Abstract
Auxin–cytokinin (CK) interactions have been extensively studied in the control of bud outgrowth in herbaceous plants. However, in temperate woody plants where the meristem of dormant buds can be repressed by either exogenous or endogenous factors, abscisic acid (ABA) has been suggested as a potential regulator of bud outgrowth. To investigate the involvement of ABA, CK, and auxin on bud sprouting in Vitis vinifera, single-bud cuttings were used under forced conditions. This artificial bud sprouting system mimics and hastens the natural sprouting process that occurs in spring. Our results showed that expression of the ABA biosynthesis gene VvNCED1 decreased during incubation, whereas expression of the ABA catabolism gene VvA8H3 remained unaltered. Expression of CK biosynthesis-related genes ISOPENTENYL TRANSFERASE (VvIPTs) and LONELY GUY (VvLOG1), CK catabolism-related gene CYTOKININ OXIDASE (VvCKX3), and key auxin biosynthesis gene VvYUC3 increased with incubation time. Moreover, treatment with hydrogen cyanamide (HC), a compound that breaks vine latency, increased expression of VvIPTs and VvLOG1 and reduced expression of VvCKX3 and VvNCED1. These results are consistent with previous reports indicating that HC increases CK levels and decreases ABA levels in grapevine buds. Taken together, the results suggest that in the vine, bud sprouting is preceded by a decrease in ABA content and an increase in CK and auxin levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many plant species, the intact main shoot apex grows predominantly and axillary bud outgrowth is inhibited. This phenomenon is called apical dominance and has been analyzed for over 70 years (Brown and others 1979; Crabbe 1984; Shimizu-Sato and others 2009). Apical dominance is regulated by the two plant hormones auxin and cytokinin (CK) (Kurakawa and others 2007; Shimizu-Sato 2009; Müller and Leyser 2011; Azizi and others 2015). However, strigolactones, a group of terpenoid lactones, was recently identified as a third type of plant hormone involved in the control of shoot branching (Gomez-Roldan and others 2008; Umehara and others 2008; Brewer and others 2015). Two mechanisms by which auxin inhibits bud outgrowth have been proposed. In the first mechanism, auxin derived from the shoot apex regulates CK levels in the stem by inducing CK degradation through the regulation of CYTOKININ OXIDASE (CKX) expression as well as by the suppression of CK biosynthesis through the regulation of ISOPENTENYL TRANSFERASE (IPT) (Shimizu-Sato and others 2009). Additionally, auxin promotes the expression of strigolactone biosynthesis-related genes (Foo and others 2005; Zou and others 2006; Hyward and others 2009). The second mechanism involves suppression of auxin transport/canalization from axillary buds into the main stem and is enhanced by the low sink for auxin in the stem (Sachs 1981; Brewer and others 2009; Müller and Leyser 2011). There is a growing body of evidence in support of this mechanism, much of which is derived from the observations that strigolactones appear to inhibit buds primarily by modulating auxin transport and canalization (Bennet and others 2006; Crawford and others 2010; Dun and others 2012). The greatest advances in understanding auxin and cytokinin interactions relative to bud outgrowth and shoot architecture have been addressed in herbaceous plant systems (Thimann and Skoog 1934; Hall and Hillman 1975; Gocal and others 1991; Li and others 1995; McIntyre and Damson 1998; Shimizu-Sato and Mori 2001; Müller and Leyser 2011).
In woody perennial plants, the inhibition of bud outgrowth has been divided into paradormancy (PD) in which axillary latent bud outgrowth is inhibited by apical dominance, endodormancy (ED) in which bud growth is inhibited by intrinsic factors located within the bud, and ecodormancy (ECD) in which bud outgrowth depends on environmental cues such as temperature and light (Lang and others 1987). PD has been studied in Vitis riparia, and it was shown that the shoot tip, summer lateral buds, and leaves in addition to node position contributed to the inhibition of axillary bud outgrowth under climate controlled conditions in the growth chamber (He and others 2012). However, in the field, under temperate climatic conditions, buds of V. vinifera transition from the PD into the ED stage when triggered by a decreased photoperiod (Kühn and others 2009; Grant and others 2013). Recently, it was reported that the plant hormone abscisic acid (ABA) plays an important role in repression of meristem activity in grapevine buds during ED (Zheng and others 2015; Parada and others 2016). In this study, single-bud cuttings of V. vinifera cv. Thompson seedless exposed to forced conditions were used to study changes in the expression of genes associated with ABA biosynthesis (VvNCED1) and degradation (VvA8H3); with auxin biosynthesis (VvAMI1, VvYUC3) and transport (VvPIN3); and with CK biosynthesis (VvIPTs, VvLOG1) and degradation (VvCKX3). Furthermore, the effect of hydrogen cyanamide (HC) on the expression of these genes was studied at different incubation times. The results suggest that on the vine, unlike in herbaceous plants, ABA and not auxin act as the principal repressor of bud outgrowth, whereas auxin and CK promote bud sprouting.
Materials and Methods
Bud Break Under Forced Conditions
The bud break response of single-bud cuttings under forced conditions is a common indicator used to describe the depth of dormancy in grapevines (Koussa and others 1994; Dennis 2003). This system allows the reproduction of ED bud development prior to bud break under controlled conditions. Canes were collected on 13 May 2015 at the ED stage according to previous studies (Vergara and others 2010). The canes were taken from 10-year-old Vitis vinifera L cv. Thompson seedless grown at the experimental station at the Chilean National Institute of Agriculture Research (INIA) located in Santiago (33°34′S latitude). Detached canes, each carrying 10 buds at positions 5–14, were transferred to the laboratory. Canes were cut into single-bud cuttings. Cuttings (10–12 cm in length) were separated into four groups and transferred to a growth chamber set at 23 ± 2 °C with a 16-h photoperiod (forced conditions). Groups 1 and 2 (30 single-bud cuttings per group) were used to study the bud break response of hydrogen cyanamide (HC) (a compound that breaks dormancy) treated and control buds, respectively, as described previously (Vergara and others 2010). Groups 3 and 4 were used to sample weekly HC-treated and control buds, respectively, from the growth chamber over a period of 3 weeks. After each time point, the samples (15 buds per date, per treatment and per biological replicate) were frozen in liquid N2 and maintained in the freezer at −80 °C until use for gene expression analysis.
RNA Purification and cDNA Synthesis
Total RNA was extracted and purified from latent buds (0.5 g fr. wt) of V. vinifera cv. Thompson seedless. Total RNA was extracted using a modification of the method of Chang and others (1993) described in Noriega and others (2007). DNA was removed by treatment with RNAase-free DNAse (1 U/µl) (Thermo Scientific, USA) at 37 °C for 30 min. First-strand cDNA was synthesized from 5 µg of purified RNA using 1 µl oligo (dT)12−18 (0.5 µg × µl−1) as a primer, 1 µl dNTP mix (10 mM), and Superscript® II reverse transcriptase (Invitrogen, CA, USA).
Gene Expression Analysis
Gene expression analysis was performed by quantitative real-time PCR using an Eco Real-Time PCR system (Illumina, Inc. SD, USA) and KAPA SYBR FAST mix (KK4602) qPCR master mix (2×). Primers suitable for the amplification of 80–200 bp products for each gene of interest were designed using PRIMER3 software (Rozen and Skaletsky 2000) (Suppl. Table S1) The amplification of cDNA was performed under the following conditions: denaturation at 94 °C for 2 min followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s. Three biological replicates with three technical replicates were performed for each treatment. The induction or repression of transcript levels was calculated using the ΔΔCq method (Livak and Schmittgen 2001); VvUBIQUITIN was used as a reference gene.
Statistical Analysis
Differences in gene expression at different incubation times were analyzed by ANOVA, and multiple comparison analysis was carried out by Duncan´s method (α = 0.05). For pairwise comparisons, the Student´s t test (α = 0.05) method was used.
Results
Bud Break Rate
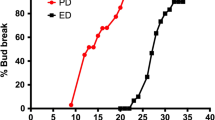
After 3 weeks of incubation in the growth chamber under forced conditions, HC-treated single-bud cuttings began to undergo bud break, and 1 week later, nearly 100% bud break was observed. Conversely, in control samples, bud break started after 5 weeks of incubation and reached nearly 60% bud break 2 weeks later (Fig. 1).
Expression of CK Metabolism Genes Increases with Incubation Time in Single-Bud Cuttings of Vine
The following CK biosynthesis-related genes were identified in the genome of V. vinifera: five paralogs that encode CK biosynthesis isopentenyl transferase isoenzymes (VvIPT1, VvIPT3a, VvIPT3b, VvIPT5a, and VvIPT5b); one gene that encodes lonely guy (VvLOG1), an enzyme that activates CKs in a single step (Herber and Kieber 2002); and one gene that encodes a cytokinin oxidase (VvCKX3), which is a CK catabolism-related gene (Brugière and others 2003). All these CK metabolism-related genes had significantly increased expression levels during incubation under forced conditions. The expression of VvIPT3a increased more than 1000-fold and peaked after 2 weeks of incubation (Fig. 2b), whereas all other gene expression levels peaked after 3 weeks of incubation (Fig. 2a, c–g).
Increased expression of CK metabolism-related genes in single-bud cuttings of Thompson seedless grapevine over time under forced conditions. Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN. Samples without incubation served as the control. Data are presented as the average of three biological replicates, each with three technical replicates, and bars represent s.d. Different lower case letters represent mean values that are significantly different at different incubation times for a given gene as determined by Duncan´s test (p ≤ 0.05)
Effect of HC on CK Metabolism Gene Expression in Single-Bud Cuttings with Different Incubation Times
To analyze the effect of HC on the expression of CK metabolism-related genes, transcript levels of these genes were compared in HC-treated and untreated buds incubated for different durations of time (Fig. 3). For gene expression analysis, untreated samples were used as controls. After 1 week of incubation in HC-treated buds, the expression of VvIPT3a increased threefold, (Fig. 3b), the expression of VvIPT3b increased 2.6-fold (Fig. 3 c), the expression of VvIPT5a increased 2.4-fold (Fig. 3d), the expression of VvLOG1 increased 1.6-fold (Fig. 3f), and the expression of VvCKX3 increased 1.7-fold (Fig. 3g) relative to control buds (Fig. 3b). However, after two weeks of incubation, the expression of VvLOG1 increased 6.5-fold (Fig. 3f), whereas after three weeks of incubation, HC repressed the expression of VvIPT5a by one-third (Fig. 3d) and of VvCKX3 by one-half (Fig. 3f).
The effect of hydrogen cyanamide (HC) on the expression of CK metabolism-related genes in single-bud cuttings of Thompson seedless grapevine during the period prior to bud break. Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN. Samples without HC treatment served as the control. Data are presented as the average of three biological replicates, each with three technical replicates, bars represent s.d., and asterisks indicate significant differences (Student´s test α = 0.05)
Relative Levels of CK Metabolism Gene Expression in Single-Bud Cuttings of Grapevine Over Time
To compare the relative levels of CK metabolism-related gene expression at different incubation times, the gene that demonstrated the greatest Ct value at each incubation time was considered as the control and the ΔCt of that gene was used to obtain the ΔΔCt for the other genes. Before incubation, buds were at the ED stage (Vergara and others 2010); at this developmental stage, the gene that showed the highest level of expression was VvCKX3, which is related to CK catabolism, whereas the gene that showed the lowest level of expression was VvIPT3b (Fig. 4a). These results suggest a low content of CK in grapevine buds during ED. However, after 1 and 2 weeks of incubation, a significant increase in the expression of VvIPT3a was observed (Fig. 4b, c), suggesting a substantial increase in CK content within the buds. After three weeks of incubation, the expression of VvIPT3a and VvCKX3 leveled, but their expression levels were still higher than the other CK related genes (Fig. 4d).
Relative expression among CK metabolism-related genes in single-bud cuttings of grapevine over time (WUFC, weeks under forced conditions). Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN. The gene with the highest Ct was used as the control in each case. Data are presented as the average of three biological replicates, each with three technical replicates, and bars represent s.d. Significant differences between transcript levels are represented by different lower case letters as determined by Duncan´s test (p ≤ 0.05)
Relative Expression Levels of VvIPT3a and VvCKX3 in the Shoot Apex and Latent Buds at Two Developmental Stages
To compare the levels of expression of VvIPT3a and VvCKX3 in the dormant buds with another organ-containing meristem, the shoot-apex, gene expression was analyzed during the active growth (27 December) and rest periods (11 March) in both organs (Vergara and others 2010). Transcript levels of both genes were significantly higher in the latent buds than in the shoot apex at both developmental stages (Fig. 5a, b). However, the difference between the expression levels of VvIPT3a in both tissues decreased in the rest period due to a strong decrease in the expression level in the latent buds, but the differences in the expression of VvCKX3 between both organs remained relatively similar at both developmental stages (Fig. 5b).
Expression analysis of CK biosynthesis-related gene (VvIPT3a) and CK catabolism-related gene (VvCKX3) at the shoot apex and latent buds of Thompson seedless grapevines during active growth and recess periods. Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN. Data are presented as the average of three biological replicates, each with three technical replicates, bars represent s.d., and asterisks indicate significant differences between shoot apex and latent buds (Student´s test α = 0.05)
VvYUC3 is Up-Regulated by HC in a Time-Dependent Manner in Single-Bud Cuttings Under Forced Conditions
Four paralogs encoding the enzyme YUCCA (flavin monooxygenase family) were identified in the genome of V. vinifera; however, VvYUC3 was most highly expressed in grapevine buds. This enzyme catalyzes the conversion of indole-3-pyruvic acid (IPA) to indole-3-acetic acid (IAA) (Mano and Nemoto 2012). Only one gene coding for the AMI enzyme, which catalyzes the conversion of indole-3-acetamide (IAM) to IAA, was identified in the genome of V. vinifera. The expression of auxin biosynthesis genes VvAMI1 and VvYUC3 and auxin transport gene VvPIN3 were analyzed in single-bud cuttings over time (Fig. 6a, b, c). The effect of HC on the expression of these genes was also analyzed over the same time course (Fig. 6d, e, f). Transcript levels of VvYUC3 increased over time (Fig. 6b) and were strongly up-regulated by HC (Fig. 6e). However, transcript levels of VvAMI1 only slightly increased (Fig. 6a), whereas those of VvPIN3 slightly decreased (Fig. 6c) over time. Therefore, HC did not affect the expression levels of both genes (Fig. 6d, f).
Gene expression analysis of auxin-related genes in single-bud cuttings of Thompson seedless grapevine prior to bud break (a, b, c). Effect of hydrogen cyanamide (HC) on the expression of auxin-related genes over time (d, e, f). Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN. Samples without incubation served as control for analyzing gene expression over time. Data are presented as the average of three biological replicates, each with three technical replicates, and bars represent s.d. Different lower case letters represent mean values that are significantly different at different incubation times for a given gene as determined by Duncan´s test (p ≤ 0.05). Pairwise comparisons between HC-treated and non-treated single-bud cuttings at each time point were performed by Student´s t test (α = 0.05), and asterisks indicate significant differences between them
ABA Biosynthesis Gene VvNCED1 is Down-Regulated, while the ABA Catabolism Gene VvA8H3 is not Affected Over Time in Single-Bud Cuttings
A previous bioinformatics analysis identified three putative homologues of NCED in grapevines (Young and others 2012). NCED encodes the enzyme 9-cis-epoxycarotenoid dioxygenase, which is a key enzyme in the synthesis of ABA (Lefebvre and others 2006). Three VvNCED genes were identified in mature grape buds; however, VvNCED1 showed the highest expression in grapevine buds (Parada and others 2016). Four ABA 8-hydroxylase genes were identified in the genome of V. vinifera; however, VvA8H3 was most highly expressed in grapevine buds. ABA has recently been involved in the release of grapevine buds from ED. Here, we analyzed the expression of the ABA biosynthesis gene VvNCED1 and the ABA catabolic gene VvA8H3 in single-bud cuttings at different incubation times (Fig. 7a, b). The effect of HC on the expression of these genes was also analyzed over time (Fig. 7c, d). Transcript levels of VvNCED1 decreased significantly after 1 week of incubation and remained at these lower levels until bud break (Fig. 7a). As expected, the application of HC to single-bud cuttings further down-regulated VvNCED1 expression (Fig. 7b). On the other hand, transcript levels of VvA8H3 did not show a clear pattern of expression during incubation (Fig. 7c). HC did not affect gene expression over this time course (Fig. 7d).
Gene expression analysis of ABA metabolism-related genes in single-bud cuttings of Thompson seedless grapevine prior to bud break (a, b). Effect of hydrogen cyanamide (HC) on the expression of ABA metabolism-related genes (c, d). Transcript levels were determined by RT-qPCR and normalized against VvUBIQUITIN. Samples without incubation served as the control for analyzing gene expression over time. Data are presented as the average of three biological replicates, each with three technical replicates, and bars represent s.d. Different lower case letters represent mean values that are significantly different at each time point for a given gene as determined by Duncan´s test (p ≤ 0.05). Pairwise comparisons between HC-treated and non-treated single-bud cuttings at the different incubation times were performed by Student´s t test (α = 0.05), and asterisks indicate significant differences between them
Discussion
Apical meristems are specialized regions found at the extremities of the stem and the root where cells remains in a non-differentiated state and have the potential to proliferate indefinitely (Gegas and Doonan 2006). Axillary and adventitious meristems are also major participants in the control of the overall plant form, but their outgrowth is controlled in various ways including dormancy. In temperate woody plants, the activity of the meristem of latent buds can be repressed either by endogenous factors leading to endodormancy (ED) or by exogenous factors leading to paradormancy (PD) (Lang and others 1987; Rohde and Bhalerao 2007). On the vine, the transition of buds from the PD to the ED is triggered mainly by the SD-photoperiod (Kühn and others 2009; Grant and others 2013), and sprouting occurs after the end of the ED. In contrast to poplar and other tree species, Vitis does not set a terminal bud, and the shoot apex does not enter into ED nor cold acclimates in response to the SD-photoperiod. However, other hallmark phenotypes such as periderm development, growth cessation, and development of bud ED are induced (Fennel and Hoover 1991; Wake and Fennel 2000; Sreekantan and others 2010; Grant and others 2013). In recent studies, the plant hormone abscisic acid (ABA) has been involved in the establishment and maintenance of ED in grapevine buds (Zheng and others 2015; Parada and others 2016). Furthermore, it has been suggested that the dormancy-breaking compound hydrogen cyanamide (HC) exerts its dormancy-breaking effect by reducing ABA content (Zheng and others 2015). Here, we show that expression of the ABA biosynthesis gene VvNCED1 is decreased throughout incubation in single-bud cuttings and that this down-regulation is enhanced by HC (Fig. 7a, c). These results agree with the data of Zheng and others (2015). Moreover, we observed increased expression of the auxin and CK biosynthesis genes VvYUC3 and VvIPT3a, respectively, in single-bud cuttings over time (Figs. 6b, 2b) with additional enhancement by HC (Figs. 6e, 3b), strongly suggesting that the plant hormones CK and auxin promote sprouting in grapevine buds. Additionally, our results suggest that HC applications might increase CK content because expression of CK biosynthesis genes VvIPTs and VvLOG1 are up-regulated by HC, whereas the expression of the CK catabolism gene VvCKX3 is down-regulated (Fig. 3). These results agree with previous results indicating that HC increases the content of CK in grapevine buds (Lombard and others 2006). The increase of more than 1000-fold in VvIPT3a expression after two weeks of incubation followed by a sixfold reduction at week three (Fig. 2b) suggests that a transient increase in CK content could be a signal (Yoshida and others 2011; Roman and others 2016) for the resumption of cell proliferation and differentiation within the bud meristem, and therefore, for the cessation of the ED. It has been shown that direct applications of CK to tendrils enhances inflorescence formation (Srinivasan and Mullins 1978, 1980); however, no information regarding the effect of exogenous applications of CK on bud break has been reported on grapevines. Interestingly, the irreversible degradation of CK by the enzyme cytokinin oxidase (CKX) has been suggested as a mechanism through which plants can regulate their CK levels (Brugière and others 2003). VvCKX3, the gene coding for cytokinin oxidase in vines, is highly expressed in the bud in relation to other organs containing meristems like the shoot apex. This high expression of VvCKX3 in dormant buds gives them a high capacity to degrade CK, which could prevent or mitigate the effect of exogenous CK applications on vine sprouting. In conclusion, the results suggest that in the endodormant buds of the vine, ABA is the main repressor of bud outgrowth, while auxin and CK promote bud outgrowth.
References
Azizi P, Rafi MY, Maziah M, Abdullah SNA, Hanafi MM, Latif MA, Rashid AA, Sahebi M (2015) Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mech Dev 135:1–15
Bennet T, Sieberer T, Willet B, Booker J, Lusching C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16:553–563
Brewer PB, Dun EA, Gui, R, Mason MG, Beveridge C (2015) Strigolactone inhibition of branching independent of polar auxin transport. Plant Physiol 168:1820–1829
Brown BT, Foster C, Phillips JN, Rattigan BM (1979) The indirect role of 2,4-D in the maintenance of apical dominance in decapitated sunflower seedlings (Helianthus annuus L.). Planta 146:475–480
Brugière N, Shuping J, Hantke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE (2003) Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, absicisic acid and abiotic stress. Plant Physiol 132:1228–1240
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Crabbe JJ (1984) Correlative effects modifying the course of bud dormancy in woody plants. Z Planzenphysiol 113: 465–469
Crawford S, Shinohara N, Siberee T (2010) Strigolactone enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913
Dennis FG (2003) Problems in standardizing methods for evaluating the chilling requirements for the breaking of dormancy in buds of woody plants. HortScience 38:347–350
Dun EA, de Saint Germain A, Rameau C, Beveridge C (2012) Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol 158:487–498
Fennel A, Hoover E (1991) Photoperiod influences growth, bud dormancy and cold acclimation of Vitis labruscana and V. riparia. J Am Soc Hort Sci 116:270–273
Foo E, Bullier E, Goussot M, Foucher M, Rameau C, Beveridge C (2005) The Branching gene RAMOUSUS1 mediates interactions among two novel signals and auxin in pea. Plant Cell 17:464–474
Gegas VC, Doonan JH (2006) Expression of cell cycle genes in shoot apical meristem. Plant Mol Biol 60: 947–961
Gocal GFW, Pharis RP, Yeung EC, Pearce D (1991) Changes after decapitation in concentration of indole-3-acetic acid and abscisic acid in the larger axillary bud of Phaseolus vulgaris L. cv tender green. Plant Physiol 95:344–350
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danous S, Portrais JC, Bowmeester H, Bécaud G, Beveridge CA, Rameau C, Rochange SF (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Grant TNL, Gargrave J, Dami IE. 2013. Morphological, Physiological, and Biochemical changes in Vitis genotypes in response to photoperiod regimes. Am J Enol Vitic 64: 466–475
Hall SM, Hillman JR (1975) Correlative inhibition of lateral bud growth in Phaseolus vulgaris L. Timing of bud growth following decapitation. Planta 123:137–143
He D, Mathiason K, Fennell (2012) Auxin cytokinin related gene expression during active shoot growth and latent bud paradormancy in Vitis riparia grapevine. J Plant Physiol 169: 643–648
Herber G, Kieber JJ (2002) Cytokinin new insights into a classical phytohormone. Plant Physiol 128:354–362
Hyward A, Stirnberg P, Beveridge C, Leyser O (2009) Interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412
Koussa T, Broquedis M, Bouard J.1994. Changes of abscisic acid level during the development of grape latent buds, particularly in the phase of dormancy break. Vitis 33:63–67
Kühn N, Ormeño-Nuñez J, Jaque-Zamora G, Pérez FJ (2009) Photoperiod modifies the diurnal expression profile of VvPHYA and VvPHYB transcripts in field-grown grapevine leaves. J Plant Physiol 166:1172–1180
Kurakawa T, Ueda N, MaeKawa M, Kobayashi K, Kojima M, Nagato Y (2007) Direct control of shoot meristem activity by a cytokinin-activating enzyme. Nature 445:555–562
Lang GA, Early JD, Martin GC, Darnell RL (1987) Endo-para and ecodormancy: physiological terminology and classification for dormancy research. HortScience 22:381–387
Lefebvre V, North H, Frey A, Sotta B, Seo M, Okamoto M, Nambara E, Marion Polla A (2006) Functional analysis of Arabidopsis NCED genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J 45:309–319
Li CJ, Guevera E, Herrera J, Bengerth F (1995) Effect of apex excision and replacement by 1-naphthylacetic acid on cytokinin concentration and apical dominance in pea plants. Physiol Plant 94:465–469
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real time quantitative PCR and the ∆∆CT method. Methods 25:402–408
Lombard PJ, Cook NC, Bellstedt DU (2006) Endogenous cytokinin levels of table grape vine during spring budburst as influenced by hydrogen cyanamide application and pruning. Sci Hortic 109:92–96
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:3853–3872
McIntyre GI, Damson EL (1988) Apical dominance in Phaseolus vulgaris. The triggering effect of shoot decapitation and leaf excision on growth of the lateral buds. Physiol Plant 74:607–614
Müller D, Leyser O (2011) Auxin, cytokinin and the control of shoot branching. Ann Bot 107:1203–1212
Noriega X, Burgos B, Pérez FJ (2007) Short-day photoperiod triggers and low temperature increase expression of peroxidase RNA transcripts and basic peroxidase isoenzyme activity in grape-buds. Phytochemistry 68: 1376–1383
Parada F, Noriega X, Dantas D, Bressan-Smith R, Pérez FJ (2016) Differences in respiration between dormant and non-dormant buds suggest the involvement of ABA in the development of endodormancy in grapevines. J Plant Physiol 201:71–78
Rohde A, Bhalereao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12:217–223
Roman H, Girault T, Barbier F, Péron T, Brouard N, Pencick A, Novák O, Vian A, Sakr S, Lothier J, Le Gourrierec, Leduc N (2016) Cytokinin are initial targets of light in the control of bud outgrowth. Plant Physiol 172:489–509
Rozen S, Skaletsky H (2000) Primer3 on the www for general users and for biologist programmers. Methods Mol Biol 132:365–386
Sachs T (1981) The control of patterned differentiation of vascular tissues. Adv Bot Res 9:151–262
Shimizu-Sato S, Mori H (2001) Control of outgrowth and dormancy in axillary buds. Plant Physiol 127:1405–1413
Shimizu-Sato S, Tanaka M, Mori H (2009) Auxin-cytokinin interactions in the control of shoot branching. Plant Mol Biol 69:429–435
Sreekantan L, Mathiason L, Grimplet J, Schlauch K, Dickerson JA, Fennell A (2010) Differential floral developmentand gene expression in grapevines during long and short photoperiods suggest a role for floral genes in dormancy transitioning. Plant Mol Biol 73:191–205
Srinivasan C, Mullins MG (1978) Control of flowering in the grapevine (Vitis vinifera L). Plant Physiol 61:127–130
Srinivasan C, Mullins MG (1980) Flowering in Vitis: effects of genotype on cytokinin-induced conversion of tendrils into inflorescence. Vitis 19:293–300
Thimann KV, Skoog F (1934) On the inhibition of bud development and other functions of growth substances in Vicia faba. Proc R Soc Lond B Biol Sci 114:317–339
Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magone H, Kamiya Y, Shirasu K, Yoneyama K, Kyozuka J, Yamaguchi S (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455:195–200
Vergara R, Pérez FJ (2010) Similarities between natural and chemically induced bud endodormancy release in grapevine Vitis vinifera L. Sci Hortic 125:648–653
Wake CMF, Fennell A (2000) Morphological, physiological and dormancy responses of three Vitis genotype to short photoperiod. Physiol Plant 109:203–210
Yoshida S, Mandel T, Kuhlemeier C (2011) Stem cell activation by light guides plant organogenesis. Genes Dev 25:1439–1450
Young PR, Lashbrooke JG, Alexandersson E, Jacobson D, Moser C, Velasco R, Vivier MA (2012) The gene and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genom 13:243–259
Zheng C, Halaly T, Acheampong AK, Takebayashi Y, Jikumaru Y, Kamiya Y, Or E (2015) Absicisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act trough modification of ABA metabolism. J Exp Bot 66:1527–1542
Zou J, Zhang S, Zhang W, Li G, Chen Z, Zhai W, Zhao X, Pan X, Xie Q, Zhu L (2006) The rice HIGH-TILLERING DWARF1 encoding an ortholog of Arabidopsis MAX3 is required for negative regulation in the outgrowth of axillary buds. Plant J 48:667–698
Acknowledgements
The financial support of FONDECYT project 1140318 is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Noriega, X., Pérez, F.J. ABA Biosynthesis Genes are Down-regulated While Auxin and Cytokinin Biosynthesis Genes are Up-regulated During the Release of Grapevine Buds From Endodormancy. J Plant Growth Regul 36, 814–823 (2017). https://doi.org/10.1007/s00344-017-9685-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-017-9685-7