Abstract
The transition metal elements like copper act as double-edged sword for living cells. Cu, a redox active metal, is essential for various biological processes, but at higher concentrations it leads to toxicity by inducing production of reactive oxygen species (ROS). Thus, the objective of the present study was to investigate the effects of exogenously applied castasterone on oxidative stress markers and redox homeostasis managers in Brassica juncea plants subject to copper stress for 30 days. Copper-exposed plants showed accumulation of free radicals (H2O2 and superoxide anion) and lipid peroxidation. However, the exogenous treatment of seeds via the seed soaking method with different concentrations of castasterone reduced H2O2 production, superoxide anion radical content, and lipid peroxidation, thus indicating improved detoxification of ROS. Enzyme activity was increased by 19.19% for guaiacol peroxidase, 16.20% for superoxide dismutase, 35.74% for glutathione peroxidase, 27.58% for dehydroascorbate reductase, and 42.75% for ascorbate peroxidase, with castasterone pre-soaking under copper stress. The levels of non-enzymatic antioxidants were also increased with castasterone pre-treatment under copper stress. It may be concluded that castasterone treatment enhanced redox homeostasis managers in addition to increased levels of osmoprotectants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The level of copper (Cu) is increasing in soil and water as a result of inappropriate agricultural practices such as overuse of fungicides with high Cu content, release of industrial wastewater, and mining activities (Sonmez and others 2006). Cu is a redox active metal that acts as a cofactor for many enzymes involved in photosynthesis, respiration, ethylene sensing, and lignification processes, such as Cu/Zn superoxide dismutase, polyphenol oxidase, ascorbate oxidase, cytochrome c oxidase, laccase, and plastocyanin (Yruela 2009). At the cellular level, Cu plays a role in signaling of transcription and protein trafficking machinery, iron mobilization, and oxidative phosphorylation (Yruela 2005). The range of Cu content in plant tissues is reported to vary between 3 and 20 ppm (Fernandes and Henriques 1991). Plants show toxicity symptoms above 20 ppm concentration. Various studies have reported the critical tissue concentration of Cu toxicity in a variety of species. O. sativa shows toxicity at 35 mg kg−1, safflower shows toxicity even at 10 mg kg−1 concentration (Borkert and others 1998). These symptoms include reduced root length, chlorosis, necrosis, leaf discoloration, and stunted growth. Cu toxicity also increases the production of reactive oxygen species (ROS) through Fenton–Haber–Weiss reactions, and thus causes a redox imbalance (Yurekli and Porgali 2006). As a result of this imbalance, damage occurs to cellular membranes and various essential biomolecules such as DNA, lipids, and proteins (Ahmad and others 2010, 2015, 2016). Maintenance of redox homeostasis and regulated production of ROS is essential for normal metabolism and growth in plants (Kasote and others 2015). To reduce the effects of these free radicals and limit functional and structural damage, plants have developed different mechanisms to cope with a high abundance of metal (Kováĉik and others 2008). Primary mechanisms for scavenging free radicals are broadly categorized into two systems, which include enzymatic and non-enzymatic antioxidants (Ahmad and others 2010, 2015, 2016). Antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (POD), ascorbate peroxidase (APOX), dehydroascorbate reductase (DHAR), and glutathione reductase (GR), play major roles in the removal of ROS via the Halliwell–Asada pathway, whereas glutathione-S-transferase (GST), and glutathione peroxidase (GPOX) act independently and reduce peroxides with the help of GSH (Kapoor and others 2014). It has been observed that short- and long-term exposure to Cu leads to significant up-regulation of various enzymes (Srivastava and others 2006). The non-enzymatic-based system is composed of ascorbic acid and glutathione, which are important antioxidants produced in cells (Gill and Tuteja 2010; Kanwar and others 2015). Plants also activate the sulfur assimilation pathway to fulfill the requirement of glutathione for the synthesis of phytochelatins, which assist further in the sequestration of heavy metals (Ederli and others 2004). Accumulation of various organic molecules such as proline, sugars, and free amino acids protects enzymes from degradation and stabilizes protein synthesis machinery (Choudhary and others 2012).
Phytohormones act as major endogenous signals for morphological, physiological, and molecular adaptive responses to soil metal toxicity. Owing to the close relationship between hormonal stimuli and improved metal toxicity, the application of hormones to plants is a sustainable strategy to enhance protective mechanisms against metal stress (Manara 2012; Poonam and others 2014). Brassinosteroids (BRs) are phytohormones involved in the regulation of several developmental and physiological processes required for vegetative and reproductive development (Gupta and others 2004; Kumar and others 2010; Poonam and others 2015; Sharma and others 2015). Castasterone (CS) belongs to the C28 group of BRs, which have strong biological activities and play major roles in the growth and development of plants (Kim and others 2004). CS is the most widely distributed BR with a presence in 53 plant species including B. juncea plants (Kanwar and others 2015). In our previous study, an increase in the growth characteristics was observed with pre-treatment of seeds with CS to Cu-stressed plants where a 102.37% increase in root length, 30.35% increase in shoot length, 59.89% increase in fresh weight, and 18.52% increase in dry weight were recorded (Poonam and others 2015). A number of studies have analyzed different BRs like homobrassinolide and epibrassinolide for their role in stress management in plants (Clouse 2011); however, the role of CS in stress management remains uncharacterized.
Brassica juncea L., also known as Indian mustard, is an important oil-producing crop grown widely in the Indian subcontinent. It is a good source of Vitamin A, C, and iron. It has medicinal potential due to the presence of different bioactive molecules like glycosides, flavonoids, phenols, etc. and is also used as a condiment. Brassica juncea species is a well-known hyperaccumulator of various metals including Cu and thus has potential to be used for phytoremediation purposes. In the present study, B. juncea plants were exposed to excess Cu metal stress and different physiological and biochemical parameters were determined. The objectives of the studies were to examine the ameliorative role of CS on Cu-induced oxidative stress and to address the hypothesis that exogenous priming of CS could remove the Cu-induced oxidative stress in the plants by improving defense mechanism of plants composed of enzymes, non-enzymatic antioxidants, and osmoprotectants.
Materials and Methods
Plant material and Experimental Design
Certified seeds of B. juncea L., variety RLC1, were surface sterilized with a 0.5% (v/v) sodium hypochlorite solution and rinsed five times with double-distilled water. The surface-sterilized seeds were treated with CS by soaking them in different concentrations of castasterone (CS) for 8 h. CS was purchased from Scitech, Prague and dissolved in HPLC grade methanol to make a stock solution (10−3 M). The stock solution was used to make working concentrations of CS (0, 10−11, 10−9, and 10−7 M). Garden soil was prepared by mixing soil and organic manure in a 3:1 ratio. Model pot experiments were performed in 10 × 12 inch pots and these pots were filled with 5.0 kg garden soil. Copper sulfate (CuSO4·5H2O) was used to make working concentrations of Cu (0 and 500 µM). The working solution of Cu was added to the pots in the ratio of 250 ml per kg soil. A randomized block design containing five replicates of each treatment was used for the experimental setup. The CS pre-soaked seeds were sown in pots and allowed to grow under natural conditions with regular watering for 30 days. After 30 days of sowing, the plants were harvested and used for biochemical analysis. The treatments were as follows:
-
1.
Control (0 µM Cu)
-
2.
Cu (500 µM)
-
3.
10−11 M CS,
-
4.
10−9 M CS,
-
5.
10−7 M CS
-
6.
10−11 M CS + 500 µM Cu,
-
7.
10−9 M CS + 500 µM Cu,
-
8.
10−7 M CS + 500 µM Cu
Growth Parameters
Plants were harvested after 30 days of sowing and analyzed for the morphological parameters, height of plants and number of leaves per plant.
Measurement of ROS Indicators
The production of superoxide anion radicals was estimated according to the method of Wu and others (2010). One gram of fresh plant leaf tissue was homogenized in 3 ml of 50 mM phosphate buffer (pH 7.8). The extract was centrifuged at 12,000×g for 15 min at 4 °C. The 0.5 ml of obtained supernatant was added to the 0.5 ml of phosphate buffer and 0.1 ml of hydroxylamine hydrochloride. This reaction mixture was incubated for 30 min at 25 °C. After incubation, 1 ml of solution was mixed with 1 ml of 1-naphthylamine and 1 ml of 3-aminobenzene. The reaction mixture was further incubated for 30 min at 25 °C. The absorbance of the reaction mixture was noted at 530 nm. A standard curve prepared using NaNO2 was used to calculate the superoxide anion content. For imaging of the superoxide anion radical, the method described by Jabs and others (1996) was followed with slight modifications. Leaves of different treatments were detached from 30-day-old plants of B. juncea and were vacuum infiltrated with sodium azide solution (10 mM in 10 mM phosphate buffer pH 7.8) for 2 min at room temperature. Following this, they were vacuum infiltrated with nitro blue tetrazolium (NBT, 0.1% in 10 mM phosphate buffer) for 2 min at room temperature and incubated for 2 h in the solution of NBT in dark at room temperature. Chlorophyll was removed from leaves using a solution of glycerol, acetic acid, and ethanol (1:1:3) at 37 °C. Leaf imaging was performed using a Nikon digital camera. H2O2 content was estimated using the method described by Jana and Choudhuri (1981). The malondialdehyde (MDA) content was measured according to the method of Heath and Packer (1968). MDA content was calculated using the extinction coefficient of 155 mM−1 cm−1.
Measurement of Heavy Metal Content
Harvested plants were separated into roots, shoots, and leaves and then washed properly with distilled water followed by washing with 0.5 M EDTA. The plant parts were blotted to remove extra water and then were dried completely in a hot air oven at 80 °C for 24 h. Dried plant tissue (1 g) was digested in a solution of HNO3 and HClO4 in the ratio 2:1 using the protocol provided by Allen and others (1976). The mixture was placed on a hot plate and incubated until only a clear solution remained. Digested samples were cooled and then diluted with double-distilled water to a final volume of 100 mL and filtered through No. 1 Whatman filter paper. The samples were then analyzed in triplicate for Cu concentration using atomic absorption spectroscopy (AAS, Shimadzu).
Elemental Analysis
Based on the results of the AAS analysis, the most effective Cu concentration and CS treatment conditions were used for elemental analysis following the method given by Ramamurthy and Kannan (2009). Dry leaf samples were used for elemental analysis via ESEM (Zeiss Supra 55 Field Emission Scanning Electron Microscope) equipped with an EDS (Energy-dispersive X-ray spectroscopy) system. Plant samples were mounted on an aluminum stub. Coating of each stub was performed with silver (Ag) using a vacuum system. Element composition was recorded as an EDS spectrum and element composition was quantified using Energy-dispersive X-ray spectroscopy (EDX) software (INCA). Triplicates of each sample were analyzed.
Measurement of Antioxidants
Enzymatic Antioxidants
The experiments were performed to determine the effect of Cu metal ions on protein content and activity of antioxidant enzymes. For the estimation of antioxidant enzymes, 1 g of frozen leaf tissue was homogenized in potassium phosphate buffer (50 mM, pH 7.0) containing 1 mM PMSF (phenylmethylsulfonyl fluoride), 0.5% triton X-100, 1 mM EDTA (ethylenediaminetetraacetic acid), and 2% PVP-30 (polyvinylpyrrolidone) using a chilled mortar and pestle. The homogenate was centrifuged at 4 °C for 20 min at 13,000×g. The resulting supernatant was transferred to a new vial and enzyme activity was estimated using a UV–visible double beam spectrophotometer (Systronics 2202).
Protein content was estimated according to the method of Bradford (1976). A standard curve was prepared using different concentrations of bovine serum albumin.
Estimation of SOD activity was performed according to the method of Kono (1978). The reaction was initiated by the addition of 0.1 mM hydroxylamine hydrochloride to the reaction mixture (50 mM sodium carbonate buffer, 24 µM NBT, 0.1 mM EDTA and 0.03% Triton X). After 2 min of incubation, the enzyme extract was added and the inhibition in reduction of NBT was estimated. The absorbance of the reaction mixture was recorded at 560 nm for 2 min. One unit of SOD activity is equivalent to the amount of enzyme required for 50% inhibition of NBT reduction min−1 g−1 FW. Hydroxylamine was autoxidized at pH 10.2 in the presence of EDTA, whereas NBT reduces leading to the production of nitrite. Due to the reduction of NBT, blue formazon is produced and accumulates leading to increase in absorbance at 560 nm. With the addition of SOD enzyme, superoxide radicals get trapped and thus cause inhibition of reduction of NBT to blue formazan formation.
CAT activity was measured according to the method of Aebi (1984). The addition of enzyme extract to the reaction mixture of 15 mM H2O2 in 50 mM phosphate buffer initiated the reaction and a decrease in absorbance at 240 nm was recorded.
The activity of POD was calculated according to the method of Putter (1974). The reaction was initiated by addition of plant enzyme extract in the reaction mixture (20 mM guaiacol, 12.3 mM H2O2, and 50 mM phosphate buffer). Production of GDHP (guaiacol dehydrogenation product) was observed by an increase in absorbance at 436 nm.
APOX activity was calculated according to the method of Nakano and Asada (1981). The reaction mixture contained 1 mM H2O2 and 0.5 mM ascorbate in 50 mM phosphate buffer (pH-7.0). The reaction was started with the addition of extract of the enzyme to the reaction mixture and decrease in absorbance per minute at 290 nm was recorded.
GR activity was determined according to the method of Carlberg and Mannervik (1975). The reaction mixture contains 1 mM oxidized glutathione, 0.1 mM NADPH, 1 mM EDTA, and 50 mM phosphate buffer. Addition of enzyme extract initiated the reaction and a decrease in absorbance was observed at 340 nm.
DHAR activity was measured according to the method of Dalton and others (1986). The reaction was started by addition of enzyme extract to the reaction mixture containing 2.5 mM reduced glutathione, 0.2 mM dehydroascorbate, 0.1 mM EDTA, and 50 mM phosphate buffer. The increase in absorbance was recorded at 265 nm.
Activity of GPOX was calculated according to the method of Flohe and Gunzler (1984). The reaction was started by addition of enzyme extract to the reaction mixture (0.5 mM EDTA, 1 mM sodium azide, 1 mM reduced glutathione, 0.15 mm NADPH, 0.15 mM H2O2, 2.4 GR units/ml, and 50 mM phosphate buffer). Change in absorbance at 340 nm per minute was recorded.
GST activity was estimated according to the method of Habig and Jacoby (1981). The activity was assayed by addition of enzyme extract to the reaction mixture containing 1 mM reduced glutathione, 1 mM 1-chloro-2,4-dinitrobenzene, and 100 mM phosphate buffer. Change in absorbance was recorded at 340 nm for 2 min.
Non-enzymatic Antioxidants
For the estimation of the content of the non-enzymatic antioxidants ascorbic acid and glutathione (GSH), a plant extract was prepared by homogenizing 1 g of plant tissues in Tris buffer (50 mM, pH 10.0) using a pre-chilled mortar and pestle. The homogenate was cleared by centrifugation at 12,000×g for 20 min and the obtained supernatant was used for assay of glutathione and ascorbic acid.
The method described by Roe and Kuether (1943) was used for the estimation of ascorbic acid content. Activated charcoal (100 mg) was added to the 4 ml DDW, 0.5 ml plant extract, and 0.5 ml 50% TCA. This reaction mixture was mixed well and filtered through No.1 Whatman filter paper. To 1 ml filtrate, 0.4 ml DNPH reagent was added. This reaction mixture was incubated at 37 °C for 3 h. After incubation, 1.6 ml of cold 65% H2SO4 was added and incubated at room temperature for 30 min. The absorbance of the reaction mixture was taken at 520 nm.
The GSH content was estimated according to the method of Sedlak and Lindsay (1968) using Ellman’s reagent. 100 µl of plant extract was mixed with 1 ml of Tris buffer and 50 µl of DTNB. Then, 4 ml of absolute methanol was mixed to this and kept at room temperature for 15 min. Then this reaction mixture was centrifuged at 3000×g for 15 min at 4 °C. The absorbance of the mixture was noted at 412 nm.
Measurement of Isozymes Through Native Polyacrylamide Gel Electrophoresis
Plant tissue (1 g) was homogenized in either sodium acetate buffer (50 mM, pH 4.0) for POD, or in phosphate buffer (50 mM, pH 7.0) for CAT and SOD isoenzymes. Equal protein amounts were loaded into each well and electrophoresed using a 4% stacking gel and a 12.5% resolving gel. For POD, following electrophoretic separation, the gel was stained according to the procedure described by Fielding and Hall (1978). For CAT, the gel was stained according to the method proposed by Woodbury and others (1971). For SOD, the gel was stained using the method of Beauchamp and Fridovich (1971).
Measurement of Amino Acids
Total free amino acids were estimated according to the method of Hamilton and Van Slyke (1943). The 1 g of leaf samples was homogenized in 50 mM phosphate buffer (pH-7.0) followed by centrifugation at 13,000×g for 15 min at 4 °C. To 1 mL of plant extract, 10% aqueous pyridine and 2% ninhydrin were added and the mixture was heated in a boiling water bath for 30 min. Following heating, the reaction mixture was diluted to 50 mL with distilled water and absorbance was measured at 570 nm. Amino acid content was calculated using a standard curve prepared using leucine.
Cysteine content was calculated according to the method of Gaitonde (1967). Samples (1 mL) were prepared by homogenizing plant tissue in 5% perchloric acid mixed with acetic acid and acid ninhydrin. Samples were then boiled in a water bath for 10 min and rapidly cooled under tap water. Samples were then diluted to 5 mL with 95% ethanol and absorbance was measured at 560 nm. Cysteine was used for preparation of the standard curve.
Proline content was measured following the procedure described by Bates and others (1973). One gram of fresh plant leaves were homogenized in 3 sulphosalicylic acid and centrifuged at 13,000×g for 15 min at 4 °C. Glacial acetic acid and acid ninhydrin were mixed with 2 mL of plant sample supernatant. This reaction mixture was incubated for 1 h in a boiling water bath. The reaction was terminated by placing the test tubes in an ice bath. Following cooling, toluene was added to the reaction mixture, which was then stirred for 20–30 s. The toluene layer was separated and brought up to room temperature. The intensity of red coloring was quantified by the absorbance measured at 520 nm. A standard curve prepared using L-proline was used for the calculation of proline content.
Measurement of Thiols
For the measurement of thiols, plant samples were extracted in 0.02 M EDTA buffer. Total thiols (TTs), non-protein thiols (NPTs), and protein thiols (PTs) were measured according to the method of Sedlak and Lindsay (1968). The absorbance of the reaction mixtures was measured at 412 nm. Protein thiols were calculated by subtracting the non-protein thiols from total thiols.
Measurement of Osmoprotectants
Total sugars were measured according to the Anthrone method described by Yem and Willis (1954). Following the measurement of absorbance at 630 nm, sugar content was calculated from a standard curve made using glucose.
Measurement of Flavonoids
Flavonoids were measured based on the method of Zhishen and others (1999). The absorbance was measured at 510 nm. Rutin was used to create a standard curve for the calculation of flavonoid content.
Statistical Analysis
The results obtained were statistically analyzed using the two-way ANOVA test. Mean values for the different treatments were compared with the control using Tukey’s HSD tests at p ≤ 0.05.
Results
Growth Parameters
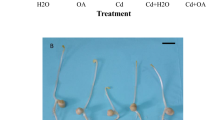
In 30-day-old plants, Cu treatment affected and reduced the growth parameters like height of plants and number of leaves in B. juncea plants in comparison to control plants. The height of plants and number of leaves decreased at the selected concentrations of copper (39.01% in height of plants and 55.55% in number of leaves) (Fig. 1a). The application of CS as seed soaking showed improvement in the height of plants and number of leaves. The maximum significant increase in the height of plants by 49.92% and the number of leaves by 116.61% was recorded in 10−7 M CS-treated seeds under Cu toxicity compared to only Cu-treated plants (Fig. 1b).
Generation of ROS
Cu treatment triggered H2O2 production, increase in superoxide radical content, and lipid peroxidation. The H2O2 production increased by 48.19%, superoxide radical content by 30.51%, and MDA content by 26.27% under Cu toxicity compared to control plants (Fig. 2a–c). A significant decrease in H2O2 content (6.35%) was observed in plants grown with 10−7 M CS-treated seeds in Cu-containing soils. Superoxide radical content decreased by 14.92% and MDA content by 5.81% in plants grown with 10−7 M CS-treated seeds under Cu toxicity with respect to Cu-only-treated plants.
ROS content was found to be most effectively decreased by the 10−7 M CS seed treatment. This concentration in conjunction with Cu-treated soil (500 µM) was therefore used to create tissues for the imaging of superoxide anion radical content in the leaves of 30-day-old B. juncea plants. The presence of superoxide ions (O2−) was also visualized in intact leaves using NBT. The intense color developed in leaves from plants under Cu treatment indicated high-level production of these ions, whereas CS seed pre-treatment resulted in a lower intensity of color development, which indicated lower accumulation of superoxide anion radicals (Fig. 3).
Cu Ion Uptake in Different B. juncea Plant Parts
Significant uptake of Cu ions was observed in 30-day-old B. juncea plants. Cu uptake in plants increased following the addition of Cu ions to the soil. Increased Cu uptake of 422.32% in roots, 74.04% in shoots, and 160.92% in leaves was observed in Cu-treated plants as compared to control (Fig. 4a–c). Plants treated with different concentrations of CS under Cu stress showed enhanced uptake of Cu compared to Cu-treated plants alone. The maximum uptake of Cu, 16.45, 10.67, and 25.44% was recorded in roots, shoots, and leaves, respectively at 10−7 CS-treated plants under Cu stress compared to only Cu-treated plants.
Elemental Analysis
Figure 5a–c shows C decreased by 21.55%, O by 16.69% but N content showed an insignificant decrease as compared to control. However, supplementation of CS (10−7) enhanced the C, N, and O content. The contents of Na, Mg, S, Cl, K, and Ca were enhanced by 87.50, 76.92, 55.35, 175.67, 462.40, and 198.85%, respectively, by Cu stress relative to control. The priming of 10−7 M CS as a pre-treatment of seeds further enhanced the above elements.
Antioxidant Defense System
Cu toxicity significantly altered the activity of antioxidant enzymes such as SOD, CAT, POD, APOX, GR, DHAR, GPOX, and GST as compared to control plants (Fig. 6a–h). Cu toxicity enhanced the SOD activity by 114.94%, CAT by 79.07%, POD by 20.00%, APOX by 29.79%, GR by 23.65%, DHAR by 63.86%, GPOX by 100.71%, and GST by 51.81% relative to control in the present study. Further increase in the activity of these enzymes was observed in plants grown with CS pre-treated seeds; however, seeds treated with 10−7 CS showed better results as compared to 10−11 and 10−9 CS. The 10−7 CS dosage was found to be most effective in enhancing the activity of SOD, CAT, POD, APOX, GR, DHAR, GPOX, and GST by 19.25, 15.64, 28.12, 9.84, 45.94 27.56, 35.99, and 16.87%, respectively, compared to plants treated with Cu only.
On the basis of these results, 10−7 M CS was selected as the dosage for the study of isoenzymes of CAT, POD, and SOD. For native polyacrylamide gel electrophoresis analysis, SOD displayed six bands of three isoenzymes in Cu-treated plants and CS treatment further enhanced their band intensity (Fig. 7a). CS treatment and Cu stress resulted in the detection of three bands of POD isoenzymes in comparison to the single isoenzyme observed in control plants (Fig. 7b). Furthermore, the band intensity was greater in samples from Cu- and CS-treated plants. Regarding CAT isoenzymes, a single band was observed for all CS and Cu treatment concentrations (Fig. 7c).
Redox Homeostasis Managers and Osmoprotectants
Various redox homoeostasis managers such as glutathione, ascorbic acid, free amino acids, cysteine, and proline were analyzed in B. juncea plants under Cu stress. Cu stress enhanced the ascorbic acid and glutathione content by 6.31% and 32.63%, respectively, when compared to control plants (Fig. 8a, b). The pre-soaking of seeds in CS further enhanced the ascorbic acid and glutathione content and the concentration of 10−7 M CS was seen to be more effective. The increase of 9.90% in ascorbic acid and 8.33% in glutathione content was observed in Cu-treated plants supplemented with 10−7 M CS.
The total free amino acid content decreased by 8.98% in Cu-stressed plants compared to control plants. Increases in the levels of total free amino acids were observed in the Cu-treated plants grown from CS pre-treated seeds as compared to Cu-only-treated plants, with maximum enhancement of 28.75% being observed with 10−7 M CS-treated plants grown under Cu toxicity (Fig. 9a).
An increase of 40.35 and 34.67% in cysteine and proline content, respectively, was observed under Cu stress relative to control plants. The pre-sowing seed treatment of plants with different levels of CS resulted in a further increase in cysteine and proline content. The 10−7 M CS treatment was found to be more effective in enhancing cysteine content (14.35%) and proline content (11.67%) compared to Cu-only-treated plants (Fig. 9a–c). The contents of TTs (30.00%), NPTs (60.41%), and PTs (28.57%) were significantly increased over control plants in Cu-treated plants (Fig. 9a–c). CS seed pre-treatment further enhanced their contents and 10−7 M CS treatment resulted in more accumulation. With 10−7 M CS treatment, 9.61, 10.38, and 8.88% increase in TTs, NPTs, and PTs, respectively, was recorded as compared to Cu-only-treated plants.
Total sugar content was observed to increase by 22.76% under Cu toxicity conditions relative to control plants. Further enhancement in sugar content by 11.09% was recorded in Cu-treated plants grown from 10−7 M CS-treated seeds in comparison to Cu-only-treated plants (Fig. 10d).
Protein content decreased by 9.43% in B. juncea plants grown in Cu-treated soil compared to protein content in control plants (Fig. 10e). Conversely, an improvement in protein content was observed in plants grown from CS pre-treated seeds. A CS seed pre-treatment at 10−7 M partially neutralized the toxic effects of Cu and enhanced protein content by 14.82% when compared to protein content in only Cu-treated plants.
The flavonoid content increased by 120.15% in Cu-stressed plants compared to control plants. Further enhancement in flavonoid content was recorded with pre-treatment of seeds with different concentrations of CS. However, the observed increase in flavonoid content in co-treatment of CS and Cu was not significant. Nevertheless, a maximum increase (6.82%) in flavonoid content was observed in the Cu-stressed plants grown from seeds pre-treated with 10−7 M CS (Fig. 10f).
Discussion
Cu is an essential micronutrient, a minimal amount of Cu is required for the normal growth and metabolism of plants. However, it is also potentially toxic in nature when present in excess and causes deleterious effects on plant production and survival. Being a redox active metal, free Cu participates in redox reactions and directly generates reactive oxygen species (ROS) through Fenton reactions and thus damages proteins, DNA, and other biomolecules. At high concentrations, Cu causes inhibition of root growth, chlorosis, necrosis, stunting, and leaf discoloration in plants. At the cell level, Cu toxicity is caused by inhibition of enzyme activity or protein function, affecting the cell transport system, deficiency of other essential ions, and oxidative damage (Yruela 2005). Plants have various stress-protective strategies and BRs have shown potential in successfully alleviating different abiotic stresses (Ahmad and others 2017; Kohli and others 2017). The growth parameters like height of plants, biomass of plants, and number of leaves indicate the toxicity induced by heavy metals. In the present study, growth parameters like height of plants and number of leaves decreased with Cu treatment. Our findings are clearly supported by Fariduddin and others (2013) and Feigl and others (2015) who observed decreased morphological parameters in C. sativus, B. juncea, and B. napus plants. However, improvement in the growth parameters of B. juncea plants was observed with CS priming. Fariduddin and others (2009) reported increased morphological characteristics by the application of 24-epibrassinolide. BRs play a role in cell elongation and modify the permeability of the plasma membrane under adverse conditions and thus improve the growth of plants.
In the present study, plants showed significant uptake of metal ions when soils were treated with Cu. Similar results were reported by Ariyakanon and Winaipanich (2006). But when CS pre-treatment was applied to seeds, Cu uptake in roots, shoots, and leaves of B. juncea was marginally increased compared to only metal-treated plants. Plants accumulate and sequester metal in roots as a defensive strategy to protect upper plant parts, specifically metabolically active photosynthetic cell vacuoles, from heavy metal damage (Rascio and Navari-Izzo 2011). Increased uptake of Cd and Ni with exogenous application of CS and epibrassinolide has been reported in B. juncea and S. nigrum (Kaur and others 2016; Soares and others 2016). It can be hypothesized that application of BRs increases the redistribution of metals in shoots and leaves as a strategy for root defense and enhances the chances of survival.
Being a redox active transition metal, Cu catalyzes the production of reactive oxygen species like H2O2, O2 −, and OH− by Haber–Weiss and Fenton reactions and thus causes an oxidative burst in cells (Halliwell and Gutteridge 1984). Our results indicate that the levels of H2O2 and O2 − increased in B. juncea plants exposed to higher levels of Cu. The present findings are supported by other authors, where heavy metal accumulation led to overproduction of ROS (Ahmad and others 2015; Kaur and others 2016). The cell membranes are the primary sites of injury under heavy metal toxicity due to the overproduction of ROS (Ahmad and others 2015). Copper stress induces structural and functional damage to cell membranes and increases lipid peroxidation through MDA production (product of cell membrane lipid peroxidation), a biomarker of stress (Yamamoto and others 2001). Other studies also have reported that Cu exposure causes an increase in MDA content and the cell membranes are the primary target of Cu toxicity (Liu and others 2014). Decreased levels of ROS with the priming of CS (seed soaking method) under Cu stress was observed in the present study, which might be due to more efficient scavenging of ROS. Brassinosteroids are well-known secondary messengers and induce the up-regulation of components of the antioxidant defense system (Choudhary and others 2012). Other than this, H2O2 acts as a signaling molecule and mediates the induction of antioxidant genes by BRs (Xia and others 2009). Because MDA content is directly linked with ROS, decreased ROS levels can also lead to reduced lipid peroxidation (Ahmad and others 2015). These findings are supported by previous studies in which MDA content decreased with BRs under heavy metal stress (Soares and others 2016). BRs are also reported to modify membrane structure and provide the membrane stability under stressful conditions; hence treatment of plants with CS showed higher membrane stability and reduced lipid peroxidation (Rady 2011).
Heavy metals affect the uptake and distribution of various mineral elements because of their interaction with these elements. In this study, the content of N, Na, Mg, K, S, Cl, and Ca increased with Cu exposure, whereas the content of C and O decreased. The increase in mineral content has been reported in various plants like Arabidopsis under Cu stress (Martinez-Penalver and others 2012), and P. vitatta under arsenic stress (Tu and Ma 2005). Heavy metals interfere with mineral uptake by affecting the permeability of the plasma membrane (Sarwar and others 2010). No direct relationship has been found for increased mineral content under metal toxicity by BRs but it can be hypothesized that BRs help in the stabilization of plant membranes and thus might help in the uptake of mineral elements. Enhanced contents of various mineral elements help in stress tolerance (Singh and others 2015). These elements are part of various mechanisms, reactions, and enzymes. Potassium ions help maintain anion–cation balance and have a role in protein synthesis enzyme activation. Phosphorus (P) is a constituent of cell membranes and nucleic acids. P helps in reducing the metal toxicity either by reducing metal mobility or by diluting the metal (Sarwar and others 2010). Similarly, sulfur (S) is part of various co-enzymes, vitamins, and ferredoxin. Increased S content helps to increase the S-containing defense molecules like GSH and phytochelatins, and increase the activity of S-containing enzymes (Leustek and Saito 1999; Khan and others 2009). Thus, the increase in mineral content helps in increasing stress tolerance induced by metal toxicity.
As stated above, the overproduction of ROS under metal stress disturbs the redox balance of cells leading to changes in the cellular signaling pathways that regulate cell division (Chiu and Dawes 2012). The exact mechanism of toxicity caused by heavy metals in plants is still not clear. Plants have evolved complex defense mechanisms like antioxidant systems to enhance their tolerance to various stresses and improve their chances of survival (Pitzschke and others 2006). The antioxidant system includes both enzymatic and non-enzymatic components. Activation of intricate metabolic activities such as antioxidant pathways, in particular ROS scavenging systems, in the cells contribute towards plant stress tolerance (El-Mashad and Mohamed 2012). The results obtained in the present study clearly showed the increase in the activities of various enzymes under Cu stress (Fig. 4). Superoxide dismutase (SOD) acts as the first line of defense and converts O2 − into H2O2 which can be converted to water molecules by catalases and peroxidases (Mourato and others 2012). The H2O2 is decomposed to water molecules with the help of ascorbate peroxidase (APOX), catalase (CAT), and guaiacol peroxidase (POD) (Mittler and others 2011). The activities of enzymes involved in the ascorbate glutathione cycle (glutathione reductase and dehydroascorbate reductase) also have been reported to be enhanced, showing the role of this metabolic cycle in providing resistance to heavy metal stress (Ahmad and others 2015; Ali and others 2015). Glutathione peroxidase (GPOX) is part of the main enzymatic defense system against oxidative membrane damage due to its capability of repairing membrane lipid peroxidation (Kuhn and Borchert 2002). Glutathione-s-transferase is known for detoxification of xenobiotic compounds by limiting oxidative damage and stress responses in plants. The increase in SOD, APOX, CAT, DHAR, GR, GST, and GPOX activities was observed in the present study in both Cu and CS treatment. These results are supported by the findings of Poonam and others (2014), and Yadav and others (2016), who also reported increases in the activities of various enzymes with metal as well BR treatment. It has been reported that BRs can regulate the activity of the antioxidant system and help in scavenging overproduced ROS (Ashraf and others 2010) and can provide tolerance by inducing expression of regulatory genes like respiratory burst oxidase homologue (RBOH), mitogen-activated protein kinase 1 (MAPK1), and mitogen-activated protein kinase 3 (MAPK3), and also activating genes involved in antioxidative defense and responses (Xia and others 2009; Li and others 2016). BRs cause a transient increase in H2O2 levels by activating the plasma membrane NADPH oxidase enzymes, and H2O2 which is also a signaling molecule induces the antioxidative defense system of plants (Xia and others 2009). The change in activity of these enzymes might be due to the enhanced protein synthesis in response to EBL, or a change in the kinetic properties of the enzymes (Lascano and others 1998). Cu treatment also altered the isoenzyme pattern of SOD and POD, but did not change the CAT isoenzyme pattern in the present study. The pre-treatment of plants with CS under Cu stress further increased the band intensity indicating the up-regulation of expression of POD, CAT, and SOD. This increase enhances plant tolerance towards metal toxicity.
Non-enzymatic low-molecular weight molecules such as ascorbate and glutathione act as major redox buffers and are enzyme cofactors that play a role in defense. These molecules have the capacity to chelate metal ions, which leads to a reduction in the metal’s catalytic ability to generate ROS and also free radical scavenging. In plant cells, the combination of ascorbate, glutathione, and tocopherol constitutes an integral part of the abiotic stress response (Szarka and others 2012). Ascorbic acid, tocopherol, and glutathione scavenge hydrogen peroxide, superoxide radicals, and hydroxyl radicals either via direct reactions or with the help of enzyme catalysis (Foyer and Noctor 2011). In the present investigation, an increase in ascorbic acid and glutathione content following both Cu and CS treatment was observed. This result is in accordance with Choudhary and others (2012), who observed an increase in ascorbic acid and glutathione content under Cu stress in addition to EBL treatment in Raphanus sativus seedlings. It has been reported that BRs play a role in controlling the activity of GalLDH (l-galactono-1,4-lactone dehydrogenase) activity which helps in catalyzing the last step of ascorbic acid formation and therefore, affects the ascorbic acid formation and promotes ascorbic acid accumulation in leaves of plants (Mazorra Morales and others 2014). Similarly, it has been found that BRs induce the synthesis of GSH by up-regulating the transcript of GSH 1–2 encoding for γ-glutamyl cysteine synthetase and glutathione synthetase, respectively (Zhou and others 2015).
In the present study, free amino acid content decreased under Cu stress and the results are in accordance with Zemanová and others (2013), who also observed decreased free amino acid content under Cd stress in Noccaea caerulescens and Arabidopsis halleri. Amino acids being an important part of primary metabolism and a product of photosynthesis and nitrogen assimilation, thus act as connecting links of nitrogen and carbon metabolism. With the priming of CS, the free amino acid content was observed to increase in the present study in Cu-stressed plants (Fig. 7). It has been observed that application of BRs enhances the activity of nitrate reductase along with higher accumulation of free amino acids in roots of Cajanus cajan under salinity stress (Dalio and others 2013). BRs maintain cell membrane integrity under adverse conditions and thus preserve nitrate uptake. The BRs also help in maintaining photosynthetic systems, it can be hypothesized that increased amino acids content may be connected with restored photosynthesis (Dalio and others 2013).
Cysteine is produced as a final product of sulfur assimilation in plants and it acts as a sulfur donor for synthesis of methionine, glutathione, phytochelatins, some vitamins, and proteins containing thiols. Cysteine also has a role in redox signaling as it is present in various cell compartments (Romero and others 2014). We have observed enhanced concentrations of cysteine with application of both Cu as well as CS. These results are in accordance with Aly and Mohamed (2012), who reported gradual increase in the cysteine content under various levels of Cu stress in maize plants. Genetic modification in the capacity for metal-induced synthesis of cysteine supported tolerance of Arabidopsis under acute cadmium toxicity (Dominguez-Solis and others 2004). Cysteine acts as antioxidant and helps in scavenging of free radicals by its own (Zhang and others 2014). The increased sulfur content might be related with the enhanced accumulation of cysteine under both Cu as well as CS application.
Proline accumulation occurs in plants as a response to different environmental stresses. This amino acid acts as a signaling molecule required for recovery of plants from adverse conditions (Szabados and Savouré 2009). It also helps in membrane stabilization, acts as an osmoprotectant, aids ROS removal, and buffers redox potential; therefore, decreasing oxidative stress produced by abiotic stress conditions of drought, salinity, and heavy metals (Gill and Tuteja 2010; Boaretto and others 2014). B. juncea plants responded to Cu stress with accumulation of proline both with or without CS treatment, as reported previously with different plant species (Fariduddin and others 2013; Poonam and others 2014). BRs are reported to induce the expression of genes involved in proline metabolism (Ozdemir and others 2004), thus the enhanced production led to increased proline content in B. juncea plants.
Enhanced levels of total thiols, non-protein thiols (NPTs), and protein thiols were observed in the present study. It has been reported that metal stress increases the sulfur reduction pathway by affecting sulfur uptake and transport. Zhang and others (2009) observed increased protein thiol content in rice seedlings under different Cu (0, 50, 100, 200 µM) concentrations. Aly and Mohamed (2012) showed a significant increase in the content of total thiols and protein thiols in maize plants, whereas a gradual decrease was observed in non-protein thiol content. The NPTs are composed of phytochelatins, glutathione, cysteine, and various acid-soluble sulfhydryl components (De Vos and others 1993). Phytochelatins are known for binding toxic metals and induction of phytochelatin synthesis enhances plant tolerance towards metal toxicity (Cobbett 2000). Pre-soaking of seeds with CS further enhanced the thiol content in the present study. Thiols contain sulfur as their integral part thus, its content depends on the amount of sulfur available. In this study, increase in the sulfur content was recorded. It can be hypothesized that enhanced sulfur content led to the formation of thiols which act as antioxidants and help in stress amelioration. This increase in thiol content with CS priming suggests a role for BRs in enhancing tolerance of plants towards Cu stress.
Osmoprotectants like sugars and flavonoids also produce and accumulate under abiotic stress and play an important role in antioxidant mechanisms to enhance tolerance under stress conditions (Nakabayashi and others 2014; Ahmad and others 2016). In the present study, an increase in sugars was observed with Cu toxicity. These results correspond with Choudhary and others (2012), who observed increased soluble sugars under Cu stress in radish plants. The priming of seeds with CS further enhanced the sugar content and was also reported by other authors in different plant species under metal stress (Bali and others 2016). BRs are known for regulating CESA (cellulose synthase) gene expression required for the synthesis of cellulose, and they are considered as major regulators of carbohydrate and sugar accumulation under stress (Kaur and others 2014).
Similarly, the content of flavonoids decreased under Cu stress with or without CS priming in the present study. Flavonoids act as secondary antioxidant compounds produced during stress conditions to resist the adverse effects either by chelating metals or by inhibiting ROS generation (Grael and others 2010; Ahmad and others 2016). The increase in flavonoid content was also reported under Cu, Cd, and Zn stress (Márquez-García and others 2012; Gautam and others 2016). It has been reported that exogenous application of BRs can increase the level of flavonoids under different environmental stresses like phenanthrene (Ahammed and others 2013), cadmium, and mercury (Kapoor and others 2014). It has also been reported that brassinosteroids may increase the activity of PAL enzymes, thereby resulting in enhanced production of flavonoids following BR application (Ahammed and others 2013).
Conclusions
The present study suggests that Cu toxicity leads to the over production of ROS, which causes an imbalance in the redox state of the cell, reduces the growth, and affects metabolic activity in plants. The soaking of seeds with CS showed a positive response by improving plant tolerance to Cu, reducing the toxicity as seen in biometric analysis. The present study revealed that the priming of CS in B. juncea plants significantly improved the level of antioxidant enzymes (SOD, CAT, POD, APOX, DHAR, GR, GST, and GPOX), co-enzymes of SOD, POD, and CAT, antioxidants (glutathione and ascorbic acid), thiol (total, protein, and non-protein), osmoprotectants (sugars and flavonoids), free amino acid, and proline and cysteine contents. An enhancement in the level of the antioxidant system aids in rectifying the redox balance under Cu stress. It can also be concluded that BRs showed ameliorative effects on Cu toxicity by improving redox homeostasis leading to improved growth of B. juncea plants. The 10−7 M CS treatment was the most effective in significantly improving most of the biochemical constituents related to the antioxidant defense system. The increase in the antioxidant potential due to priming of CS was further reflected by a reduction in H2O2, superoxide radicals, and MDA content.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahammed GJ, Zhou YH, Xia XJ, Mao WH, Shi K, Yu JQ (2013) Brassinosteroid regulates secondary metabolism in tomato towards enhanced tolerance to phenanthrene. Biol Plant 57:154–158
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–175
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LP (2015) Alleviation of cadmium toxicity in Brassica juncea L. (Czern & Coss) by calcium application involves various physiological and biochemical strategies. PLoS ONE 10:e0114571
Ahmad P, Allah EA, Hashem A, Sarwat M, Gucel S (2016) Exogenous application of selenium mitigates cadmium toxicity in Brassica juncea L. (Czern & Cross) by up-regulating antioxidative system and secondary metabolites. J Plant Growth Regul 6:1–15
Ahmad A, Ahanger MA, Egamberdieva D, Alam P, Alyemeni MN, Ashraf M (2017) Modification of osmolytes and antioxidant enzymes by 24-epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J Plant Growth Regul doi. 10.1007/s00344-017-9730-6)
Ali B, Gill RA, Yang S, Gill MB, Farooq MA, Liu D, Daud MK, Ali S, Zhou W (2015) Regulation of cadmium-induced proteomic and metabolic changes by 5-aminolevulinic acid in leaves of Brassica napus L. PLoS ONE 10(4):e0123328
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C, Roberts JD (1976) Chemical analysis. In: Chapman SB (ed) Methods in plant ecology. Blackwell Scientific Publications, Oxford, pp 424–426
Aly AA, Mohamed AA (2012) The impact of copper ion on growth thiol compounds and lipid peroxidation in two maize cultivars (Zea mays L.) grown in vitro. Aust J Crop Sci 6:541–549
Ariyakanon N, Winaipanich B (2006) Phytoremediation of copper contaminated soil by Brassica juncea (L.) Czern and Bidens alba (L.) DC. var. radiate. J Sci Res Chulalongkorn Univ 31:50–56
Ashraf M, Akram NA, Arteca RN, Foolad MR (2010) The physiological, biochemical and molecular roles of brassinosteroids and salicylic acid in plant processes and salt tolerance. Crit Rev Plant Sci 29:162–190
Bali S, Poonam, Kohli SK, Kaur H, Bhardwaj R (2016) Improvement in photosynthetic efficiency of Brassica juncea under copper stress by plant steroid hormone. J Chem Pharmaceut Res 8(5):464–470
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem Rev 44:276–287
Boaretto LF, Carvalho G, Borgo L, Creste S, Landell MGA, Mazzafera P, Azevedo RA (2014) Water stress reveals differential antioxidant responses of tolerant and non-tolerant sugarcane genotypes. Plant Physiol Biochem 74:165–175
Borkert CM, Cox FR, Tucker MR (1998) Zinc and copper toxicity in peanut, soybean, rice, and corn in soil mixtures. Commun Soil Sci Plant Anal 29:2991–3005
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Chiu J, Dawes IW (2012) Redox control of cell proliferation. Trend Cell Biol 22:592–601
Choudhary SP, Oral V, Bhardwaj R, Yu J-Q, Tran LS (2012) Interactions of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J Expt Bot 63:5659–5675
Clouse SD (2011) Brassinosteroid signal transduction: from receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 23:1219–1230
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy metal detoxification. Curr Opin Plant Biol 3:211–216
Dalio RJD, Pinheiro HPP, Sodek L, Haddad CRB (2013) 24-epibrassinolide restores nitrogen metabolism of pigeon pea under saline stress. Bot Stud 54:9
Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ (1986) Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci USA 83:3811–3815
De Vos C, Ten Bookum W, Vooijs R, Schat H, DeKok L (1993) Effect of copper on fatty acid composition and peroxidation of lipids in the roots of copper tolerant and sensitive Silene cucubalus. Plant Physiol Biochem 31:151–158
Dominguez-Solis JR, Lopez Martin MC, Ager MC, Ynsa MD, Romero LC, Gotor C (2004) Increased cysteine availability is essential for cadmium tolerance and accumulation in Arabidopsis thaliana. Plant Biotechnol J 2:469–476
Ederli L, Reale L, Ferranti F, Pasqualini S (2004) Responses induced by high concentration of cadmium in Phragmites australis roots. Physiol Plant 121:66–74
El-Mashad A, Mohamed H (2012) Brassinolide alleviates salt stress and increases antioxidant activity of cowpea plants (Vigna sinensis). Protoplasma 249:625–635
Fariduddin Q, Yusuf M, Hayat S, Ahmad A (2009) Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environ Expt Bot 66:418–424
Fariduddin Q, Khalil RR, Mir BA, Yusuf M, Ahmad A (2013) 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ Monit Assess 185(9):7845–7856
Feigl G, Kumar D, Lehotai N, Pető A, Molnár Á, Rácz É et al (2015) Comparing the effects of excess copper in the leaves of Brassica juncea (L. Czern) and Brassica napus (L.) seedlings: growth inhibition, oxidative stress and photosynthetic damage. Acta Biol Hung 66(2):205 – 21
Fernandes JC, Henriques FS (1991) Biochemical, physiological and structural effects of excess copper in plants. Bot Rev 57:246–273
Fielding JL, Hall JL (1978) A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum: II. Distribution of enzymes in relation to root development. J Expt Bot 29:983–991
Flohe L, Gunzler WA (1984) Assays of glutathione peroxidase. Methods enzymol 105:114 – 21
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18
Gaitonde M (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Gautam S, Anjani K, Srivastava N (2016) In vitro evaluation of excess copper affecting seedlings and their biochemical characteristics in Carthamus tinctorius L. (variety PBNS-12). Physiol Mol Biol Plants 22:121
Gill SS, Tuteja N (2010) Polyamines and abiotic stress tolerance in plants. Plant Signal Behav 5:26–33
Grael CFF, Kanashiro A, Kabeya LM, Jordao CO, Takeara R, Gobbo-Neto L, Polizello ACM, Lucisano-Valim YM, Lopes NP, Lopes JLC (2010) In vitro study of antioxidant and scavenger properties of phenolic compounds from Lychnophora species. Química Nova 33:867‒870
Gupta D, Bhardwaj R, Nagar PK, Kaur S (2004) Isolation and characterization of brassinosteroids from leaves of Camellia sinensis (L.) O. Kuntze. Plant Growth Regul 43(2):97–100
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405
Halliwell B, Gutteridge JM (1984) Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J 19(1):1–14
Hamilton PB, Van Slyke DD (1943) Amino acid determination with ninhydrin. J Biol Chem 150:231–233
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 273(5283):1853–1856
Jana S, Choudhuri M (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aqua Bot 12:345–354
Kanwar MK, Poonam, Bhardwaj R (2015) Arsenic induced modulation of antioxidative defense system and brassinosteroids in Brassica juncea L. Ecotoxicol Environ Saf 115:119–125
Kapoor D, Rattan A, Gautam V, Kapoor N, Bhardwaj R (2014) 24-Epibrassinolide mediated changes in photosynthetic pigments and antioxidative defense system of radish seedlings under cadmium and mercury stress. J Stress Physiol Biochem 10(3):110–121
Kasote DM, Katyare SS, Hegde MV, Bae H (2015) Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int J Biol Sci 11(8):9892–9991
Kaur H, Sirhindi G, Bhardwaj R, Sharma P, Mudasir M (2014) 28-Homobrassinolide modulate antenna complexes and carbon skeleton of Brassica juncea L. under temperature stress. J Stress Physiol Biochem 10:186–196
Kaur R, Yadav P, Thukral AK, Walia A, Bhardwaj R (2016) Co-application of 6-ketone type brassinosteroid and metal chelator alleviates cadmium toxicity in B. juncea L. Environ Sci Pollut Res. doi:10.1007/s11356-016-7864-x
Khan NA, Anjum NA, Nazar R, Iqbal N (2009) Increased activity of ATP-sulfurylase and increased contents of cysteine and glutathione reduce high cadmium- induced oxidative stress in mustard cultivar with high photosynthetic potential. Russ J Plant Physiol 56:670–677
Kim T-W, Chang SC, Lee JS, Takatsuto S, Yokota T, Kim S-K (2004) Novel biosynthetic pathway of castasterone from cholesterol in tomato. Plant Physiol 135(3):1231–1242
Kohli SK, Handa N, Sharma A, Gautam V, Arora S, Bhardwaj R, Alyemeni MN, Wijaya L, Ahmad P (2017) Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma. doi:10.1007/s00709-017-1124-x)
Kono Y (1978) Generation of superoxide radical during autooxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys 186:189–195
Kováĉik J, Gruz J, Backor M, Tomko J, Strnad M, Repcak M (2008) Phenolic compounds composition and physiological attributes of Matricaria chamomilla grown in copper excess. Environ Expt Bot 62:145–152
Kuhn H, Borchert A (2002) Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med 33:154–172
Kumar M, Sirhindi G, Bhardwaj R, Kumar S, Jain G (2010) Effect of exogenous H2O2 on antioxidant enzymes of Brassica juncea L. seedlings in relation to 24-epibrassinolide under chilling stress. Indian J Biochem Biophys 47(6):378–382
Lascano HR, Gomez LD, Casano LM, Trippi VS (1998) Changes in glutathione reductase activity and protein content in wheat leaves and chloroplasts exposed to photooxidative stress. Plant Physiol Biochem 36:321–329
Leustek T, Saito K (1999) Sulfate transport and assimilation in plants. Plant Physiol 120:637–644
Li J, Yang P, Kang J, Gan Y, Yu J, Calderón-Urrea A, Lyu J, Zhang G, Feng Z, Xie J (2016) Transcriptome analysis of pepper (Capsicum annuum) revealed a role of 24-epibrassinolide in response to chilling. Front Plant Sci 7:1281
Liu P, Kerr BJ, Chen C, Weber TE, Johnston LJ, Shurson GC (2014) Methods to create thermally oxidized lipids and comparison of analytical procedures to characterize peroxidation. J Anim Sci 92:2950–2959
Manara A (2012) Plant responses to metal stress. In: Furinin A (ed) Plants and heavy metals. Springer, New York, pp 27–53
Márquez-García B, Fernández-Recamales MA, Córdoba F (2012) Effects of cadmium on phenolic composition and antioxidant activities of erica andevalensis. J Bot. doi:10.1155/2012/936950
Martinez-Penalver A, Grana E, Reigosa MJ, Sanchez-Moreiras AM (2012) The early response of Arabidopsis thaliana to cadmium and copper-induced stress. Environ Exp Bot 78:1–9
Mazorra Morales LM, Senn ME, Grozeff GEG, Fanello DD, Carrión CA, Núñez M, Bishop GJ, Bartoli CG (2014) Impact of brassinosteroids and ethylene on ascorbic acid accumulation in tomato leaves. Plant Physiol Biochem 74:315–322
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, van Breusegem F (2011) ROS signaling: the new wave? Trend Plant Sci 16:300–309
Mourato M, Reis R, Martins L (2012) Characterization of plant antioxidative system in response to abiotic stresses: a focus on heavy metal toxicity. In: Montanaro G, Dichio B (eds) Advances in selected plant physiology aspects. Intech, Croatia, pp 23–44
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K et al (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Ozdemir F, Bor M, Demiral T, Turkan I (2004) Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul 42:203–211
Pitzschke A, Fornazi C, Hirt H (2006) Reactive oxygen species signalling in plants. Antioxid Redox Sign 8:1757–1764
Poonam, Kaur R, Bali S, Singh R, Pati PK, Bhardwaj R (2014) Treatment of 24-EBL to B. juncea plants under Cu-metal stress lowers oxidative burst by activity of antioxidant enzymes. J Stress Physiol Biochem 10(2):315–327
Poonam, Kaur R, Bhardwaj R, Sirhindi G (2015) Castasterone regulated polyphenolic metabolism and photosynthetic system in Brassica juncea plants under copper stress. J Pharmacog Phytochem 4:282–289
Putter J (1974) Peroxidase. In: Bergemeyer HU (ed) Methods of enzymatic analysis. Academic Press, London, pp 685–690
Rady MM (2011) Effect of 24-epibrassinolide on growth, yield, antioxidant system and cadmium content of bean (Phaseolus vulgaris L.) plants under salinity and cadmium stress. Sci Hortic 129:232–237
Ramamurthy N, Kannan S (2009) SEM-EDS analysis of soil and plant (Calotropis gigantea Linn) collected from an industrial village, Cuddalore Dt, Tamil Nadu, India. Rom J Biophys 19(3):219–226
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180(2):169 – 81
Roe JH, Kuether CA (1943) The determination of ascorbic acid in whole blood and urine through 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J Biol Chem 147:399
Romero LC, Aroca MA, Laureano-Marin AM, Moreno I, Garcia I, Gotor C (2014) Cysteine and cysteine-related signaling pathways in Arabidopsis thaliana. Mol Plant 7:264–276
Sarwar N, Saifullah Sukhdev SM, Munir HZ, Asif N, Sadia B, Ghulam F (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants (Review). J Sci Food Agric 90:925–937
Sedlak J, Lindsay RH (1968) Estimation of total protein bond and non-protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25:192–205
Sharma I, Bhardwaj R, Pati PK (2015) Exogenous application of 28-homobrassinolide modulates the dynamics of salt and pesticides induced stress responses in an elite rice variety Pusa Basmati-1. J Plant Growth Regul 34(3):509–518
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2015) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143
Soares C, de Sousa A, Pinto A, Azenha M, Teixeira J, Azevedo RA et al (2016) Effect of 24-epibrassinolide on ROS content antioxidant system lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ Exp Bot 122:115–125
Sonmez S, Kaplan M, Sonmez NK, Kaya H, Uz I (2006) High level of copper application to soil and leaves reduce the growth and yield of tomato plants. Sci Agric 63(3):213–218
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Gupta DK (2006) Copper-induced oxidative stress and responses of antioxidants and phytochelatins in Hydrilla verticillata (Lf) Royle. Aqua Toxicol 80:405–415
Szabados L, Savouré A (2009) Proline: a multifunctional amino acid. Trend Plant Sci 15:89–97
Szarka A, Tomasskovies B, Banhegyi G (2012) The ascorbate-glutathione-α-tocopherol triad in abiotic stress response. Int J Mol Sci 13:4458–4483
Tu C, Ma L (2005) Effects of arsenic on concentration and distribution of nutrients in the fronds of the arsenic hyperaccumulator Pteris vittata L. Environ Pollut 135(2):333–340
Woodbury W, Spencer AK, Stahmann MA (1971) An improved procedure using ferricyanide for detecting catalase isozymes. Anal Biochem 443:301–305
Wu GL, Cui J, Tao L, Yang H (2010) Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicol 19:124–132
Xia XJ, Wang YJ, Zhou YH, Tao Y, Mao WH, Shi K, Asami T, Chen Z, Yu JQ (2009) Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol 150(2):801–814
Yadav P, Kaur R, Kohli SK, Sirhindi G, Bhardwaj R (2016) Castasterone assisted accumulation of polyphenols and antioxidant to increase tolerance of B. juncea plants towards copper toxicity. Cogent Food Agric 2:1–14
Yamamoto Y, Yukiko Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yem EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57:769–773
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–156
Yruela I (2009) Copper in plants: acquisition transport and interactions. Funct Plant Biol 36:409–430
Yurekli F, Porgali ZB (2006) The effects of excessive exposure to copper in bean plants. Acta Biolo Cracovien Ser Bot 48(2):7–13
Zemanová V, Pavlík M, Pavlíková D, Tlustoš P (2013) The changes of contents of selected free amino acids associated with cadmium stress in Noccaea caerulescens and Arabidopsis halleri. Plant Soil Environ 59:417–422
Zhang H, Lian C, Shen Z (2009) Proteomic identification of small copper-responsive proteins in germinating embryos of Oryza sativa. Ann Bot 103(6):923–930
Zhang D, Liu D, Lv X, Wang Y, Xun Z, Liu Z, Li F, Lu H (2014) The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 26:2939–2961
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mudberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559
Zhou Y, Xia X, Yu G, Wang J, Wu J, Wang M, Yan Y, Shi K, Yu Y, Chen Z, Gan J, Yu J (2015) Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci Rep 5(9018):1–7
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group No (RG-1438-039). Authors also acknowledge the UGC, New Delhi, India, for providing assistance through the RGNF program.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yadav, P., Kaur, R., Kanwar, M.K. et al. Ameliorative Role of Castasterone on Copper Metal Toxicity by Improving Redox Homeostasis in Brassica juncea L.. J Plant Growth Regul 37, 575–590 (2018). https://doi.org/10.1007/s00344-017-9757-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9757-8