Abstract
TCP genes encode plant-specific transcription factors in various species that they play important roles in plant growth and development. In the present study, we cloned the TCP transcription factor, TCP13, from Chrysanthemum nankingense (CnTCP13). CnTCP13 belongs to the class II CIN subfamily of the TCP family and harbors an atypical basic-helix-loop-helix motif, that was preferentially expressed in the leaf. CnTCP13 transcription was significantly inhibited 24 h after exogenous application of 6-BA. The CnTCP13 protein was localized in the nuclei of transformed onion epidermal cells and did not exhibit transcriptional activation. Heterologous expression of CnTCP13 in Arabidopsis thaliana reduced leaf size. qRT-PCR analysis revealed that the transcription levels of cell division-related genes were altered in transgenic A. thaliana containing CnTCP13. CnTCP2 and CnF-box were identified as putative interaction proteins of CnTCP13 by a yeast two-hybrid assay and bimolecular fluorescence complementation. CnF-box belongs to the F-box family and is abundantly expressed in roots. The CnF-box protein was localized in the nucleus and had no transcriptional activation. In A. thaliana, CnF-box overexpression led to strong crinkling of leaves. Taken together, CnTCP13 is involved in leaf development through the regulation of cell division-related genes, and likely by its interaction with CnTCP2 and CnF-box.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leaves provide the basis for plant growth through photosynthesis. In plants, leaves develop from a mass of pluripotent cells at the shoot apical meristem, then display marginal meristem activity, and finally expand to form a mature leaf (Bar and Ori 2014). Leaf growth is maintained by cell proliferation and cell expansion. Various phases of the cell cycle depend on a number of proteins, including cyclins, cyclin-dependent kinases (CDKs) and CDK inhibitors (Breuer et al. 2010). Leaf development is a complex molecular network regulated by intercellular signaling molecules, including plant hormones, sugars and transcription factors (Kalve et al. 2014).
TEOSINTE-LIKE1, CYCLOIDEA and PROLIFERATING CELL FACTOR 1 (TCP) transcription factors constitute a small family of plant-specific transcription factors whose members share functions in plant development (Martín-Trillo and Cubas 2010). The TCP domain harbors a 59 amino acid basic helix-loop-helix (bHLH) motif that is involved in DNA binding and protein-protein interactions (Martín-Trillo and Cubas 2010). Populus euphratica, Brassica rapa and Medicago truncatula have 33, 39 and 21 TCP genes, respectively (Ma et al. 2016; Du et al. 2017; Wang et al. 2018). Based on the motif, members of the TCP family are classified into class I (PCF or TCP-P class) and class II (TCP-C class) subfamilies (Navaud et al. 2007). Class I and II TCP genes act antagonistically in modulating plant cell proliferation and expansion by competing for similar targets or partners. As such, while class I TCP genes promote cell proliferation, class II TCP genes prevent cell proliferation (Martín-Trillo and Cubas 2010). Inhibition of the class I gene AtTCP11 at the C-terminal domain resulted in curly rosette leaves with margins tending to fold upwards (Viola et al. 2011). Arabidopsis TCP14 and TCP15 regulate trichromes development on stems and leaves, and increase cuticle permeability (Camoirano et al. 2020). The cell cycle genes, CYCA2;3 and CYCB1;1, are known to be the targets of AtTCP14 (Daviere et al. 2014). The class I gene, TCP9, acts with TCP20 to positively regulate leaf senescence through jasmonic acid signaling pathway (Danisman et al. 2012). The class II gene, AtTCP4, directly activates MIR396b, which encodes a miRNA that prevents cell proliferation in leaves (Schommer et al. 2014). BrrTCP2 overexpression in wild-type A. thaliana reduces leaf size and also restores the leaf phenotype of the triple mutants of tcp2/4/10 to wild-type (Du et al. 2017). In Chinese cabbage, BrTCP3, BrTCP4a and BrTCP24a are highly expressed at the rosette stage (Liu et al. 2018). AtTCP13 inhibits leaf growth by repressing Arabidopsis thaliana homeobox 12 (ATHB12) expression, which promotes leaf growth mainly during the cell expansion phase (Hur et al. 2019). CIN is expressed in the lamina, proximal to and, perhaps, overlapping the cell-cycle arrest front, and serves as a suppressor of cell proliferation in leaves (Nath et al. 2003).
F-box proteins (FBPs) constitute one of the largest protein families in the plant kingdom. FBPs harbor a roughly conserved F-box motif composed of 40–60 amino acid residues with few invariant positions (Abd-Hamid et al. 2020). Based on the C-terminal region, FBPs are classified into several subfamilies, including leucine-rich repeats, WD-40, Kelch repeats, F-box associated and TUBBY. FBPs are involved in various plant biological processes, including plant development and morphogenesis, cell signaling, circadian clock, cell cycle, phytohormone signaling, biotic and abiotic stress responses (Dharmasiri et al. 2005; Cao et al. 2008; Chen et al. 2014). The FBP STERILE APETALA gene positively regulates cell proliferation through degradation of the plant-specific factors PEAPOD1 and PEAPOD2 in A. thaliana (Wang et al. 2016). Also in Arabidopsis, FBXL negatively regulates vein pattern formation (Cui et al. 2016), and FBX92 suppresses leaf growth by negatively regulating several cell cycle genes (Baute et al. 2017).
Chrysanthemum nankingense Hand.-Mazz. (Anthemideae, Asteraceae) is a diploid species native to China and is closely related to the important ornamental species C. morifolium (Song et al. 2015). Among the C. morifolium TCP genes, BRC1 inhibits lateral branching (Chen et al. 2013), CmCYC regulates the ray floret growth (Huang et al. 2016), and CmTCP14 reduces organ size (Zhang et al. 2017). However, little is known regarding TCP genes in C. nankingense. Therefore, in this study, we cloned the TCP transcription factor TCP13 from C. nankingense (CnTCP13), revealed the transcriptional behavior of CnTCP13, and studied the phenotypic effect of heterologously expressing it in A. Thaliana.
Materials and methods
Plant materials
The accession of C. nankingense used was obtained from the Chrysanthemum Germplasm Resource Preserving Center, Nanjing Agricultural University, China. Rooted cuttings were grown in a greenhouse (23 °C during the day and 15 °C at night; 70–75 % relative humidity; natural light). A. thaliana (ecotype Col-0) plants were grown in a 1:1:1 (v/v/v) mixture of soil, perlite, and vermiculite under a 16 h photoperiod (80–110 µmol m− 2 s− 1 illumination) at a day/night temperature of 21 °C/18°C.
Isolation and sequencing of CnTCP13 cDNA
Total RNA was isolated from the C. nankingense leaves using the RNAiso reagent (TaKaRa, Tokyo, Japan) according to the manufacturer’s instructions. The first-strand cDNA was synthesized from 1 µg of total RNA using an M-MLV RTase cDNA Synthesis Kit (TaKaRa), following the manufacturer’s instructions. The full-length CnTCP13 cDNA was amplified using the specific primer pair CnTCP13-F/R (Table S1) that are based on the sequence identified from the C. nankingense transcriptome database (Wang et al. 2013). The amplified products were purified using the AxyPrep DNA Gel Extraction Kit (Axygen, Shanghai, China) and then inserted into the plasmid pMD19-T (TaKaRa) for sequencing.
Phylogenetic analysis
From the Arabidopsis Tair Database (https://www.arabidopsis.org/), A. thaliana TCP amino acid sequences were downloaded. The AtTCP sequences were combined with the obtained CnTCP13 and used to conduct a multiple sequence alignment using ClustalW (Larkin et al. 2007). The phylogenetic analysis was performed using the neighbor-joining method and a graphical representation was drawn using MEGA V6 (Tamura et al. 2013). Bootstrap values were estimated using 1000 bootstrap replicates.
Transcription profiling of CnTCP13 using qRT-PCR
Total RNA was extracted from various tissues of C. nankingense using the RNAiso reagent (Takara) according to the manufacturer’s instructions. Transcription profiling was performed using qRT-PCR with the primer pair CnTCP13-QF/QR (Table S1). CnEF1α was used as the reference (Table S1) (Gao et al. 2015). Three biological replicates and three technical replicates were used for qRT-PCR. The cycling conditions were as follows: initial denaturing at 95 °C for 2 min, followed by 40 cycles of 95 °C for 15 s, 55 °C for 15 s, and 72 °C for 20 s. Relative expression levels were measured by the 2−△△CT method (Livak and Schmittgen 2012).
Phytohormone treatment
Young plants (6–8 leaf stage) were used to evaluate CnTCP13 transcription in response to cytokinin treatment as previously described (Gao et al. 2015). The cytokinin treatment involved spraying the leaves with 5.0 µM 6-benzyladenine (6-BA) (Wang et al. 2017; Qi et al. 2019). Control plants were treated with distilled water. Leaves were sampled before and 1, 2, 4, 8, 12, and 24 h after phytohormone application. The 6-BA was applied to three plants at each time point, and three biological replicates were used for each time point. The 6-BA treatment experiment was replicated three times (Li et al. 2015). The sampled second true leaves were frozen in liquid nitrogen immediately and stored at -80 °C for further usage (Gao et al. 2015).
Subcellular localization of CnTCP13
The primer pair CnTCP13-pENTR1A-F/R (Table S1) was used to amplified the CnTCP13 ORF without the stop codon using the Phusion High-Fidelity PCR Kit (New England Biolabs, MA, USA). Both the pENTR1A vector (Invitrogen, Carlsbad, CA, USA) and amplicon were cleaved with the restriction enzymes SalI and NotI, and the products were ligated with T4 DNA ligase (New England Biolabs). The pENTR1A-CnTCP13 fusion product was subsequently sequenced for validation. pMDC43 was recombined with pENTR1A-CnTCP13 to generate the fusion vector p35S::GFP-CnTCP13 using the LR Clonase™ II Enzyme Mix (Invitrogen). The empty pMDC43 vector and p35S::GFP-CnTCP13 were transformed into onion epidermal cells using the He-driven particle accelerator PDS-1000 (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions. After bombardment, the onion peels were incubated at 22 °C for 16 h on MS medium in the dark (Li et al. 2015). Green fluorescence was observed using a confocal laser microscope (Leica SP2, Germany).
Transactivation activity
A yeast one-hybrid assay was used to test the transactivation activity of CnTCP13 (Li et al. 2015). pENTR1A-CnTCP13 was recombined with pGBKT7 to produce pGBKT7-CnTCP13 using the LR Clonase™ II Enzyme Mix (Invitrogen). The pGBKT7-CnTCP13 construct, pCL1 (positive control), and pGBKT7 (negative control) were individually introduced into Y2H Gold yeast cells (Clontech, Mountain View, CA, USA), following the manufacturer’s protocol. Transformants carrying pGBKT7-CnTCP13 or pGBKT7 were selected by culturing on SD/-Trp medium, and the pCL1 transformants were selected by culturing on SD/-Leu medium. All three transformed cell lines were then plated on SD/-His-Ade + 20 mg/mL X-α-gal medium and incubated at 30 °C to observe cell growth.
Arabidopsis thaliana transformation and expression level of cell cycle marker genes
Arabidopsis thaliana (Col-0) was transformed with Agrobacterium tumefaciens (strain EHA105) carrying p35S::GFP-CnTCP13 using the floral dip method (Clough and Bent 1998). The transformed progenies were selected by plating on 1/2 MS + 20 µg/mL hygromycin agar medium, and the T3 generation was obtained by self-pollination. Transgene zygosity was identified by RT-PCR using the primer pair CnTCP13-ORF-F/R (Table S1). The cell size of the seventh leaves at 35 days was observed using phase contrast microscopy (Olympus BX41, Tokyo, Japan). Leaves and cell sizes were measured using Image J software (version 1.8.0_172). All measurements represent the mean of 10 replications. Total RNA was extracted from the seventh leaves of 4-week-old Col-0 and 35 S::CnTCP13 transgenic plants using the RNAiso reagent (Takara) according to the manufacturer’s instructions. Changes in the expression levels of cell cycle marker genes were detected using qRT-PCR. All primer pairs are listed in Table S1, and AtActin was used as the reference (Table S1).
Yeast two-hybrid assay
The bait pGBKT7-CnTCP13 construct was transformed into yeast strain AH109 (Clontech) to screen the prey Chrysanthemum yeast cDNA library according to the manufacturer’s protocol, followed by the selection of interacting partners on SD/-Trp-Leu-His-Ade medium and incubation at 30 °C for 3 days. All clones were selected and cultured on SD/-Trp-Leu-His-Ade and SD/-Trp-Leu-His-Ade + 20 mg/mL X-α-gal medium and incubated at 30 °C for 3 days to observe cell growth. Positive clones with blue color were amplified using Mighty Amp® DNA Polymerase PCR (TaKaRa) based on the pGADT7 plasmid primer pair AD-F/R (Table S1). Clones containing only one prey were sequenced. Sequence analysis was performed using BLASTX (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
A gene-specific primer pair (CnF-box-ORF-F/R, Table S1) was designed to amplify the ORF sequence of CnF-box. The amplified ORF sequence was cloned into the pENTR1A vector to generate pENTR1A-CnF-box (CnF-box-pENTR1A-F/R, Table S1). pENTR1A-CnF-box was then recombined with both pGBKT7 and pGADT7 plasmids. The transactivation activity of CnF-box was tested by growing on SD/-His-Ade medium.
The interactions between CnTCP13, CnTCP2, and CnF-box were identified by testing combinations of CnTCP13-bait and CnTCP2-prey, and CnTCP13-bait and CnF-box-prey constructs co-expressed in the yeast strain AH109 (Clontech) following the manufacturer’s instructions. The pGADT7-T vector was combined with the pGBKT7-53 vector as the positive interaction control and with the pGBKT7-Lam vector as the negative interaction control. Interactions were identified by culturing on SD/-Trp-Leu-His-Ade and SD/-Trp-Leu-His-Ade + 20 mg/mL X-α-gal medium.
BiFC assay
In the bimolecular fluorescence complementation (BiFC) constructs, the ORF of CnTCP13 was cloned as the C-terminal fusion of the fluorescent protein fragments in the pSAT4A-cEYFP-N1 and pSPYCE vectors at the KpnI/SmaI and XhoI/KpnI cloning sites using the primer pairs CnTCP13-BiFC-F/R and CnTCP13-BiFC-f/r, respectively (Table S1). The coding region of CnTCP2 was cloned as the N-terminal fusion in the pSAT4A-nEYFP-N1 and pSPYNE vectors at the KpnI/SmaI and BamHI/KpnI cloning sites using the primer pairs CnTCP2-BiFC-F/R and CnTCP2-BiFC-f/r, respectively (Table S1). The full-length cDNA encoding the CnF-box protein was cloned as the N-terminal fusion in the pSAT4A-nEYFP-N1 and pSPYNE vectors at the KpnI/SmaI and BamHI/KpnI cloning sites using the primer pairs CnF-box-BiFC-F/R and CnF-box-BiFC-f/r, respectively (Table S1).
Transient gene expression in onion epidermal cells was performed using the PDS-1000 biolistic transformation system (Bio-Rad) according to the manufacturer’s instructions. After bombardment, the onion peels were incubated at 22 °C for 16 h on MS medium in the dark (Song et al. 2013). YFP fluorescence was tested using a confocal laser scanning microscope (Leica SP2). An in planta transient assay was used to investigate whether CnTCP13 interact with CnTCP2 or CnF-box in N. benthamiana. A. tumefaciens strain GV3101 cells harboring pSPYCE-CnTCP13, pSPYNE-CnTCP2, pSPYNE-CnF-box, pSPYCE or pSPYNE were suspended in infiltration buffer (2 mM NaH2PO4, 100 µM acetosyringone, 50 mM MES and 0.5 % Glc) to obtain an OD600 of 0.2, and then spot-infiltrated into the leaves of 7-week-old N. benthamiana plants, following the protocol described by Wang et al. (2014). At 48–96 h after infiltration, YFP fluorescence was observed using a confocal laser scanning microscope (Leica SP2).
Statistical analysis
Data were analyzed by analysis of variance and for significance with means separated by Duncan’s Multiple Range tests at p = 0.05 using SPSS V17.0 software (SPSS Inc., Chicago, IL, USA). Results are presented as means ± standard errors (SE) of three biological replicates.
Results
Identification and characterization of CnTCP13
A TCP transcript with the full-length of 1,240 nt and a 1,032 nt ORF, which encodes a 343 amino acids, was isolated from the C. nankingense transcriptome database (SRS591679) (Wang et al. 2013). Homology BLAST showed that CnTCP13 belonged to the class II CIN subfamily of the TCP family and was the most similar to AtTCP13 (Fig. 1a). The CnTCP13 protein harbored an atypical bHLH motif (Fig. 1b).
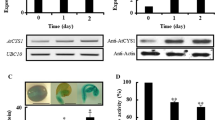
qRT-PCR revealed that CnTCP13 was highly expressed in the leaf, and its expression was the lowest in roots (Fig. 2a). CnTCP13 transcription was significantly inhibited (two-fold) 24 h after exogenous application of 6-BA (Fig. 2b). To determine the subcellular localization of CnTCP13, a transient assay involving the bombardment of the p35S::GFP-CnTCP13 fusion construct into onion epidermal cells was used. The CnTCP13-GFP fusion protein was localized in the nucleus (Fig. 2c). CnTCP13 transcriptional activity was tested using a yeast one-hybrid assay. Yeast cells harboring the positive control pCL1 were able to grow on SD/-His-Ade + 20 mg/mL X-α-gal medium, whereas the cells harboring the negative control pGBKT7 and pGBKT7-CnTCP13 failed to grow (Fig. 2d). These results indicated that CnTCP13 exhibits no transcriptional activity.
Expression patterns of CnTCP13. a Differential expression patterns of CnTCP13 in various tissues of C. nankingense. b Effects of 5.0 µM 6-BA on CnTCP13 transcription. Columns headed by a different letter indicate significantly different transcript abundances (p < 0.05) compared with the control (CK). The x-axis indicates the time point of the assay following the spray treatment. c GFP activity generated by the p35S::GFP-CnTCP13 transgene introduced into onion epidermal peels. Fluorescence: images obtained in the green fluorescence channel; DIC: images obtained in bright light; Merge: overlay plots. Bar: 50 μm. d Yeast one-hybrid assay was used to detect the transcriptional activation of CnTCP13. In a and b, CnEF1α was used as the reference gene, and values are presented as mean ± SE (n = 3)
Phenotype of A. thaliana plants heterologously expressing CnTCP13
To investigate the function of CnTCP13, two independent overexpression lines, OX-1 and OX-2, from the T3 generation were used for subsequent analysis (Fig. 3a). The transgenic plants showed crinkled middle-layer leaves (Fig. 3b). The growth of transgenic plants was greatly restricted at the vegetative stage (Fig. 3c), although they had a large number of rosette leaves (Fig. 3d). While, their leaf length (Fig. 3e) and width (Fig. 3f) were significantly reduced. The size of the lower epidermal cells was larger in the transgenic lines than in the wild-type plants (Fig. 3g and h). These results indicated that overexpression of CnTCP13 reduced leaf size in A. thaliana.
Phenotype of A. thaliana constitutively expressing CnTCP13. a RT-PCR-based identification of the transgenic A. thaliana lines OX-1 and OX-2. b The appearance of seventh rosette leaves. Bar: 0.5 cm. c he appearance of 35-day-old wild-type Col-0, OX-1, and OX-2 plants. Bar: 1 cm. Quantification of leaf growth. Bar: 1 cm. d Number of rosette leaves. e Leaf length of the seventh leaf. f Leaf width of the seventh leaf. g Epidermal cell size of the seventh leaf. Values in (d) through (g) are presented as mean ± SE (n = 10). h Appearance of epidermal cells sampled from the seventh leaf. Bar: 50 μm. Col-0: wild-type plants; OX-1, OX-2: transgenic lines
It reported that AtTCP4 protein blocks cell cycle of budding yeast, specifically at G1→S transition, by regulating G1 checkpoint control pathway, and the expression of a number of cell cycle genes is altered (Aggarwal et al. 2011). To further reveal the role of CnTCP13 in regulating A. thaliana leaf development, the expression of 12 cell division-related genes was analyzed (Fig. 4). Transcription levels of the positive regulators of cell cycle, including AtCYCA1;1, AtCYCA3;1, AtCYCB2;4, AtCDKB1;2, AtCDKB2;2, AtCDKD;2 and AtCDKD;3 were downregulated in the transgenic lines. The transcription level of AtCYCA3;2 remained unchanged. Transcription levels of the negative regulators of cell cycle, including AtCDKC;1, AtCDKG;2, AtKRP5 and AtE2Fc were upregulated in the transgenic lines. The above results suggested heterologous expression of CnTCP13 in A. thaliana alters the transcription levels of cell division-related genes.
Effects of constitutive CnTCP13 expression on the transcription of cell cycle marker genes in A. thaliana. Col-0: wild-type plants; OX-1, OX-2: transgenic lines. Values are presented as mean ± SE (n = 3). Columns headed by different letters indicate significantly different transcript abundances (p < 0.05) compared with the wide-type
Putative interaction proteins of CnTCP13
Yeast two-hybrid assay was used to identify putative interaction proteins of CnTCP13. The CnTCP13 gene was fused to the DNA-binding domain of the bait plasmid pGBKT7. A Chrysanthemum-yeast cDNA library was used as the prey. Sixty-four positive clones were amplified (Fig. S1), and 44 clones containing only one prey were sequenced. Twenty-four positive clones were identified as candidate interaction protein of CnTCP13 using NCBI BLASTX (Table 1). Taking the number of clones, their predicted subcellular location and proteins that typically interact with the bait in this system into consideration, two candidates, CnTCP2 and CnF-box, were considered as putative interaction proteins of CnTCP13. Interactions were validated using yeast two-hybrid assay again. CnTCP2 and CnF-box genes were cloned into the pGADT7 plasmid. pGADT7-T co-transformed with the pGBKT7-53 vector, as the positive interaction control, was able to grow on SD/-Trp-Le-His-Ade medium and show X-α-galactosidase activity, whereas the negative interaction control failed to grow on this medium or show X-α-galactosidase activity (Fig. 5a); CnTCP2 and CnF-box showed significantly growth when co-transformed with CnTCP13 and exhibited X-α-galactosidase activity. The interactions among CnTCP13, CnTCP2 and CnF-box were further confirmed by BiFC assays in onion and N. benthamiana. YFP fluorescence signals were observed in the onion epidermal cells co-expressing nEYFP-CnTCP2 and cEYFP-CnTCP13 or nEYFP-CnF-box and cEYFP-CnTCP13, but not in cells co-expressing nEYFP and cEYFP-CnTCP13, nEYFP-CnTCP2 and cEYFP, nEYFP-CnF-box and cEYFP or nEYFP and cEYFP (controls) (Fig. 5b). In addition, YFP fluorescence signals were observed in N. benthamiana leaves co-expressing pSPYNE-CnTCP2 and pSPYCE-CnTCP13 or pSPYNE-CnF-box and pSPYCE-CnTCP13, but not in cells co-expressing pSPYNE and pSPYCE-CnTCP13, pSPYNE-CnTCP2 and pSPYCE, pSPYNE-CnF-box and pSPYCE or pSPYNE and pSPYCE (controls) (Fig. 5c).
Verification of CnTCP13 interaction with CnTCP2 and CnF-box. (a) Yeast two-hybrid assay verifying the interactions among CnTCP13, CnTCP2, and CnF-box. The left panel shows the selection of yeast colonies on SD/-T/-L/-H/-A medium; the right panel shows the selection of yeast colonies on SD/-T/-L/-H/-A medium containing X-α-gal. SD/-T/-L/-H/-A: SD/-Trp/-Leu/-His/-Ade; X-α-gal: SD/-T/-L/-H/-A + X-α-gal. (b) BiFC assay verifying the interactions among CnTCP13, CnTCP2, and CnF-box in transiently transfected onion cells. YFP: images obtained in the yellow fluorescence channel; DIC: images obtained in bright light; Merge: overlay plots. Bar: 50 μm. (c) BiFC assay verifying the interactions among CnTCP13, CnTCP2, and CnF-box in transiently transfected N. benthamiana leaves. mRFP-NLS: nuclear location shown by RFP activity; YFP: images obtained in the yellow fluorescence channel; DIC: images obtained in bright light; Merge: overlay plots. Ba: 50 μm
Characterization of the CnF-box sequence
CnF-box harbored an 897 nt ORF predicted to encode a 298 amino acid residue product. CnF-box belongs to the FBP family and harbored an F-box motif (Fig. S2a). It was the most closely related to CcF-box (Fig. S2b). CnF-box was abundantly expressed in the root (Fig. S3). It was localized in the nucleus (Fig. S4a), and showed no transcriptional activity (Fig. S4b).
To explore the function of CnF-box, two independent overexpression lines, namely OX-1 and OX-2, from the T3 generation were selected (Fig. 6a). The growth of transgenic plants was slightly restricted at the vegetative stage (Fig. 6b). CnF-box overexpression plants bore the same number of rosette leaves as Col-0 plants, but the middle-layer leaves were strongly crinkled (Fig. 6c).
Phenotype of A. thaliana constitutively expressing CnF-box. a RT-PCR-based identification of the transgenic A. thaliana lines OX-1 and OX-2. b The appearance of 35-day-old wild-type Col-0, OX-1, and OX-2 lines. Bar: 1 cm. c Appearance of rosette leaves. Bar: 1 cm. Col-0: wild-type plants; OX-1, OX-2: transgenic lines
Discussion
The TCP family comprises plant-specific transcription factors that are involved in multiple processes during plant growth and development, such as leaf (Aguilar-Martínez and Sinha 2013; Ma et al. 2016; Bresso et al. 2017), flower (Crawford et al. 2004; Es et al. 2018) and nodule (Wang et al. 2018) development, as well as hormonal pathways (Koyama et al. 2010; Danisman et al. 2012; Es et al. 2018). In the present study, CnTCP13 in C. nankingense was identified. CnTCP13 belongs to the class II subfamily of the TCP family and harbors an atypical bHLH motif. In Antirrhinum cin mutants, leaves are larger with an undulating edge because of excessive growth in marginal regions (Crawford et al. 2004). LA (a CIN ortholog in tomato) activity in young leaf primordia is increased in a LA gain-of-function mutant (La-2); this mutation confers resistance to miR319, and leads to the formation of small, simple tomato leaves instead of large, compound ones (Ori et al. 2007). In Brassica rapa, Brp-MIR319a2 overexpression inhibites BrpTCP4 expression, leading to the formation of crinkly leaves (Mao et al. 2014). Compared with wild-type A. thaliana, the tcp2/tcp4 mutant borns enlarged flat leaves, and the tcp2/tcp3/tcp4/tcp10 plants born strongly crinkled leaves (Bresso et al. 2017). Constitutive CnTCP4 expression suppresses cell proliferation in fission yeast and reduced the leaf size in A. thaliana (Qi et al. 2019). Here, plants with heterologously expressing CnTCP13 were smaller and bore crinkly middle-layer leaves (Fig. 3), indicating that CnTCP13 suppresses leaf development.
CIN-like TCP transcription factors regulate plant growth through their involvement in hormone-associated pathways. For instance, AtTCP3 directly upregulates the expression of the auxin signaling repressor IAA3/SHY2 and modulates auxin responses (Koyama et al. 2010). In A. thaliana, TCP4 promotes the expression of LIPOXYGENASE2 (LOX2), a gene involved in jasmonate (JA) biosynthesis, consequently, the increased JA content inhibites cell proliferation (Danisman et al. 2012). AtTCP4 physically interacts with the SWI/SNF chromatin remodeling ATPase BRAHMA (BRM), and then TCP4-BRM binds to the promoter and activates the CK response inhibitor ARABIDOPSIS RESPONSE REGULATOR16 (ARR16) (Efroni et al. 2013). In the present study, 6-BA treatment inhibited CnTCP13 expression (Fig. 2b), which was in consistent with previous reports, suggesting the involvement of CnTCP13 in the hormonal regulation of plant growth.
AtTCP4 prevents cell proliferation in leaves by positively regulating miR396 and negatively regulating GROWTH-REGULATING FACTORs (GRFs) (Rodriguez et al. 2010). In A. thaliana, plants expressing a hyper-activated form of TCP4 shows decreased cell proliferation and reduced leaf size with cup-shaped lamina in extreme cases (Sarvepalli and Nath 2011). In the present study, constitutive CnTCP13 expression in A. thaliana reduced leaf size but enlarged epidermal cells on the abaxial leaf surface (Fig. 3c, g). In A. thaliana tcp20 mutant leaves, there is a significant increase in average epidermal cell size, but no obvious size or shape alterations can be observed, due to a reduction in total number of cells in the leaf (Danisman et al. 2012). Average epidermal cell size is significantly increased in transgenic CnTCP2/4 A. thaliana leaves, but there are no obvious leaf size or shape alterations in these transgenic plants as the cell size effect was compensated by a reduction in total cell number in the leaf (Qi et al. 2019). Smaller leaves could be observed in transgenic A. thaliana containing CnTCP13, implying that the effect of enlarged epidermal cells was compensated for by a decrease of the total cell number in the leaf. These results suggest that CnTCP13 suppresses cell proliferation but promotes cell expansion.
The development of multicellular organisms is controlled by precise cell proliferation and expansion. The mechanisms regulating plant organ size through the cell cycle are rather complex, as they must suit fluctuating environments (Mizukami 2001). The cell cycle involves a series of phases, and every process is regulated by specific cyclins and CDKs (Breuer et al. 2010). Mitotic A- and B-type cyclins show expression peaks at the G2-to-M boundary, and plant-specific B-type CDKs positively control the entry into and passed through mitosis (Leene et al. 2010). In the quintuple mutant tcp8/tcp15/tcp21/tcp22/tcp23, CYCLINA1;1 (CYCA1;1) and CYCA2;3 are regulated, resulting in larger leaf blades than the wild-type (Aguilar-Martínez and Sinha 2013). Arabidopsis expresses four CDK-activating kinases (CDKDs): CDKD;1, CDKD;2, CDKD;3 and CDKF;1. The complex of CDKD;2 or CDKD;3 with CYCH;1 positively regulates the cell cycle by phosphorylating CDKA at the T161 residue (Gutierrez 2009). In the present study, AtCYCA1;1, AtCYCA3;1, AtCYCB2;4, AtCDKB1;2, AtCDKB2;2, AtCDKD;2 and AtCDKD;3 were downregulated in transgenic lines (Fig. 4) likely suppressing cell proliferation, thus leading to the formation of small leaves. CDKCs phosphorylate the C-terminal domain (CTD) of RNA polymerase II. The CDKC-CycT complex negatively regulates the positive transcription elongation factor b (P-TEFb) (Kitsios et al. 2008), whereas the loss of CDKC;2 promotes cell division in A. thaliana (Zhao et al. 2017). There are two CDKGs, namely CDKG;1 and CDKG;2, in A. thaliana. CDKG;2 forms a complex with CYCL1, which negatively regulates cell cycle onset (Leene et al. 2010). Kip-related protein 5 (KRP5) prevents cell cycle progression by inhibiting the activity of the CYCD2-CDKB complex kinase and reconstitutes CYCD2-associated kinases at both G1/S and G2/M transitions (Nakai et al. 2006). E2FC inhibites cell division by negatively regulating the entry into G1/S, and E2FC overexpression obviously reduces the root meristem cell number (del Pozo et al. 2006). In the present study, AtCDKC;1, AtCDKG;2, AtKRP5 and AtE2Fc upregulation likely suppressed cell proliferation, resulting in small leaves in transgenic A. thaliana. These results indicated that heterologous expression of CnTCP13 in A. thaliana alters the transcription levels of cell division-related genes and reduces the size of leaves.
Some TCP transcription factors are not transcriptional activators per se, but require interaction with other proteins to form homo- and heterodimers to control transcription (Martín-Trillo and Cubas 2010). A yeast two-hybrid assay confirms that AtPURα interacts with AtTCP20, suggesting that these two proteins act together at the promoter of ribosomal genes (Trémousaygue et al. 2003). The TCP transcription factor CCA1 HIKIHG EXPEDITION (CHE) interacts with the CAA1 transcriptional activator TIMING OF CAB EXPRESSION1 (TOC1), which downregulated CAA1 by binding to its promoter (Pruneda-Paz et al. 2009). CmTCP14 reduces plant stature by interacting with CmDELLA1, CmDELLA2 and CmDELLA3 in the gibberellic acid signaling pathway (Zhang et al. 2017). AtTCP24 interacts with ABAP1 and negatively regulates the transcription of AtCDT1α and AtCDT1b, thus limiting mitotic DNA replication and repressing cell proliferation in leaves (Masuda et al. 2008). In the present study, we isolated factors interacting with CnTCP13 using yeast two-hybrid system. The interactions between CnTCP13 and CnTCP2 or CnTCP13 and CnF-box were confirmed by a yeast two-hybrid assay and by BiFC (Fig. 5). The F-box protein is part of an SCF complex, and harbors a roughly conserved F-box motif that binds to SKP1, and diverse typical protein-protein interaction domains involved in various aspects of plant biology (Lechner et al. 2006). In A. thaliana, CnF-box overexpression plants showed strongly crinkled leaves, similar to CnTCP13 transgenic plants with crinkled middle-layer leaves (Figs. 3b and 6c). Moreover, in A. thaliana, CnTCP2 overexpression plants shows smaller leaves, similar to CnTCP13 transgenic plants, which plays negative roles in leaf development by suppressing cell proliferation and promoting cell expansion (Qi et al. 2019). These results indicated that CnTCP13 controls leaf development in C. nankingense maybe through its interaction with CnTCP2 and CnF-box.
In conclusion, heterologous CnTCP13 expression in A. thaliana reduced leaf size. CnTCP13 was involved in leaf development by modulating the expression of cell cycle marker genes, and maybe through its interaction with CnTCP2 and CnF-box, which remains further investigation in more details.
References
Abd-Hamid NA, Ahmad-Fauzi MI, Zainal Z, Ismail I (2020) Diverse and dynamic roles of F-box proteins in plant biology. Planta 251:68
Aggarwal P, Padmanabhan B, Bhat A, Sarvepalli K, Sadhale PP, Nath U (2011) The TCP4 transcription factor of Arabidopsis blocks cell division in yeast at G1→S transition. Biochem Bioph Res Co 410:276–281
Aguilar-Martínez JA, Sinha N (2013) Analysis of the role of Arabidopsis class I emopenTCPemclose genes emopenAtTCP7emclose, emopenAtTCP8emclose, emopenAtTCP22emclose, and emopenAtTCP23emclose in leaf development. Front Plant Sci 4:406
Bar M, Ori N (2014) Leaf development and morphogenesis. Development 141:4219–4230
Baute J, Polyn S, De Block J, Blomme J, Lijsebettens MV, Inze D (2017) F-Box protein FBX92 affects leaf size in Arabidopsis thaliana. Plant Cell Physiol 58:962–975
Bresso EG, Chorostecki U, Rodriguez RE, Palatnik JF, Schommer C (2017) Spatial control of gene expression by miR319-regulated TCP transcription factors in leaf development. Plant Physiol 176:1694–1708
Breuer C, Ishida T, Sugimoto K (2010) Developmental control of endocycles and cell growth in plants. Curr Opin Plant Biol 13:654–660
Camoirano A, Arce AL, Ariel FD, Alem AL, Viola IL (2020) Class I TCP transcription factors regulate trichome branching and cuticle development in Arabidopsis. J Exp Bot 71:5438–5453
Cao Y, Yang Y, Zhang H, Li D, Zheng Z, Song F (2008) Overexpression of a rice defense-related F-box protein gene OsDRF1 in tobacco improves disease resistance through potentiation of defense gene expression. Physiol Plantarum 134:440–452
Chen R, Guo W, Yin Y, Gong ZH (2014) A novel F-Box protein CaF-box is involved in responses to plant hormones and abiotic stress in pepper (Capsicum annuum L.). Int J Mol Sci 15:2413–2430
Chen XL, Zhou XY, Lin X, Li JX, Zhao RY, Nan M, Zhao LJ (2013) Roles of DgBRC1 in regulation of lateral branching in Chrysanthemum (Dendranthema ×grandifloracv. Jinba). PLoS ONE 8:e61717
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Crawford BCW, Nath U, Carpenter R, Coen ES (2004) CINCINNATA controls both cell differentiation and growth in petal lobes and leaves of Antirrhinum. Plant Physiol 135:244–253
Cubas P, Lauter N, Doebley J, Coen E (1999) The TCP domain: a motif found in proteins regulating plant growth and development. Plant J 18:215–222
Cui XH, Xu XF, He YY, Du XL, Zhu J (2016) Overexpression of an F-box protein gene disrupts cotyledon vein patterning in Arabidopsis. Plant Physiol Biochem 102:43–52
Danisman S, van der Wal F, Dhondt S, Waites R, de Folter S, Bimbo A, van Dijk AD, Muino JM, Cutri L, Dornelas MC (2012) Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol 159:1511–1523
Daviere JM, Wild M, Regnault T, Baumberger N, Eisler H, Genschik P, Achard P (2014) Class I TCP-DELLA interactions in inflorescence shoot apex determine plant height. Curr Biol 24:1923e1928
Del Pozo JC, Diaz-Trivino S, Cisneros N, Gutierrez C (2006) The balance between cell division and endoreplication depends on E2FC-DPB, transcription factors regulated by the ubiquitin-SCFSKP2A pathway in Arabidopsis. Plant Cell 18:2224–2235
Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005) Plant development is regulated by a family of auxin receptor F box proteins. Develop Cell 9:109–119
Du JC, Hu SM, Yu Q, Wang CD, Yang YQ, Sun H, Yang YP, Sun XD (2017) Genome-wide identification and characterization of BrrTCP transcription factors in Brassica rapa ssp. rapa. Front Plant Sci 8:1588
Efroni I, Han SK, Kim HJ, Wu MF, Sang Y, Steiner E, Birnbaum KD, Hong JC, Eshed Y, Wagner D (2013) Regulation of leaf maturation by chromatin-mediated modulation of cytokinin responses. Dev Cell 24:438–445
Es SWV, Silveira SR, Rocha DI, Bimbo A, Martinelli AP, Dornelas MC, Angenent GC, Immink RGH (2018) Novel functions of the Arabidopsis transcription factor TCP5 in petal development and ethylene biosynthesis. Plant J 94:867–879
Gao CY, Li PL, Song AP, Wang HB, Wang YJ, Ren LP, Qi XY, Chen FD, Jiang JF, Chen SM (2015) Isolation and characterization of six AP2/ERF transcription factor genes in Chrysanthemum nankingense. Int J Mol Sci 16:2052–2065
Gutierrez C (2009) The Arabidopsis cell division cycle. Arabidopsis Book 7:e0120
Huang D, Li XW, Sun M, Zhang TX, Pan HT, Cheng TR, Wang J, Zhang QX (2016) Identification and characterization of CYC-like genes in regulation of ray floret development in Chrysanthemum morifolium. Front Plant Sci 7:1633
Hur YS, Kim J, Kim S, Son O, Kim WY, Kim GT, Ohme-Takagi M, Cheon CI (2019) Identification of TCP13 as an upstream regulator of ATHB12 during leaf development. Genes 10:644
Kalve S, De Vos D, Beemster GTS (2014) Leaf development: a cellular perspective. Front Plant Sci 5:362
Kitsios G, Alexiou KG, Bush M, Shaw P, Doonan JH (2008) A cyclin-dependent protein kinase, CDKC2, colocalizes with and modulates the distribution of spliceosomal components in Arabidopsis. Plant J 54:220–235
Koyama T, Mitsuda N, Seki M, Shinozaki K, Ohme-Takagi M (2010) TCP transcription factors regulate the activities of ASYMMETRIC LEAVES1 and miR164, as well as the auxin response, during differentiation of leaves in Arabidopsis. Plant Cell 22:3574–3588
Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, Mcwilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lechner E, Achard P, Vansiri A, Potuschak T, Genschik P (2006) F-box proteins everywhere. Curr Opin Plant Biol 9:631–638
Leene JV, Hollunder J, Eeckhout D, Persiau G, Slijke EVD, Stals H, Isterdael GV, Verkest A, Neirynck S, Buffel Y (2010) Targeted interactomics reveals a complex core cell cycle machinery in Arabidopsis thaliana. Mol Syst Biol 6:397
Li PL, Song AP, Gao CY, Jiang JF, Chen SM, Fang WM, Zhang F, Chen FD (2015) The over-expression of a chrysanthemum WRKY transcription factor enhances aphid resistance. Plant Physiol Biochem 95:26–34
Liu Y, Guan XY, Liu SN, Yang M, Ren JH, Guo M, Huang ZH, Zhang YW (2018) Genome-wide identification and analysis of TCP transcription factors involved in the formation of leafy head in Chinese cabbage. Int J Mol Sci 19:847
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 –∆∆CT method. Methods 25:402–408
Ma XD, Ma JC, Fan D, Li CF, Jiang YZ, Luo KM (2016) Genome-wide identification of TCP family transcription factors from Populus euphratica and their involvement in leaf shape regulation. Sci Rep 6:32795
Mao YF, Wu FJ, Yu X, Bai JJ, Zhong WL, He YK (2014) microRNA319a-targeted Brassica rapa ssp. pekinensis TCP genes modulate head shape in Chinese Cabbage by differential cell division arrest in leaf regions. Plant Physiol 164:710–720
Martín-Trillo M, Cubas P (2010) TCP genes: a family snapshot ten years later. Trends Plant Sci 15:31–39
Masuda HP, Cabral LM, De Veylder L, Tanurdzic M, De Almeida Engler J, Geelen D, Inzé D, Martienssen RA, Ferreira PCG, Hemerly AS (2008) ABAP1 is a novel plant Armadillo BTB protein involved in DNA replication and transcription. EMBO J 27:2746–2756
Mizukami Y (2001) A matter of size: developmental control of organ size in plants. Curr Opin Plant Biol 4:533–539
Nakai T, Kato K, Shinmyo A, Sekine M (2006) Arabidopsis KRPs have distinct inhibitory activity toward cyclin D2-associated kinases, including plant-specific B-type cyclin-dependent kinase. FEBS Lett 580:336–340
Nath U, Crawford BC, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299:1404–1407
Navaud O, Dabos P, Carnus E, Tremousaygue D, Herve C (2007) TCP transcription factors predate the emergence of land plants. J Mol Evol 65:23–33
Ori N, Cohen AR, Etzioni A, Brand A, Yanai O, Shleizer S, Menda N, Amsellem Z, Efroni I, Pekker I (2007) Regulation of LANCEOLATE by miR319 is required for compound-leaf development in tomato. Nat Genet 39:787–791
Pruneda-Paz JL, Breton G, Para A, Kay SA (2009) A functional genomics approach reveals CHE as a novel component of the Arabidopsis circadian clock. Science 323:1481–1485
Qi XY, Qu YX, Gao R, Jiang JF, Fang WM, Guan ZY, Zhang F, Zhao S, Chen SM, Chen FD, Wang HB (2019) The heterologous expression of a Chrysanthemum nankingense TCP transcription factor blocks cell division in yeast and Arabidopsis thaliana. Int J Mol Sci 20:4848
Rodriguez RE, Mecchia MA, Debernardi JM, Schommer C, Weigel D, Palatnik JF (2010) Control of cell proliferation in Arabidopsis thalianaby microRNA miR396. Development 137:103–112
Sarvepalli K, Nath U (2011) Hyper-activation of the TCP4 transcription factor in Arabidopsisthaliana accelerates multiple aspects of plant maturation. Plant J 67:595–607
Schommer C, Debernardi JM, Bresso EG, Rodriguez RE, Palatnik JF (2014) Repression of cell proliferation by miR319-regulated TCP4. Mol Plant 7:1533–1544
Song AP, Lou WH, Jiang JF, Chen SM, Sun ZX, Guan ZY, Fang WM, Teng NJ, Chen FD (2013) An isoform of eukaryotic initiation factor 4E from Chrysanthemummorifolium interacts with Chrysanthemum Virus B coat protein. PLoS ONE 8:e57229
Song AP, Wang LX, Chen SM, Jiang JF, Guan ZY, Li PL, Chen FD (2015) Identification of nitrogen starvation-responsive microRNAs in Chrysanthemum nankingense. Plant Physiol Biochem 91:41–48
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol 30:2725–2729
Trémousaygue D, Garnier L, Bardet C, Dabos P, Hervé C, Lescure B (2003) Internal telomeric repeats and ‘TCP domain’ protein-binding sites co-operate to regulate gene expression in Arabidopsis thaliana cycling cells. Plant J 33:957–966
Viola IL, Uberti Manassero NG, Ripoll R, Gonzalez DH (2011) The Arabidopsis class I TCP transcription factor AtTCP11 is a developmental regulator with distinct DNA-binding properties due to the presence of a threonine residue at position 15 of the TCP domain. Biochem J 435:143–155
Wang CQ, Guthrie C, Sarmast MK, Dehesh K (2014) BBX19 interacts with CONSTANS to repress FLOWERING LOCUS T transcription, defining a flowering time checkpoint in Arabidopsis. Plant Cell 26:3589–3602
Wang HB, Jiang JF, Chen SM, Qi XY, Peng H, Li PR, Song AP, Guan ZY, Fang WM, Liao Y, Chen FD (2013) Next-generation sequencing of the Chrysanthemum nankingense (Asteraceae) transcriptome permits large-scale unigene assembly and SSR marker discovery. PLoS ONE 8:e62293
Wang HF, Wang HW, Liu R, Xu YT, Lu ZC, Zhou CE (2018) Genome-wide identification of TCP family transcription factors in Medicago truncatula reveals significant roles of miR319-targeted TCPs in nodule development. Front Plant Sci 9:774
Wang JJ, Wang HB, Ding L, Song AP, Shen F, Jiang JF, Chen SM, Chen FD (2017) Transcriptomic and hormone analyses reveal mechanisms underlying petal elongation in Chrysanthemummorifolium ‘Jinba’. Plant Mol Biol 93:593–606
Wang ZB, Li N, Jiang S, Gonzalez N, Huang XH, Wang YC, Inze D, Li YH (2016) SCFSAP controls organ size by targeting PPD proteins for degradation in Arabidopsis thaliana. Nat Commun 7:11192
Zhang T, Qu YX, Wang HB, Wang JJ, Song AP, Hu YH, Chen SM, Jiang JF, Chen FD (2017) The heterologous expression of a chrysanthemum TCP-P transcription factor CmTCP14 suppresses organ size and delays senescence in Arabidopsis thaliana. Plant Physiol Biochem 115:239–248
Zhao LN, Li YQ, Xie Q, Wu YR (2017) Loss of CDKC;2 increases both cell division and drought tolerance in Arabidopsis thaliana. Plant J 91:816–828
Acknowledgements
This research was supported by the National Key Research and Development Program of China (2018YFD1000401), the National Natural Science Foundation of China (31,872,149), the Natural Science Fund of Qinghai Province, China (2018-HZ-819), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Contributions
XYQ, HBW and FDC designed the experiment. XYQ, YXQ, APS and PPC performed the experiment. XYQ, ZYG and WMF analyzed the data and wrote the manuscript. XYQ, JFJ, YXG and SMC revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Communicated by Paul Holford.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10725_2021_743_MOESM2_ESM.tif
Fig. S2. Characterization of the CnF-box. (a) Amino-acid comparison of CnF-box and F-box homologues from other species. (b) Phylogenetic relationship of CnF-box and other F-box family proteins. The bar (0.02) indicated branch length. The GenBank accession numbers of the amino acid sequences used were: HaF-box (Helianthus annuus, XP_022020705.1), CcF-box (Cynara cardunculus var. Scolymus, KVH89818.1), SiSKIP31(Sesamum indicum, XP_011082189.1), HuSKIP31(Herrania umbratica, XP_021273565.1), NnSKIP31-like (Nelumbo nucifera, XP_010275142.1), MnSKIP31 (Morus notabilis, XP_010111779.1), McSKIP31(Momordica charantia, XP_022140264.1), PpSKIP31 (Prunus persica, XP_007222722.1), PaSKIP31(Prunus avium, XP_021810235.1), CfSKIP31(Cephalotus follicularis, GAV80251.1), GmSKIP31-like (Glycine max, XP_003517102.1), MtSKIP31(Medicago truncatula, XP_003612486.1), AiSKIP31(Arachis ipaensis, XP_016201304.1), AdSKIP31(Arachis duranensis, XP_015963458.1), CqSKIP31(Chenopodium quinoa, XP_021744740.1), SoSKIP31(Spinacia oleracea, XP_021848668.1), HbSKIP31(Hevea brasiliensis, XP_021685246.1), JcSKIP31(Jatropha curcas, XP_012079765.1). Supplementary material 2 (TIF 3352.0 kb)
10725_2021_743_MOESM3_ESM.tif
Fig. S3. Differential expression patterns of CnF-box in various tissues of C. nankingense. Supplementary material 3 (TIF 1100.8 kb)
10725_2021_743_MOESM4_ESM.tif
Fig. S4. Subcellular localization of the CnF-box protein in onion epidermal cells and the transcriptional activation of CnF-box. (a) GFP activity generated by the p35S::GFP-CnCnF-box transgene introduced into onion epidermal peels. Fluorescence: images obtained in the green fluorescence channel; DIC: images obtained in bright light; Merge: overlay plots. Bar: 50 μm. (b) Yeast one-hybrid assay was used to detect transcriptional activation of CnF-box.. Supplementary material 4 (TIF 3504.1 kb)
Rights and permissions
About this article
Cite this article
Qi, X., Qu, Y., Jiang, J. et al. Heterologous expression of Chrysanthemum nankingense TCP13 suppresses leaf development in Arabidopsis thaliana. Plant Growth Regul 95, 331–341 (2021). https://doi.org/10.1007/s10725-021-00743-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-021-00743-3