Abstract
Expansin (EXP) plays an important role in plant root formation. The EXP genes associated with chrysanthemum roots have not yet been reported. Here we isolated a root-specific EXP gene in chrysanthemum (Chrysanthemum morifolium), namely CmEXPA4. Bioinformatics analysis showed that CmEXPA4-encoded protein has a conserved DPPB (Double-Psi Beta-Barrel) domain in the N-terminal with a series of Cys residues, an HFD (His-Phe-Asp) motif in the central region, and a pollen allergen domain in the C-terminal. The protein also has a specific α-insertion of WCNP (Trp-Cys-Asn-Pro), which suggests that it belongs to the A-subgroup of the EXP family. In the present study, we cloned the 1,129 bp promoter region upstream of CmEXPA4, and the analysis revealed an abundance of cis-acting elements associated with hormones, light and stress-related responses, and some root-specific regulatory elements in particular. Subcellular localization results indicated that CmEXPA4 locates in the cell wall. Exogenous indole butyric acid induced the up-regulation of CmEXPA4 expression, whereas exogenous abscisic acid inhibited its expression. Tissue expression analysis showed that CmEXPA4 was preferentially expressed in the roots and was synchronized with the rapid emergence of the root. These results suggested that CmEXPA4 may act on the growth and development of chrysanthemum roots. The function of CmEXPA4 was further tested by virus-induced gene silencing, and the results showed that CmEXPA4 silencing inhibited the normal development of the chrysanthemum root system. The roots appeared thinner and shorter, and several important root parameters, including total length, average diameter, surface area, total volume, and root tip number, decreased significantly. The cortical cells of the transgenic plant roots were significantly smaller and shorter than those of the control. Collectively, our results demonstrated that CmEXPA4 gene plays a key role in the growth and development of chrysanthemum roots and affects the root system by acting on the individual cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant roots have important physiological functions such as anchoring plants in the soil, acquiring mineral nutrients and water, synthesizing a plethora of metabolites (Schmidt 2014). The morphological structure and physiological activity of the root will thus directly affect the growth and nutrient uptake of the individual plant, thereby affecting the yield and quality of crops.
The expansin protein was firstly isolated from cucumber and named by McQueen-Mason et al. (1992) and was determined to be involved in cell wall relaxation and cell enlargement, facilitating the rapid buffering of the structural tension of the plant cell wall to loosen it in acidic environments (Cosgrove 2005). Numerous studies have shown that EXPs are involved in almost all stages of plant growth and development (Marowa et al. 2016), playing important roles in seed germination (Xu et al. 2013), leaf growth (Kuluev et al. 2014), petal expansion (Liu et al. 2014), pollen tube growth (Valdivia et al. 2007), fruit ripening and softening (Palapol et al. 2015), and other aspects.
Certainly, EXPs also play a very important role in the formation and growth of plant roots. Lee et al. (2003) successfully cloned the first root-specific expansin gene GmEXP1, which mainly expresses in the epidermis cortex of the roots and plays an important role in the growth and development of roots in soybean (Glycine max L.), particularly the formation and elongation of primary and secondary roots. Furthermore, its super-expression in tobacco (Nicotiana tabacum L.) accelerated root growth. EXPAs of AtEXPA4, AtEXPA14, and AtEXPA17 in Arabidopsis thaliana L. are involved in the formation of lateral root primordia and the separation of cortical cell-coated side root primordia (Lee and Kim 2013; Lee et al. 2013). Li et al. (2015a, b) used the root-specific promoter PYK10 to root-specific expressing TaEXPB23 in tobacco and found that the PYK10-TaEXPB23 lines exhibited an increase in lateral roots and a higher root biomass. Li et al. (2015a, b) demonstrated that the overexpression of GbEXPATR enhanced root hair development in transgenic A. thaliana. In addition, the expression of EXPs is related to plant hormones. In tomato (Solanum lycopersicum L.), cyanamide (CA) could break down the auxin-ethylene balance by affecting cell division, resulting in the expression of some EXP genes that affect plant root growth (Soltys et al. 2012). Abscisic acid (ABA), IAA, and other plant growth regulators (PGRs) also induce the expression of EXPs (Han et al. 2012).
Chrysanthemum, as a traditional Chinese flower, is one of four of the world’s major cut flowers (Guo et al. 2017). Assessing the functions of EXPs in chrysanthemum root is important not only for clarifying the molecular mechanism of root morphogenesis, but also for improving the yield and quality of chrysanthemum via the genetic improvement of the root system. In the present study, we cloned CmEXPA4 along with its promoter, a gene from the EXPA family, and preferentially expressed it in chrysanthemum root. We detected its expression patterns in different tissues, performed PGR treatments, and investigated its function in chrysanthemum root development using virus-induced gene silencing (VIGS). The potential application of CmEXPA4 in the genetic improvement in chrysanthemum is ultimately discussed.
Materials and Methods
Plant Materials and Treatments
Using ‘Hangzhou White Chrysanthemum’ as the experimental material, root cuttings of similar growth morphology were transferred to an artificial climate chamber (light 16 h, 25 °C; dark 8 h, 20 °C; relative humidity 75%). Short-day treatment (8 h light and 16 h dark) promoted flower bud differentiation in the mature stage of vegetative growth. For the spatial expression analysis of CmEXPA4, the roots (mature roots and new roots), stems, leaves (mature leaves and new leaves), alabastra (pre-blooming buds), buds, and inflorescences (early flowering period, full flowering period, and aging period; indicated in Supplemental Fig. S1) were collected at the flowering phase. The roots were harvested at 0, 1, 3, 7, 14, and 21 days after culture. The total length, average diameter, surface area, total volume, and root tip number of the roots were detected using an EPSON root scanner with WinRHIZO software (G780B, Seiko Epson Corp., Tokyo, Japan).
For the exogenous PGRs treatments, the cuttings were treated in Hoagland’s nutrient solution (Guo et al. 2017) with 10 µM 6-BA (6-benzylaminopurine), 10 µM GA3 (gibberellin A3), 10 µM IBA (indole butyric acid), and 10 µM ABA, respectively. Plants grown in Hoagland’s nutrient solution lacking PGR addition were used as a blank control (CK). All root samples were obtained 48 h after treatment. Each treatment had three biological replicates. All the samples were plunged in liquid nitrogen immediately and stored at − 80 °C for RNA extraction.
RNA Isolation and the Full-Length Cloning of CmEXPA4
Total RNA isolation was carried out using the Ultrapure RNA Kit (CWBIO, Beijing, China) and was then converted into cDNA for RACE (rapid amplification of cDNA ends) using the SMARTerTM RACE cDNA Amplification Kit (TaKaRa, Japan). Together with the adaptor primer UPM in the amplification kit and the 5′ and 3′ RACE specific primers, the full-length cDNA of CmEXPA4 was cloned. The PCR products of the full-length CmEXPA4 were ultimately inserted into the pMD18-T vector (TaKaRa, Japan) and sequenced.
Bioinformatics Analysis of CmEXPA4
The amino acid sequence was deduced by DNAMAN software (Lynnon Corporation). The functional domains were searched using the SMART protein online analysis program (http://smart.embl-heidelberg.de/smart/set_mode.cgi), CDD, the conserved domain database of the NCBI Web site, and the InterProScan Web site (http://www.ebi.ac.uk/interpro/search/sequence-search). Multiple amino acid sequences were aligned in ClustalW and DNAMAN. A phylogenetic tree was constructed based on sequence alignments using ClustalW and MEGA 7.0 software (Kumar et al. 2016). Signal peptide sequences were predicted by SignalP online Web site (http://www.cbs.dtu.dk/services/SignalP/).
Cloning and Sequence Analysis of the Promoter of CmEXPA4 Gene
Chrysanthemum genomic DNA extracted with a Plant Genomic DNA Kit (CWBIO, Beijing, China) was used as the template. Based on the gene sequence, three nested primers were designed and synthesized as follows: SP1: 5′-AGGAGGGCAAAAGTTTGTAGCAGTG-3′; SP2: 5′-GCTAAACCCCTTGTTGAACAAAGC-3′; and SP3: 5′-CCATAGAATGTAGCATGAGCACCTTG-3′. The steps of chromosome walking were based on the Genome Walking Kit manual (TaKaRa, Japan). After three episodes of PCR, the produced fragments were recovered and sequenced. PLACE (Higo et al. 1999) and PLANTCARE (Lescot et al. 2002) online software were used to analyze the sequences of the promoter.
Subcellular Localization of CmEXPA4
The complete open reading frame (ORF) of CmEXPA4 was cloned into the vector pC1301 to generate the CmEXPA4-GFP fusion protein (35S::CmEXPA4-GFP). The cell-wall-specific marker was built on the basis of N. tabacum expansin: EXPA6 (GenBank: KJ730251.1) which only expressed only on the cell wall. An empty vector containing green fluorescent protein (GFP) was used as a negative control. The experiment of onion epidermis cell infection was carried out, and the fluorescence signal was detected using a laser confocal microscope (PerkinElmer, America).
Real-time Quantitative PCR Analysis (qRT-PCR)
The cDNA was synthesized using the same method as above. The primers for the qRT-PCR analysis were designed and pre-tested by general PCR to ensure the accuracy: EFY: 5′-GGGGACCACAACACACTTCAC-3′ and ERY: 5′-GACAATGCCAGCACGATACTCA-3′. The PCRs used the UltraSYBR Mixture (CWBIO, Beijing, China) and a Light Cycler 480 system (Roche Diagnostics). The PCR cycling conditions were: one cycle for 10 min at 94 °C, 40 cycles for 20 s at 94 °C, 30 s at 60 °C, and two cycles as above to analyze the melting curves. All reactions were performed in triplicate replications, and the chrysanthemum Ubiquitin gene was used as the loading control.
VIGS of CmEXPA4 in Chrysanthemums and Morphological Detection of Transgenic Plants
VIGS of CmEXPA4 was performed as described by Lü et al. (2014). Using specific primers (EFS: 5′-TCTAGAGGGGACCACAACACACTTCAC-3′ and ERS: 5′-TGAGTATCGTGCTGGCATTGTCCTCGAG-3′; underlined parts are the Xbal and Xhol recognition sites, respectively), a specific fragment of CmEXPA4 was cloned and inserted into the pTRV2 vector. The root parameters were detected and recorded using an LA-S Plant Root Analysis System (Wan Shen, Hangzhou, China). Root paraffin sections were made according to the method of Qin et al. (2013) and finally observed by microscope (NIKON Eclipse Ci, Japan). Twenty cells were randomly selected to measure the length and width of the cells in different fields of vision. Each treatment was repeated three times.
Statistical Analysis
All the data obtained from the study were evaluated by one-way analysis of variance (ANOVA) using the statistical program SPSS (version 17.0). Duncan’s multiple range test was used to compare the differences between treatment means at P < 0.05.
Results
Identification of CmEXPA4
The resulting isolated gene was 1130 bp with an ORF encoding a polypeptide of 257 amino acids containing N-terminal secretory signal peptides ranging from 1 to 20 residues. The predicted molecular weight and isoelectric point were 27.9 kD and 9.66, respectively.
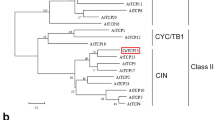
As indicated in Fig. 1, CmEXPA4 showed strong sequence similarity to the expansins of other species, such as AtEXPA4 of A. thaliana and PtEXPA4 of white poplar (Populus tomentosa Carrière). All contain a DPBB domain with six Cys residues in the N terminal, an HFD motif in the middle of the sequence, and a pollen allergen domain with four Try (W) residues in the C terminal. Additionally, all possess WCNP (Trp-Cys-Asn-Pro) residues inserted in front of the HFD motif, which is regarded as a unique α-insertion in the EXPA subfamily (Lu et al. 2016). As shown in Fig. 2, CmEXPA4 was significantly diverged from other EXPBs, which complemented the above analysis. It clustered into a subgroup with AtEXPA4 and also closely to RhEXPA4.
Multiple sequence alignment of CmEXPA4. Identical and similar amino acids are shaded in colors. Underlined parts are putative signal peptide sequence. The two functional domains are marked with arrows: DPBB domain and Pollen allergen domain. The HFD motif, and conserved Cys (C) and Trp (W) residues are showed with rectangular boxes. The α-insertion (WCNP) is marked with five-pointed star. GsEXPA4: Gossypium schwendimanii, AEN70893.1; PtEXPA4: P. tomentosa, AFZ78606.1; CmEXPA4: Chrysanthemum morifolium, KY315238.1; HhEXPA4, Hedera helix, APV45520.1; MeEXPA4, Manihot esculenta, XP_021619783.1; TcEXPA4, Theobroma cacao, EOY07514.1; CumEXPA4, Cucumis melo, NP_001284471.1; PpEXPA4, Prunus persica, XP_007218834.1; ATEXPA4, A. thaliana, NP_181500.1
Phylogenetic tree analysis of CmEXPA4. CmEXPA4 (marked with red square) is significantly diverged from other EXPBs and clustered into a subgroup with AtEXPA4. AtEXPA10, A. thaliana, AT1G26770; ATEXPA4, A. thaliana, NP_181500.1; RhEXPA4, Rosa hybrida cultivar, AFQ21787.1; AtEXPA7, A. thaliana, NP_172717.1; AtEXPA17, A. thaliana, NP_192072.1; AtEXPA18, A. thaliana, NP_176486.1; OsEXPA8, Oryza sativa Japonica Group, XP_015632452.1; OsEXPA17, O. sativa Japonica Group, XP_015642083.1; OsEXPA1, O. sativa Japonica Group, XP_015634193.1; GmEXPA1, G. max, NP_001237850.2; AtEXPB1, A. thaliana, NP_179668.1; OsEXPB2, O. sativa Japonica Group, XP_015614021.1; OsEXPB4, O. sativa Japonica Group, XP_015614992.1; OsEXPB6, O. sativa Japonica Group, XP_015614019.1; OsEXPB11, O. sativa Japonica Group, XP_015623786.1; GmEXPB2, G. max, ACA83732.1; TaEXPB23, Triticum aestivum, AAP84631.1; HvEXPB1, Hordeum vulgare, AAQ57591.1
Bioinformatics Analysis of the CmEXPA4 Promoter
Table 1 displays various motifs along with their function and location site on the analyzed promoter sequence described in the previous literature. The results showed that the sequence contained typical core promoter regions of eukaryotes, including the core promoter element TATA-box, the enhancer components of CAAT-box, and the light responsive elements of GATA-box. Many important cis-elements were also found in the regulatory region of the gene. Plant hormone response elements were also detected, such as the auxin-induced elements ASF1MOTIFCAMV (also induced by salicylic acid) and NTBBF1ARROLB; the ABA response elements ABRE, DPBFCOREDCDC3, and DRE1COREZMRAB17; the ethylene response element ERE, and the gibberellin response element PYRIMIDINEBOXHVEPB1. The cis-elements involved in the response to abiotic stresses in plants, such as LTRE (low-temperature stress), CCAATBOX1 (heat shock), MYB1AT, MYBCORE, and MYCCONSENSUSAT (water stress), MBS (drought-inducibility), and the wound-responsive element WUN-motif, were detected. Additionally, we found several important elements associated with root-specific expression, including four ROOTMOTIFTAPOX1, one RHERPATEXPA7, two OSE1ROOTNODULE, and one WUSATAg.
Subcellular Localization of CmEXPA4
SignalP program analysis showed that CmEXPA4 had a 20 bp signal peptide sequence in the N-terminal, which can guide the protein into the secretory pathway, and the Plant-mPLoc Web site predicted that CmEXPA4 was localized to the cell wall. As indicated in Fig. 3, the GFP fluorescence of the control cells was visualized in the cell wall and nucleus, while cells transformed with 35S::CmEXPA4-GFP only displayed green fluorescence in the cell wall, according to the cell wall-specific marker. Based on the articles on EXPs reported earlier and the sequence analysis, CmEXPA4 was determined to be localized in the cell wall.
Expression Characteristics of CmEXPA4
Expression of CmEXPA4 in the Different Chrysanthemum Tissues
The expression of CmEXPA4 differed in the various tissues (Fig. 4). The expression level of CmEXPA4 in the new roots was the highest, followed by the old roots. Additionally, there was a very small amount of expression in the stem, leaf, and initial opening flowers, whereas almost no expression was detected in the other tissues. The results also showed that the expression level of the gene in the vigorous growing region was higher than that in the mature tissue. The data calculated that the expression of CmEXPA4 in the new roots was 1.53 times higher than that in the mature roots, whereas the expression level in the young leaves was also higher than that of mature leaves, up to 2.2 times (Fig. 4). The same trend also appeared in the inflorescence.
Expression of CmEXPA4 in the different chrysanthemum tissues. From left to right: young root (YR), mature root (MR), stem (S), young leaf (YL), mature leaf (ML), bud (B), alabastra (A), flowers at initial stage (IF), flowers at full bloom stage (BF), flowers at senescence stage (SF). Different symbols on bars indicate a significant difference at P = 0.05. Values represent the means ± SE, n = 3. The same as below
Expression of CmEXPA4 at Different Stages of Root Development
The chrysanthemum roots presented significant morphological changes during the three weeks of development (Fig. 5a), and the associated parameters are presented in supplemental Table S1. As shown in Table S1, the parameters did not differ significantly during the first 2 days of the experiment; on the third day, except for total length and surface area, the other three parameters increased slightly, but exhibited marked increases by the seventh day: the total length, average diameter, surface area, total volume, and root tip number increased by 119.6%, 31.1%, 89.3%, 88.4%, and 60.3%, respectively. Furthermore, the expression of CmEXPA4 also showed a significant increase of 79.0% (Fig. 5b). The roots continued to grow steadily over the next 2–3 weeks. During the 3 weeks of root development, the expression of CmEXPA4 increased first and then continued a high level (Fig. 5b).
Expression of CmEXPA4 Under Various Exogenous PGR Treatments
As shown in Fig. 6, both IBA and 6-BA could induce the expression of CmEXPA4 to different degrees, with IBA indicating a clear increase in up to 87.3%. ABA treatment significantly inhibited the expression of CmEXPA4, and its expression level was 51.7% of the control group. However, there were no obvious differences between GA3 treatment and CK.
Expression of CmEXPA4 under various exogenous hormone treatments. Chrysanthemum cuttings were treated in Hoagland’s nutrient solution with either 10 µM 6-BA (6-benzylaminopurine), 10 µM gibberellin A3 (GA3), 10 µM indole butyric acid (IBA), or 10 µM ABA for 48 h. Samples treated with Hoagland’s nutrient solution lacking phytohormone addition were used as controls. Compared with the blank control, IBA and 6-BA induce the expression of CmEXPA4, while ABA inhibited it and there were no obvious differences between GA3 treatment and control
Silencing CmEXPA4 Affects Root System Architecture and Plant Growth in Chrysanthemum
The qRT-PCR assay indicated that a total of 11 effectively silenced lines were obtained following treatment. We selected the representative transgenic plants for further experimentation. Figure 7a indicates their relative suppression of expression: silent line E26Footnote 1 decreased by 67.2% compared to the TRV blank control, whereas E41Footnote 2 decreased by 35.2%.
Silencing CmEXPA4 affects root system architecture and plant growth in chrysanthemum. a Expressions of CmEXPA4 in control (CK) and CmEXPA4-silenced chrysanthemums (E26, E41). b Comparison of the phenotype of roots between control (CK) and CmEXPA4-silenced plants (E26, E41). Bar = 4 cm. c Comparison of the phenotype of plant between control (CK) and CmEXPA4-silenced plants (E26, E41). Bar = 4 cm. d Aboveground biomass growth indexes of control (CK) and CmEXPA4-silenced chrysanthemums (E26, E41)
Silencing of CmEXPA4 resulted in a visible alteration in root architecture. The changes in root morphology of E26 and E41 compared to TRV control are indicated in Fig. 7b. In comparison with CKFootnote 3, the transgenic plants had less developed root systems and the roots appeared shorter and sloppier. The total root length of E26 was significantly reduced by 34.6%, whereas E41 was reduced by 16.2% (Table 2). The average root diameters of E26 and E41 differed significantly from the control and were reduced by 18.3% and 14.0%, respectively. Genetic disruption also affected the surface area and total volume of the root. In terms of surface area, E26 and E41 decreased by 24.2% and 12.4%, respectively, while the total volume reduced by 27.1% and 14.7%, respectively. Changes in the root tips of E41 were not significant, but E26 exhibited an obvious decrease in 32. The data showed that root development in the two silenced chrysanthemums was reduced by more than that of control, and root growth in E26 was generally more strongly inhibited.
As shown in Fig. 7c, the aboveground biomass growth of the VIGS plants was lower than the control plants, and the stem became thinner and more delicate. Figure 7d shows the measured indexes of the different treatment groups. The plant height, leaf width, and stem diameter of E41 were 2.7%, 5.6%, and 5.6% less than that of the control. E26 was more greatly affected, and these three indexes were reduced by 10.8%, 18.8%, and 18.5%, respectively.
Effect of CmEXPA4 Silencing on the Cortical Cells of the Roots
The cell length and width of the root cortical cells in the CmEXPA4-silenced plants were significantly reduced compared with the control (Fig. 8a, b). The average length and width of the transgenic cortical cells had been reduced to approximately 11–29% and 5–27% that of the control groups (Fig. 8c). The data showed that cell length was more greatly inhibited in addition to the above root parameters. The diameter of the vascular bundle did not show any obvious changes.
Suppression of CmEXPA4 inhibits the expansion of cortical cells in chrysanthemum roots. a Cross section observations of the root cortical cells in control (CK) and CmEXPA4-silenced plant. Arrows indicate cortical cells. (× 200, Bar = 100 µm). b Longitudinal section observations of the root cortical cells in control (CK) and CmEXPA4-silenced plant. Arrows indicate cortical cells. (× 200, Bar = 100 µm). c Root cortical cell width and length of control and CmEXPA4-silenced plants (E26, E41)
Discussion
Root growth can strongly affect the growth of the aboveground parts of chrysanthemum and further influence stress resistance (Wu et al. 2017). It is thus of practical significance that the molecular mechanisms of chrysanthemum root development are explored. EXP is an important regulator of plant root development. Here we isolated an expansin gene and explore its role in chrysanthemum root development.
Sequence analysis showed that CmEXPA4 protein contains two signal domains (Fig. 1). The DPBB domain has significant homology to glycoside hydrolase family-45 (GH45) proteins, where the HFD motif is considered to be part of the catalytic site that constitutes the family-45 endoglucanases and the Cys residues are considered to be key sites for the formation of disulfide bonds that contribute to the structural stability of proteins (Gaete-Eastman et al. 2015). The pollen allergen domain is highly homologous to grass pollen allergen proteins and contains a fiber-binding domain based on the conserved aromatic and polar residues on the surface of the protein (Sampedro and Cosgrove 2005). The four try (W) residues are related to the association of cellulose and polysaccharides (Cosgrove 2015). As indicated in the phylogenetic tree, CmEXPA4 is closely associated with RhEXPA4, AtEXPA17, and OsEXPA8. RhEXPA4 was reported that its overexpression in Arabidopsis influence lateral root formation, and leaf growth. (Lü et al. 2013). The overexpression and knock-down of AtEXPA17 will enhance and reduce lateral root formation in Arabidopsis (Lee and Kim 2013). Ma et al. (2013) demonstrated that the overexpression of OsEXPA8 in rice could increase root mass, leaf number and size, as well as plant height. Then promoter analysis showed that the gene possessed a variety of root-specific expression-related elements (Table 1). RHERPATEXPA7 (root hair-specific cis-elements) involved in root hair distribution patterns has been identified from some EXPs important for plant root development (Zou et al. 2015). WUSATAg could regulate a WUS-type homeobox gene of rice, which is related to the specification and maintenance of the stem cells in the root apical meristem (Kamiya et al. 2003). Sequence and promoter analysis preliminarily revealed the functional correlation of CmEXPA4.
The organ, tissue, and cell specificity of EXP expression has been reported in a large number of studies (Meng et al. 2015). Lu et al. (2016) analyzed the transcription profiles of 23 members in tomato, and most of the tested genes showed an organ-preferential expression pattern. As observed in this study, CmEXPA4 was highly expressed in the roots, exhibiting a relative expression level of 14.5–43.2 times the expression in other organs (Fig. 4). Moreover, we found that the gene expression level was also related to the degree of organ maturity. This might be because the young tissue is generally associated with rapid growth and development, during which the cell division and expansion of the physiological process is relatively active, and the expansin, as a protein promoting cell wall relaxation, is bound to actively participate in the regulation of these processes. Lee et al. (2003) found that GmEXPA1 in soybean seedlings constitutes a root rapid cell elongation site for the occurrence of a high level of expression and has enrichment in the shoot area opposite to the mature area where root extension stops. However, this does not mean that all EXPs are prioritized in active tissues; some fruit ripening-related expansin genes only specifically express in the mature fruit but not in the vegetative organs (Lovisetto et al. 2015). Further studying the relationship between CmEXPA4 and chrysanthemum root development, we found that the relative expression level of CmEXPA4 was almost synchronized with root system development, which firstly indicated rapid growth and then tended to slow down. A similar finding was noted in a previous report: Lee et al. (2003) studied the GmEXP1 gene, a soybean root-specific expansin protein, and found that its expression was altered in the different developmental stages of the root and reached its maximum expression in the roots of 5-day-old seedlings. This suggested that some EXPs may be differentially regulated at various plant developmental stages.
Phytohormones are necessary in regulating plant development and protecting against adverse environmental changes. Promoter sequence analysis showed that the CmEXPA4 promoter sequence possessed hormone-induced related components, such as ABRE (Table 1). And the hormone induction experiment results showed that IBA could significantly induce the expression of CmEXPA4. IBA, as an exogenous auxin analogue commonly used in chrysanthemum cutting technology, is beneficial to rapid rooting and elongation, and its application of in Malus hupehensis Rehd. could induce the expression of the MhEXP1 gene in plant roots (Xudong et al. 2008). Therefore, in this study, CmEXPA4 was strongly induced by IBA, which not only corroborated its relationship with the growth of the root system in chrysanthemum, but also provided us with a new perspective: whether CmEXPA4 responds to plant endogenous hormones and thus transcriptional expression to promote root growth, and the associated pathway of this response. For instance, reports have suggested that the accumulation of auxin can increase the activity of auxin susceptible genes, such as LAX3, thereby inducing the expression of a set of cell wall-remodeling genes, such as polygalacturonase and xyloglucan endotransglucosylase, which are involved in pectin polymer cleavage and cell wall loosening, respectively, thereby coordinating cell separation and organ emergence (Porco et al. 2016). In addition, CmEXPA4 was strongly suppressed by ABA. It has been reported that ABA can inhibit the secretion of cell H+, prevent cell wall acidification and cell elongation, and thus inhibit the hypocotyls, shoots, roots, and other organs of the elongation growth process (Davies 2010). It suggested that CmEXPA4 is not regulated by a single hormone, but rather that it interacts with many hormones to ultimately affect the physiological processes of the plant.
Silencing of CmEXPA4 resulted in blocking the growth of chrysanthemum root system (Fig. 7b; Table 2). Similar attempts have succeeded in identifying the function of EXPs by establishing RNA interference. For instance, in rice, the inhibition of OsEXPA8 expression significantly damaged the root structure, leading to shorter roots and fewer lateral roots (Wang et al. 2014). In recent years, root research also focused on the study of root morphological structure. Guo et al. (2017) found that chrysanthemum could improve the absorption and utilization of nitrate by adjusting the root system configuration. Here the silencing of CmEXPA4 resulted in a significant reduction in root parameters, such as total length and average diameter, which have become an important index for evaluating the nutrient uptake of plants in recent research reports (Xiao et al. 2015; Luo et al. 2016). The decrease in the growth of the silenced plants in this experiment indirectly supported this. Root growth inhibition is certainly not conducive to the absorption of nutrients, leading to poor plant growth in nutritional terms. A healthy plant body is the first line of response to environmental adversity. In the production and landscape application of chrysanthemum, the expression of this gene can be modified using genetic engineering technology to breed novel cultivars based on root system characteristics.
Previous studies have also reported that the impact of expansins on plant organ size is based on the accumulation of individual cell effects. Azeez et al. (2010) found that the increase in pistil style length was a consequence of increased cell expansion. Zou et al. (2015) noted that the cell size of root cortical cells in OsEXPB2-suppressed rice lines was significantly smaller than that of their counterparts in wild-type plants. A similar phenomenon was found in this study upon observation of the root tissue sections of infected and control plants. Cortical cells in the roots of CmEXPA4-silenced plants were arranged in a more chaotic manner and indicated a significant reduction in length and width (Fig. 8a–c). In rice, the down-regulation of OsEXPA8 also severely limits the size of root vascular cells (Wang et al. 2014); however, in this study, the size of the vascular bundles did not change much. This indicates that the functional mechanisms of different genes from the expansin family differ. The anatomical observations revealed the relationship between phenotypic changes and the role of gene operation from the cell level. When plants respond to different external factors, the most obvious change is morphological, and morphological changes in the roots are important regulatory mechanisms for responding to changes in the environment, especially under stress conditions. Some of the root-specific EXPs will thus become indispensable in improving the root configuration in order to cope with adversity. For instance, the overexpression of TaEXPB23 enhanced root system development in wheat and improved plant resistance (Li et al. 2015a, b; Han et al. 2015). The promoter analysis results showed that there was a series of cis-acting elements related to adverse stress in the regulatory region sequence at the 5′ of CmEXPA4, including TC-rich repeats and LTRE (Table 1). Thus, the next research direction of CmEXPA4 can begin from here and further explore the interactory mechanisms with some stress-regulating hormones.
In summary, CmEXPA4, a typical EXPA family gene that expressed preferentially in chrysanthemum roots, participates in the root growth process, especially in the rapid growth stage. The expression of this gene is regulated by hormones and its down-regulation could inhibit root growth and plant development by affecting cell growth. Our results enriched the relevant research foundation.
Notes
E26: CmEXPA4-silent line 26;
E41: CmEXPA4-silent line 41;
CK: blank control.
References
Azeez A, Sane AP, Tripathi SK, Bhatnagar D, Nath P (2010) The gladiolus GgEXPA1 is a GA-responsive alpha-expansin gene expressed ubiquitously during expansion of all floral tissues and leaves but repressed during organ senescence. Postharvest Biol Technol 58(1):48–56
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850
Cosgrove DJ (2015) Plant expansins: diversity and interactions with plant cell walls. Curr Opin Plant Biol 25:162–172
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Plant hormones. Springer, Dordrecht, pp 1–15
Gaete-Eastman C, Morales-Quintana L, Herrera R, Moya-León MA (2015) In-silico analysis of the structure and binding site features of an α-expansin protein from mountain papaya fruit (VpEXPA2), through molecular modeling, docking, and dynamics simulation studies. J Mol Model 21(5):115
Guo YH, Yu YY, Wen LZ, Sun CH, Fan HM, Sun XZ, Zheng CS (2017) Up-regulation of CmNRTs and CmANR1 genes expression contribute to root configuration changes for efficient capturing NO3 – in the roots of chrysanthemum. Sci Hortic 225:438–444
Han YY, Li AX, Li F, Zhao rong, Wang M, W (2012) Characterization of a wheat (Triticum aestivum L.) expansin gene, TaEXPB23, involved in the abiotic stress response and phytohormone regulation. Plant Physiol Biochem 54:49–58
Han Y, Chen Y, Yin S, Zhang M, Wang W (2015) Over-expression of TaEXPB23, a wheat expansin gene, improves oxidative stress tolerance in transgenic tobacco plants. J Plant Physiol 173:62–71
Higo K, Ugawa Y, Iwamoto M et al (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27(1):297–300
Kamiya N, Nagasaki H, Morikami A et al (2003) Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J 35(4):429–441
Kuluev BR, Knyazev AV, Nikonorov YM, Chemeris AV (2014) Role of the expansin genes NtEXPA1 and NtEXPA4 in the regulation of cell extension during tobacco leaf growth. Rus J Genet 50(5):489–497
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870
Lee HW, Kim J (2013) EXPANSINA17 up-regulated by LBD18/ASL20 promotes lateral root formation during the auxin response. Plant Cell Physiol 54(10):1600–1611
Lee DK, Ahn JH, Song SK, Choi Y, Lee JS (2003) Expression of an expansin gene is correlated with root elongation in soybean. Plant Physiol 131(3):985–997
Lee HW, Kim MJ, Kim NY, Lee SH, Kim J (2013) LBD18 acts as a transcriptional activator that directly binds to the EXPANSIN14 promoter in promoting lateral root emergence of Arabidopsis. Plant J 73(2):212–224
Lescot M, Déhais P, Moreau Y, De Moor B, Rouzé P, Rombauts S (2002) PlantCARE: a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30(1):325–327
Li AX, Han YY, Wang X, Chen YH, Zhao MR, Zhou SM, Wang W (2015a) Root-specific expression of wheat expansin gene TaEXPB23 enhances root growth and water stress tolerance in tobacco. Environ Exp Bot 110:73–84
Li W, Wang F, Wang J, Fan F, Zhu J, Yang J, Zhong W (2015b) Overexpressing CYP71Z2 enhances resistance to bacterial blight by suppressing auxin biosynthesis in rice. PLoS ONE 10(3):e0119867
Liu XW, Jiang GM, Liu JT et al (2014) The expression and functional analysis of expansin genes during flower opening in cut rose. Acta Hortic Sin 41(08):1673–1681
Lovisetto A, Masiero S, Rahim MA, Mendes MAM, Casadoro G (2015) Fleshy seeds form in the basal angiosperm Magnolia grandiflora and several MADS-box genes are expressed as fleshy seed tissues develop. Evol Dev 17(1):82–91
Lu Y, Liu L, Wang X, Han Z, Ouyang B, Zhang J, Li H (2016) Genome-wide identification and expression analysis of the expansin gene family in tomato. Mol Genet Genom 291(2):597–608
Lü P, Kang M, Jiang X, Dai F, Gao J, Zhang C (2013) RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta 237(6):1547–1559
Lü P, Zhang C, Liu J, Liu X, Jiang G, Jiang X, Gao J (2014) RhHB1 mediates the antagonism of gibberellins to ABA and ethylene during rose (Rosa hybrida) petal senescence. Plant J 78(4):578–590
Luo J, Hou YY, Cheng JH, Wang NN, Chen BL (2016) Root morphological characteristics of cotton genotypes with different phosphorus efficiency under phosphorus stress. Sci Agric Sin 49(12):2280–2289
Ma N, Wang Y, Qiu S, Kang Z, Che S, Wang G, Huang J (2013) Overexpression of OsEXPA8, a root-specific gene, improves rice growth and root system architecture by facilitating cell extension. PLoS ONE 8(10):e75997
Marowa P, Ding A, Kong Y (2016) Expansins: roles in plant growth and potential applications in crop improvement. Plant Cell Rep 35(5):949–965
McQueen-Mason S, Durachko DM, Cosgrove DJ (1992) Two endogenous proteins that induce cell wall extension in plants. Plant Cell 4(11):1425–1433
Meng YJ, L J, Xu J, Zhang KJ, Lou QF, Chen JF (2015) Cloning and expression analysis of CsEXPb1 gene of cucumber dismantoin gene. Horticulture 42(4):679–688
Palapol Y, Kunyamee S, Thongkhum M, Ketsa S, Ferguson IB, van Doorn WG (2015) Expression of expansin genes in the pulp and the dehiscence zone of ripening durian (Durio zibethinus) fruit. J Plant Physiol 182:33–39
Porco S, Larrieu A, Du Y, Gaudinier A, Goh T, Swarup K, Casimiro I (2016) Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3. Development 143(18):3340–3349
Qin Z, Lv H, Zhu X et al (2013) Ectopic expression of a wheat WRKY transcription factor gene TaWRKY71-1 results in hyponastic leaves in Arabidopsis thaliana. PLoS ONE 8(5):e63033
Sampedro J, Cosgrove DJ (2005) The expansin superfamily. Genome Biol 6(12):242
Schmidt W (2014) Root systems biology. Front Plant Sci 5:215
Soltys D, Rudzińska-Langwald A, Gniazdowska A, Wiśniewska A, Bogatek R (2012) Inhibition of tomato (Solanum lycopersicum L.) root growth by cyanamide is due to altered cell division, phytohormone balance and expansin gene expression. Planta 236(5):1629–1638
Valdivia ER, Wu Y, Li LC, Cosgrove DJ, Stephenson AG (2007) A group-1 grass pollen allergen influences the outcome of pollen competition in maize. PLoS ONE 2(1):e154
Wang Y, Ma N, Qiu S, Zou H, Zang G, Kang Z, Huang J (2014) Regulation of the α-expansin gene OsEXPA8 expression affects root system architecture in transgenic rice plants. Mol Breed 34(1):47–57
Wu PT, Wang JM, Shen JY, Yang YC, Guan ZY, Fang WM, Chen FD (2017) Analyses on related indexes of root, above-ground part and leaf of different cultivars of Chrysanthemum morifolium and stress resistance evaluation. J Plant Resour Environ 26(2):46–54
Xiao Y, Peng F, Dang Z et al (2015) Influence of rhizosphere ventilation on soil nutrient status, root architecture and the growth of young peach trees. Soil Sci Plant Nutr 61(5):775–787
Xu B, Gou JY, Li FG, Shangguan XX, Zhao B, Yang CQ, Chen XY (2013) A cotton BURP domain protein interacts with α-expansin and their co-expression promotes plant growth and fruit production. Mol Plant 6(3):945–958
Xudong S, Hongqiang Y, Shaochong W (2008) Cloning and expression of a full-length cDNA of expansin gene from new root of Malus hupehensis Rehd. Sci Agric Sin 5:1548–1553
Zou H, Wenwen Y, Zang G, Kang Z, Zhang Z, Huang J, Wang G (2015) OsEXPB2, a β-expansin gene, is involved in rice root system architecture. Mol Breed 35(1):41
Acknowledgements
This work was supported by Grants from Shandong Forestry Science and Technology Innovation Project of China (Grant No. LYCX06-2018-33).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
344_2019_9940_MOESM1_ESM.tif
Supplementary material 1—Inflorescence sampling period display. From left to right: IF (Flowers at initial stage), BF (Flowers at full bloom stage), SF (Flowers at senescence stage). Bar=1 cm (TIF 672 KB)
Rights and permissions
About this article
Cite this article
Ren, H., Wen, Lz., Guo, Yh. et al. Expressional and Functional Verification of the Involvement of CmEXPA4 in Chrysanthemum Root Development. J Plant Growth Regul 38, 1375–1386 (2019). https://doi.org/10.1007/s00344-019-09940-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-09940-x