimodal imaging and detection strategy with 124 I-labeled chimeric monoclonal antibody cG250 for accurate localization and confirmation of extent of disease during laparoscopic and open surgical resection of clear cell renal cell carcinoma. Surg Innov 20:59–69
Stillebroer AB, Boerman OC, Desar IM, Boers-Sonderen MJ, van Herpen CM, Langenhuijsen JF, Smith-Jones PM, Oosterwijk E, Oyen WJ, Mulders PF (2013) Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur Urol 64:478–485
Beisland C, Dabestani S, Stewart G, Bensalah K, Gudmundsson E, Lam TB, Gietzmann W, Zakikhani P, Marconi L, Fernandez-Pello S, Monagas S, Williams SP, Torbrand C, Powles T, Van Werkhoven E, Meijer RP, Volpe A, Staehler M, Ljungberg B, Bex A (2018) Imaging modalities used for follow-up of localized renal cell carcinoma (RCC) and subsequent effect on overall survival after recurrence: RECUR-database analysis. J Clin Oncol 36:Abstract 637
Dabestani S, Beisland C, Stewart GD, Bensalah K, Gudmundsson E, Lam TB, Gietzmann W, Zakikhani P, Marconi L, Fernandez-Pello S, Monagas S, Williams SP, Torbrand C, Powles T, Van Werkhoven E, Meijer R, Volpe A, Staehler M, Ljungberg B, Bex A (2018) Long-term outcomes of follow-up for initially localised clear cell renal cell carcinoma: RECUR database analysis. Eur Urol Focus. https://doi.org/10.1016/j.euf.2018.02.010 (epub ahead of print)
Author information
Authors and Affiliations
Contributions
SD: project development, data collection, manuscript writing and editing. LM: data collection, manuscript writing and editing. TK: data collection, manuscript writing and editing. AB: project development, data collection, manuscript writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
The following manuscript is a review of existing data. Therefore, this article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study (review) formal consent is not required.
Rights and permissions
About this article
Cite this article
Dabestani, S., Marconi, L., Kuusk, T. et al. Follow-up after curative treatment of localised renal cell carcinoma. World J Urol 36, 1953–1959 (2018). https://doi.org/10.1007/s00345-018-2338-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2338-z