Abstract
Purpose
To compare the oncologic outcomes of partial nephrectomy (PN) and radical nephrectomy (RN) for cT1-2/N0 renal tumors and pathologically confirmed pT1-pT3a-pNx clear cell (cc)-renal cell carcinoma (RCC). Few studies compared the oncologic outcomes of PN and RN for renal tumors >7 cm.
Methods
A prospective “renal cancer” database was queried for cT<3-cN0-cM0 and pT1a-pT3a-pNx cc-RCC. Out of 1650 cases treated between 2001 and 2013, 921 were cc-RCC and 666 met inclusion criteria, 232 of which treated with minimally invasive RN and 434 with MIPN. A 1:1 propensity score-matched (PSM) analysis was employed to minimize the selection bias of non-random assignment of patients to PN as opposed to RN. Kaplan–Meier method was used to compare the oncologic outcomes of the PSM cohorts. Survival rates were computed at 2, 5, and 10 years after surgery, and the log-rank test was applied to assess statistical significance between the two PSM groups.
Results
RN tumors were significantly larger (p < 0.001), with higher pT stages (p < 0.001), higher Fuhrman grades (p = 0.002) and a more frequent sarcomatoid differentiation (p = 0.04). After applying the PSM analysis, the two cohorts of 155 RN and 155 PN cases did not differ for all clinical and pathologic covariates (all p ≥ 0.32). PN and RN cohorts displayed comparable 5-year metastasis-free survival (88.9 vs 89.9 %, p = 0.811), local recurrence-free survival (94.2 vs 95.9 %, p = 0.283), overall survival (94.5 vs 96.8 %, p = 0.419) and cancer-specific survival (96 vs 98.6 %, p = 0.907) rates.
Conclusions
PN and RN for patients with cc-RCC larger than 7 cm provided equivalent oncologic outcomes. Safety and reproducibility of our findings should be further investigated in larger multicentric cohorts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Renal cell carcinoma (RCC) represents 2–3 % of all malignancies with an incidence of 5.8/100,000 [1]. In recent years, there has been an ever-increasing occurrence of incidental small-sized tumors [2] with advances in radiology. Here, surgeons are encouraged to opt for organ-sparing techniques.
The only prospective randomized trial was by Van Poppel et al. [3]. It showed comparable oncologic outcomes between Partial Nephrectomy (PN) and Radical Nephrectomy (RN) for small (<5 cm), low-stage RCC.
In addition, in the same trial, Scosyrev et al. [4] assessed the impact of PN and RN on kidney function by evaluating changes in the estimated glomerular filtration rate (eGFR) in patients with renal masses <5 cm and a normal contralateral kidney. They found that PN decreased the risk of developing both stages A (eGFR <60) and B (eGFR <45) chronic kidney disease (CKD) according to Modifications of Diet in Renal Disease 2 (MDRD 2) study [5].
Previous retrospective studies have highlighted a potential correlation between the development of CKD and an increased incidence of major adverse cardiovascular events (MACE) following RN [6, 7].
The higher risk of CKD following RN [4] has influenced the current recommendations on PN as a first-option treatment for cT1 renal tumors [8, 9].
Retrospective studies did not show any significant differences between PN and RN in terms of cancer control and oncologic outcomes for cT1b renal tumors (4–7 cm) [10, 11]. There is even worse evidence for cT2 and incidental pT3a tumors treated with elective PN [12–15]. These data are affected by significant selection biases, because larger (>7 cm) and high risk (Fuhrman grade 3–4) cc (clear cell)-RCCs are usually treated with RN under the perception that PN for tumors >7 cm might offer suboptimal cancer control. Thus, RN should be considered as the first-option treatment for cT2 renal tumors [8].
In this study, we used a propensity score-matched (PSM) analysis to compare the oncologic outcomes of minimally invasive (MI) PN and MIRN for cT1-2/N0/M0 renal tumors and pT1-pT3a-pNx cc-RCC.
Patients and methods
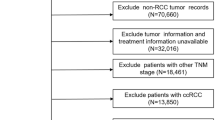
Data were prospectively collected in an institutional single-center renal surgery database from January 2001 to December 2013. Of 1650 cases, 921 were cc-RCC and 666 patients met inclusion criteria (cT<3/N0/M0 and histologically confirmed cc-RCC pT1a–3a/Nx); 232 were treated with MIRN and 434 with MIPN (Fig. 1). Tumors were classified according to the 2009 tumor–node–metastasis staging system [16]. Tumor size was defined as the greatest tumor diameter on pathologic specimens. Histologic subtypes were reported according to the 2002 American Joint Committee on Cancer/Union Internationale Contre le Cancer classifications. Pathologic reports were carried out by a single uropathologist. None of the cases included urothelial carcinoma or non-cortical renal tumors. Preoperative staging included blood tests and whole-body computed tomography (CT) scans, or magnetic resonance imaging (MRI) when CT was contraindicated.
Surgical treatment (PN or RN) was performed based on tumor stage, surgeon preference and robotic platform availability (Si Da Vinci available since June 2010). All procedures were performed with an MI approach (laparoscopic or robotic) by the same surgical team.
The RCC follow-up schedule included physical examination and routine blood assays at 3, 6, 12, 18 and 24 months postoperatively. Alternatively, abdominal ultrasonography and chest X-ray or CT scans were performed at 6-month intervals for the first 2 years and an annual CT scan thereafter.
Survival data were obtained from institutional “renal cancer” database or from the treating physicians and death certificate.
A 1:1 PSM analysis was used to minimize the potential biases of retrospective analysis of data. This allows the investigators to account for differences in covariates between the groups. This provides an adjustment for known factors. The analysis was performed with the type of surgery (PN vs RN) as the dependent variable; age, gender, tumor size, Fuhrman grade, pT stage, sarcomatoid differentiation and positive surgical margins were the independent variables. The analysis provides a standardized mean difference <10 % between covariates.
Continuous and discrete variables were reported as the mean ± SD and proportions. These were compared with Student’s t test and the χ2 test, respectively. The Kaplan–Meier method was performed to compare the oncologic outcomes of the PSM cohorts. Survival rates were computed at 2, 5, and 10 years after surgery, and the log-rank test was used to assess the statistical significance between the two groups. All tests were two-sided, and statistical significance was defined as p < 0.05. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS v.21, IBM Corp., Armonk, NY, USA) as well as the R statistical package (v.2.14.2).
Results
Demographic, clinical and pathologic data are summarized in Table 1. The two groups were comparable in terms of age (p = 0.82), gender (p = 0.64) and positive surgical margins (p = 0.301). Tumors treated with RN were significantly larger (mean size 5.54 vs 3.6 cm, p < 0.001). They exhibited higher pT stage (pT ≥ 2: 31.9 vs 7.1 %, p < 0.001), a higher Fuhrman grade (grade ≥3: 38.8 vs 27.9 %, p = 0.002), and a more frequent sarcomatoid differentiation (2.2 vs 0.5 %, p = 0.04).
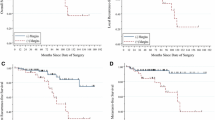
For the purpose of this analysis, 155 RN patients were matched with 155 PN cases. In the PSM-selected cohorts, the identified covariates did not show any significant imbalance (all p values ≥0.32; Table 1). The Kaplan–Meier analysis of the PN and RN cohorts displayed comparable 5-year local-recurrence-free survival (LRFS) (94.2 vs 97.9 %, respectively, p = 0.283), metastasis-free survival (MFS) (88.9 vs 89.9 %, p = 0.811), cancer-specific survival (CSS) (96.0 vs 98.6 %, p = 0.907), and overall survival (OS) (94.5 vs 96.8 %, p = 0.419) probabilities (Fig. 2).
Discussion
According to EAU guidelines [8], PN is the first treatment option for cT1a tumors (<4 cm), and a viable option for cT1b lesions (>4 cm) “when technically feasible”.
With regard to the oncologic effectiveness of PN and RN for cT1a tumors, a phase three prospective randomized trial of PN versus RN displayed comparable oncologic effectiveness with 10-year progression rates of 4.1 % (95 % CI 1.7–6.5) and 3.3 % (95 % CI 1.2–5.4), respectively (Gray’s test p = 0.48) [3]. In a subsequent analysis from the same prospective cohort, focused on the functional outcomes of either PN or RN, the authors observed a 21.0 % (95 % CI 13.8–28.3; p = 0.001) increase in the incidence of newly onset moderate (eGFR <60 ml/min/1.73 m2) renal dysfunction for the RN group (85.7 %) versus the PN group (64.7 %) [4].
As a negative consequence of the renal function deterioration, Huang et al. [7] found that RN was associated with significantly higher risks of both overall mortality (OM) (HR 1.38, 95 % CI 1.13–1.69; p < 0.01) and of MACE after surgery (95 % CI 1.06–1.89; p < 0.05).
These evidences supporting the superiority of PN in terms of renal function preservation have led surgeons to expand PN indications to larger and more complex renal tumors; in the last decade, we observed a treatment paradigm shift toward increased adoption of organ-sparing techniques [17]. Moreover, the wide diffusion of robotic platforms has made indications of PN even more frequent—especially in high-volume tertiary referral centers [18].
However, there are not evidences supporting the oncologic effectiveness of PN for tumors larger than 4 cm from prospective randomized trials. In a matched-pair cohort of patients treated with either open PN (n = 73) or RN (n = 100) for tumors >4 cm, Roos et al. [19] demonstrated comparable 5-year progression-free survival (89 vs 92 %, p = 0.558), OS (83 vs 86 %, p = 0.357), and CSS (5 years 95 vs 97 %, p = 0.239) probabilities. At multivariable regression analysis, surgical treatment and tumor size had a negligible impact on OS.
In a multi-institutional retrospective study including 3480 patients with cT1b renal tumors, Antonelli et al. [10] reported comparable 10-year CSS probabilities (87 vs 90 %, p = 0.89) following RN and PN, respectively.
However, both oncologic and functional outcomes might be affected by intrinsic selection biases. Although oncologic effectiveness of PN may be questionable due to intrinsic selection biases due to a non-random allocation of patients with exophytic lesions to PN and of patients with endophytic lesions to RN, the functional benefits of PN are strong (reduced MACE events and OM). In a propensity model evaluating more than 1000 patients who underwent either PN or RN for renal masses between 4 and 7 cm, RN cohort exhibited a 25 % (95 % CI 3–73; p < 0.0001) increased risk of cardiac death and a 17 % (95 % CI 12–27; p < 0.0001) increased risk of death from any cause on multivariable analysis [6].
From a technical standpoint, the complexity of PN is not uniform for all tumors, and several variables may affect its feasibility. Clearly, tumor size is a strong predictor of perioperative complications after PN [increased blood loss and postoperative complication rates in cT1b compared to T1a tumors (18.6 vs 6.4 %, p = 0.04)], [20]; however, PN for totally endophytic 1-cm renal masses can be a challenging surgery, and achieving negative surgical margins can be easier for large and exophytic masses.
In a single-center analysis, the utilization rate of PN was affected by several variables such as age and preoperative GFR, surgeon volume, fellowship training, academic setting, use of MI techniques and nephrometric scores [21].
In our series, nephrometry scores [22] were not available for most cases. Consequently, the analysis did not account for this variable. Nevertheless, in the report by Lane et al., tumor size was a reliable predictor of nephrometry score. The low, intermediate, and high-nephrometry score tumors >4 cm were seen in 7, 39, and 86 % of cases, respectively [21].
If indications to PN for cT1b renal tumors remain controversial and based on surgeon’s skill, the appropriateness of PN for cT2 renal tumors is still a debatable issue, and the EAU guidelines on renal cancer acknowledge locally advanced tumor growth and unfavorable tumor location as limiting factors to perform PN. In fact, RN is considered the treatment of choice for T2 renal tumors [8].
Reports on the feasibility of PN for tumors >7 cm are scarce with little evidence of effectiveness. In 2009, Jeldres and colleagues first assessed whether PN might undermine cancer control in three PSM cohorts of 17 PN versus 45 RN patients with renal tumors larger than 7 cm, 72 versus 142 patients with high Fuhrman grade (3–4) and 30 versus 63 patients with pT3a renal tumors, respectively [14].
In this analysis, tumor size >7 cm was the only independent predictor of increased risk of mortality in patients receiving PN instead of RN (HR 5.3; p = 0.025). No significant cancer-specific survival differences were recorded after PN for Fuhrman grade 3–4 (HR 0.7; p = 0.5) or for pT3a lesions (HR 2.5; p = 0.9).
More recently, Hansen et al. [12] performed a similar analysis on a larger US population employing 1:1 PSM analyses in three large cohorts of “high risk” patients from the SEER database. The goal was to test whether PN could affect the oncologic outcomes in these patients. Of 8847 patients with T larger than 7 cm, 490 were selected. From 11547 Fuhrman G3–4 cases, 3832 were selected. From 5232 pT3a patients, 954 were selected. On these PSM-selected populations, a survival analysis failed to demonstrate different Cancer-Specific Mortality (CSM) probabilities between PN and RN cohorts [90.67 (95 % CI 0.39–1.17; p = 0.2), 0.81 (95 % CI 0.58–1.12; p = 0.21) and 0.99 (95 % CI 0.61–1.61; p = 1.0), respectively].
Poor but encouraging data concerning oncologic outcomes of PN performed in cT1 renal masses incidentally upstaged to pT3a on final pathology are also available from a small retrospective series. Ramaswamy et al. [15] reported the mid-term oncologic results of a single-center series of 66 patients with cT1 tumors incidentally upstaged to pT3a; 44 of these were treated with PN and 22 with RN. With a median follow-up of 50 months, none of these patients developed recurrences suggesting that incidental upstaging of small renal masses to pT3a on final pathology does not undermine oncologic outcomes.
Given the lack of prospective randomized trials to assess the oncologic equivalence of PN and RN for tumors >5 cm, we compared here two groups of patients with cT1-2/N0/M0 renal tumors treated with either PN or RN for pathologically confirmed cc-RCC. Potential biases of retrospective analysis of data were mitigated with a 1:1 PSM analysis to select two cohorts of patients comparable for demographic variables (age, gender) and established prognostic factors (pT, tumor size, Fuhrman grade, sarcomatoid differentiation, and positive surgical margins). The PSM cohorts supported PN as a viable surgical option with comparable oncologic effectiveness compared with RN in terms of LRFS (5-year rate: 94.2 vs 97.9 %, respectively, p = 0.283), MFS (88.9 vs 89.9 %, p = 0.811), CSS (96.0 vs 98.6 %, p = 0.907) and OS (94.5 vs 96.8 %, p = 0.419) probabilities (Fig. 1).
To the best of our knowledge, this study is the first report on the oncologic equivalence of PN and RN for cT1-2/N0/M0 renal tumors with histologically confirmed pT1-3a/Nx cc-RCC from a single-center series of consecutive cases treated with MI approach. Our findings provide evidence supporting MIPN as a viable option in selected cc-RCCs > 7 cm and confirms the negligible effects of incidental pT3a cc-RCCs on oncologic outcomes after PN.
However, our study does have some limitations. Despite the intent to minimize the effects of selection biases with the employment of a PSM analysis, the increased adoption of PN in the last years for larger and more complex tumors might be a potential confounder [17]. This might be because of the availability of robotic platforms since June 2010 and the high incidence of “complex PNs” due to a selective referral from low-volume and mid-volume centers.
Also, the feasibility of minimally invasive PN for tumors >4 cm cannot be generalized. Over this yearly caseload, the laparoscopic and robotic skills of the surgical team are the main determinants of technical feasibility in this surgical treatment. Notably, the oncologic equivalence of PN and RN in our report is based on a 0 % positive surgical margin rate either in RN or in PN PSM cohorts. The achievement of negative surgical margins in complex PN cases can be challenging especially without proper surgical training and advanced surgical skills. Therefore, the reproducibility of our findings remains in question, especially due to the missing data about validated anatomical and morphometric scores to assess tumor complexity as well as perioperative complication rates. Further analyses are warranted to assess potential functional benefits of PN for tumors larger than 4 cm.
Conclusions
This study highlights the oncologic equivalence of PN and RN for cT1-2/N0/M0 cc-RCC. After adjusting for established prognosticators, and when negative surgical margins are ensured, the PN and RN for patients with cc-RCC larger than 7 cm provided equivalent oncologic outcomes . The safety and reproducibility of our findings in centers with lower surgical volume should be further investigated in larger multi-center cohorts.
References
Jemal A, Bray F, Center M et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Cho E, Adami H, Lindblad P (2011) Epidemiology of renal cell cancer. Hematol Oncol Clin N Am 25:651–665
Van Poppel H, Da Pozzo L, Albrecht W et al (2011) A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 59:543–552
Scosyrev E, Messing EM, Sylvester R et al (2014) Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol 65:372–377
Levey AS, Bosch JP, Lewis JB et al (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470
Weight CJ, Larson BT, Fergany AF et al (2010) Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 1183:1317–1323
Huang WC, Elkin EB, Levey AS, Jang TL, Russo P (2009) Partial nephrectomy versus radical nephrectomy in patients with small renal tumors—is there a difference in mortality and cardiovascular outcomes? J Urol 181:55–62
Ljungberg B, Bensala K, Canfield S et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913–924
Campbell SC, Novick AC, Belldegrun A et al (2009) Guideline for management of the clinical T1 renal mass. J Urol 182:1271–1279
Antonelli A, Ficarra V, Bertini R et al (2011) Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: results of a retrospective, comparative, multi-institutional study. BJU Int 109:1013–1018
Antonelli A, Cozzoli A, Nicolai M et al (2008) Nephron sparing surgery versus Radical nephrectomy in the treatment of intracapsular renal cell carcinoma up to 7 cm. Eur Urol 53:803–809
Hansen J, Sun M, Bianchi M et al (2012) Assessment of cancer control outcomes in patients with high-risk renal cell carcinoma treated with partial nephrectomy. Urology 80:347–353
Breau RH, Crispen PL, Jimenez RE et al (2010) Outcome of stage T2 or greater renal cell cancer treated with partial nephrectomy. J Urol 183:903–908
Jeldres C, Patard JJ, Capitanio U et al (2009) Partial versus radical nephrectomy in patients with adverse clinical or pathologic characteristics. Urology 73:1300–1305
Ramaswamy K, Kheterpal E, Pham H et al (2015) Significance of pathologic T3a upstaging in clinical T1 renal mass undergoing nephrectomy. Clin Genitourin Cancer 13:344–349
Brierley JD, Gospodariwicz MK, Wittekind C (2009) TNM classification of malignant tumors. UICC International Union Against Cancer, 7th edn. Wiley-Blackwell, New York
Simone G, De Nunzio C, Ferriero M et al (2016) Trends in the use of partial nephrectomy for cT1 renal tumors: analysis of a 10-year European multicenter dataset. EJSO. doi:10.1016/j.ejso.2016.03.022
Poon SA, Silberstein JL, Chen LY et al (2013) Trends in partial and radical nephrectomy: an analysis of case logs from certifying urologists. J Urol 190:464–469
Roos FC, Brenner W, Muller M et al (2011) Oncologic long-term outcome of elective nephron-sparing surgery versus radical nephrectomy in patients with renal cell carcinoma stage pT1b or greater in a matched-pair cohort. Urology 77:803–808
Papalia R, Simone G, Ferriero M et al (2012) Laparoscopic and robotic partial nephrectomy without renal ischaemia for tumours larger than 4 cm: perioperative and functional outcomes. World J Urol 30:671–676
Lane BR, Golan S, Eggener S et al (2013) Differential use of partial nephrectomy for intermediate and high complexity tumors may explain variability in reported utilization rates. J Urol 189:2047–2053
Ficarra V, Novara G, Secco S et al (2009) Preoperative aspects and dimensions used for an anatomical (PADUA) classification of renal tumours in patients who are candidates for nephron-sparing surgery. Eur Urol 56:786–793
Authors contribution
G Simone: project development, data analysis, manuscript writing, manuscript editing, G Tuderti: data analysis, manuscript writing, manuscript editing, U Anceschi: data management, manuscript editing, R Papalia: data collection, M Ferriero: data collection, L Misuraca: data collection, F Minisola: data collection, R Mastroianni : data collection, M Costantini: data analysis, S Guaglianone: data collection, supervision, S Sentinelli: data management, supervision, M Gallucci: supervision.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
For this type of study, formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Simone, G., Tuderti, G., Anceschi, U. et al. Oncological outcomes of minimally invasive partial versus minimally invasive radical nephrectomy for cT1-2/N0/M0 clear cell renal cell carcinoma: a propensity score-matched analysis. World J Urol 35, 789–794 (2017). https://doi.org/10.1007/s00345-016-1923-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-016-1923-2