Abstract
Daylength is an important environmental cue for synchronizing growth, flowering, and dormancy with seasonality. As many floral development genes are photoperiod regulated, it has been suggested that they could have a regulatory role in bud endodormancy. Therefore, the influence of photoperiod was studied on inflorescence primordia differentiation and floral pathway related gene expression during the development of overwintering buds in Vitis riparia and V. spp. ‘Seyval’. Photoperiod treatments were imposed 35 days after budbreak, and histological and transcriptomic analyses were conducted during the subsequent 42 days of bud development. Long day (LD, 15 h) and short day (SD, 13 h) buds were floral competent by 21 days of photoperiod treatment (56 days after budbreak); however, the floral meristem developed faster in LD than in SD buds. Analysis of 132 floral pathway related genes represented on the Affymetrix Grape Genome array indicated 60 were significantly differentially expressed between photoperiod treatments. Genes predominantly related to floral transition or floral meristem development were identified by their association with distinct grape floral meristem development and an expression pattern in LD consistent with their previously identified roles in flowering literature. Genes with a potential dual role in floral development and dormancy transitioning were identified using photoperiod induced differences in floral development between LD and SD buds and uncharacteristic gene expression trends in relation to floral development. Candidate genes with the potential to play a dual role in SD dormancy induction include circadian rhythm or flowering transition related genes: AP2, BT1, COL-13, EIN3, ELF4, DDTR, GAI and HY5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perennial plants rely on seasonal cues such as changes in photoperiod and temperature to regulate the growth cycle, endodormancy, and floral competence (Chouard 1960). It has been suggested that genes in the floral development pathway may play a role in regulating seasonal growth cessation and dormancy induction (Horvath et al. 2008). Böhlenius et al. (2006) demonstrated that constitutive expression of FLOWERING LOCUS T (FT), a floral regulatory gene, prevented seasonal short photoperiod growth cessation in poplar (Populus trichocarpa). Using poplar lines that were ABSCISIC ACID INSENSITIVE 3 (ABI3) overexpressing, ABI3 down regulated and wild-type, Ruttink et al. (2007) examined the role of 50 floral related genes during short photoperiod induced growth cessation and found that several were differentially expressed in response to changing photoperiod. Recently, Ruonala et al. (2008) noted that altering light signaling by over-expression of PHYTOCHROME A prevented short photoperiod endodormancy induction and the repression of PtFT2 and CENTRORADIALIS-LIKE 1, a gene that is involved in stem elongation and is typically down regulated during SD induction of growth cessation. These studies support the hypothesis that floral related pathway genes may also have a role in regulating the bud growth cycle (Horvath et al. 2003; Rohde and Bhalerao 2007).
In Arabidopsis and poplar, a short photoperiod induces transitions in the plant from an active vegetative growth cycle to flowering or terminal bud set and dormancy, respectively. Observations of FT expression common to both of these growth phase transitions has led to the suggestion that flowering regulatory genes could play a key role in the photoperiod response and growth cycling timing mechanisms in plants. In grapevines, it has been reported that photoperiod does not affect inflorescence induction in the latent bud (Srinivasan and Mullins 1980); however, there is evidence that the number of inflorescence primordia developed per bud is greater in long photoperiods than in short photoperiods. American species such as Vitis labrusca and V. riparia go dormant at longer daylengths than V. vinifera L. (Fennell and Hoover 1991; Kobayashi et al. 1965, 1966; Sugiura et al. 1975). Delaware vines (V. vinifera × V. labrusca) grown in long photoperiods formed nearly three times as many inflorescences as those grown in short days (Butrosse 1970). In controlled environment under high light intensities, development of inflorescence primordia depended on the photoperiod (Butrosse 1974). In contrast, dry matter accumulation was related to total incident light energy rather than photoperiod (Butrosse 1968). Therefore, inflorescence primordium development is not dependent on dry weight accumulation although both require high light energy (Srinivasan and Mullins 1980), suggesting that floral development could be photoperiod regulated.
Floral development in grapevines differs from the well studied annual model systems such as Arabidopsis or rice (Sreekantan and Thomas 2005). In grapevines, the shoot grows indeterminately, producing first and second order buds (termed prompt and compound latent buds, respectively) in the leaf axil until low temperature or short photoperiods (Fennell and Hoover 1991) terminate growth. Thus the continuous development of the grapevine latent bud presents a model different not only from the herbaceous flower models of Arabidopsis or rice, but also from the woody perennial model of poplar, which undergoes the morphological transition of terminal bud set in response to short photoperiods.
A unique feature of floral development in grapevine is that tendrils and inflorescences are essentially homologous structures, since the tendril and floral tissues arise from the same primordia type (Srinivasan and Mullins 1979; Boss and Thomas 2002). In the developing latent grapevine bud, the apical meristems produce a regular pattern of leaf primordia and uncommitted primordia (Boss and Thomas 2002; Boss et al. 2006). Depending on the cultivar and environmental conditions, the first one, two or three uncommited primordia formed in the primary and secondary meristems within the compound latent bud undergo repeated branching and develop into inflorescence primordia before the bud enters dormancy (Srinivasan and Mullin 1981). These immature inflorescences survive winter in a quiescent state. Budburst occurs in the following spring under favorable growing conditions, and the immature inflorescences continue differentiation to form individual flowers.
The grapevine morphology and growth habit with tendrils and prompt buds, in addition to latent buds, make grapevines a very different (in comparison to poplar and leafy spurge) but excellent perennial model system for exploring the role of floral pathway genes in mediating photoperiod response. Many genes homologous to the Arabidopsis floral development genes pathways or involved in photoperiod or vernalization responses can be found in the grapevine genome (Chatelet et al. 2007). However, most molecular studies in grapevines have focused on the identification of grapevine genes homologous to Arabidopsis flowering signal integrators, floral meristem identity genes, and flower organ identity genes (Carmona et al. 2002; Calonje et al. 2004; Fernandez et al. 2007; Joly et al. 2004; Boss et al. 2006; Sreekantan et al. 2006; Sreekantan and Thomas 2006). Therefore, this study explores the floral pathway in relation to grapevine latent bud development and photoperiod response to identify floral related genes that may also have a potential role in endodormancy induction.
Materials and methods
Plant materials
Potted, spur-pruned 2 to 6-year-old vines of Vitis spp. Seyval and V. riparia were removed from cold storage and grown in long photoperiod (LD, 15 h) at 25/20 ± 3°C day/night temperatures (D/N) with 600–1,400 μmol m−2 s−1 photosynthetic photon flux (PPF) in a climate-controlled unshaded glass greenhouse (En Tech Control Systems Inc., Montrose, Minn.) in Brookings, SD (44.3°N). When the grapevines reached 12–15 nodes (30 days post budbreak), they were randomized into two groups for photoperiod treatments: LD or short photoperiod (SD,13 h). Five days after randomization (35 days post budbreak), one group of plants continued in LD and the SD photoperiod treatment was started with the same temperature conditions. SD was imposed using an automated white covered black out system (Van Rijn Enterprises LTD; Grassie, Ontario). Buds were harvested at 1, 3, 7, 14, 21, 28 and 42 days of photoperiod treatments between 8:30 and 11:30 a.m., from nodes 3 to 12 from the shoot base. The buds were immediately frozen in liquid nitrogen and placed at −80°C for future RNA extraction. Three replications (5 vines/replication) were harvested between May and June 2007.
Bud histology
Buds from nodes 4 and 8 from the base of one cane were chosen from each plant in each replication at all time points for histology studies. Buds were excised from canes into tubes containing Carnoy’s fluid (Johansen 1940), fixed under vacuum for 2 h, and then held in the fixative for 10 h. Thereafter, buds were washed with 70% ethanol, dehydrated in an ethanol series (80, 90 and 100% with 1 h interval between each), and washed twice with 100% ethanol. Buds were then transferred through a series of ethanol:histoclear washes (3:1, 1:1, 1:3) to 100% histoclear, with 1 h in each mixture. After two changes of 100% histoclear the buds were left overnight at room temperature in a mixture (50/50 v/v) of histoclear and paraffin pellets. The next day the mixture was replaced with molten paraffin at 60°C, and the samples were transferred to a 60°C incubator. Buds were then taken through six changes of pure paraffin at 60°C with at least 4 h between each change. Buds were paraffin embedded in cassettes and sectioned using a microtome (Olympus CUT 4060E rotary microtome) yielding serial sections of 10 μm thickness. Sections were affixed on Probe-on Plus slides (Thermo-Fisher Scientific) by drying on a slide warmer at 42°C for 48 h. Slides were deparaffinised in histoclear, stained with safranin and fast green with differentiation in picric acid (Johansen 1940), and coverslipped. Sections were observed by light microscopy (Olympus AX70 upright compound microscope) to study the pattern of bud development, and images were captured with an Olympus DP70 digital camera.
RNA extraction
Total RNA was extracted using a modified method of Chang et al. (1993) as described in Mathiason et al. (2009). DNA was removed by incubation with 1 unit per microgram (μg) RNase-free DNase (Promega, Madison WI) at 37°C for 30 min. RNA was purified using RNeasy plant mini columns (Qiagen, Valencia CA). RNA quality and quantity were verified with an Agilent (Santa Clara, CA) 2100 Bioanalyzer RNA 6000 nano chip.
Microarray hybridization, data processing and verification
Affymetrix Vitis vinifera (Grape) Genome arrays were hybridized with biological triplicates for each photoperiod (LD and SD), time point (1, 3, 7, 14, 21, 28 and 42 days) and cultivar (Seyval and V. riparia). Hybridizations were conducted as previously described by Cramer et al. (2007). Expression data were subjected to a series of rigorous quality control steps to ensure data reproducibility and overall quality. Average background and noise metrics were examined for consistency across all 84 arrays, as indicated by the Affymetrix GeneChip® Operating Software Users Guide. Raw intensity values were processed first by RMA (Robust Multi-Array Average) (Irizarry et al. 2003) using the R package affy (Gautier et al. 2004). After pre-processing and normalization, all 84 arrays exhibited consistent expression distributions. Data from the 15,244 non-control probesets that were found to be present in all of the 84 array measurements were retained for further analyses.
A three-way ANOVA was performed on the RMA-processed data to identify those probesets with significant main and interaction effects. A statistically significant Photoperiod × Time effect yielded 3,892 probes that showed differential expression over the time course (Fennell et al. 2009). After performing the ANOVA and a multiple testing correction (Benjamini and Hochberg 1995) on the mixed effects Photoperiod × Time P-values, a Tukey’s Test was performed on all probesets with an adjusted Photoperiod × Time effect P-value of P < 0.05. The raw and processed transcriptomic data have been deposited in Gene Expression Omnibus (Accession#GSE17502, http://www.nih.ncbi.gov).
The microarray data set was verified using real-time PCR. Primers for candidate and reference genes were designed with PrimerQuest (Integrated DNA Technologies, http://www.idtdna.com), using default parameters for real-time PCR. Candidate genes included EARLY LIGHT-INDUCABLE PROTEIN (ELIP1), histone H3, stress enhanced protein 2 (SEP2), phosphoenolpyruvate carboxykinase (PEPCK), and indoleacetic acid-induced protein 6 (IAA6). The reference gene was V. riparia eukaryotic initiation factor (eIF4A). Candidate genes were chosen randomly from the entire group of 3,892 significantly differentially expressed genes, based on exhibition of different expression patterns across all time points. First strand cDNA production, primer optimization, amplification efficiency determination, and real-time PCR reactions (and parameters) were conducted as previously described (Mathiason et al. 2009). Expression levels of candidate and reference genes at 1, 3, 7, 14, 21, 28 and 42 days of LD and SD were determined using three technical and three biological replicates. The RNA from each biological rep was analyzed simultaneously, and results for each gene were then averaged. Data analysis was performed by MxPro QPCR software (Stratagene, LaJolla CA) and MS Excel software. Candidate gene expression levels were computed relative to the reference gene expression level using the Pfaffl method (Pfaffl 2001). Every microarray expression value was normalized to its respective day 1 value. LD to SD ratios of real-time PCR and microarray expression values for every timepoint (i.e. day 1 LD/day 1 SD) were calculated and log2 transformed. A linear regression was plotted between the PCR ratios and the microarray ratios (Online Resource 1).
Floral pathway genes
Flowering and floral pathway related genes and their corresponding Affymetrix Vitis vinifera (Grape) Genome array probeset numbers were identified from a manual curation of the grape genome (Grimplet et al. 2009). Significantly differentially expressed floral pathway related probesets from the significant Photoperiod × Time interaction were then used for comparison with bud development. All gene expression data is described for SD buds relative to LD buds of the same age (i.e. up regulated in SD = down regulated in LD and down regulated in SD = up regulated in LD).
Results
Inflorescence development
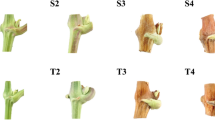
In this study grapevines were grown at optimal temperatures for 35 days after budbreak, at which point LD and SD photoperiod treatments were started using the same temperature conditions. At the start of the differential photoperiod treatments, the latent buds had a well developed primary bud with four to five leaf primordia present (Fig. 1a). Fourteen days after the differential photoperiod treatment was initiated (49 days post budbreak) uncommitted primordia were observed in both LD and SD latent buds. Seven days later (21 days of photoperiod treatment, 56 days post budbreak) inflorescence primordia branch meristems were present in some LD buds. The same age SD buds contained only the two arms and a subtending bract that could develop into tendrils or inflorescences (Fig. 1b), indicating that although not as well developed, the SD buds were also competent to flower at this time. At 42 days of SD treatment (77 days post budbreak), greater inflorescence meristem development was observed than at 21 days SD, but the inflorescence meristem was still in the early stages of development in the form of two arms and subtending bracts (Fig. 1c). In contrast, the same age (42 days) LD buds contained a more developed inflorescence primordia with strong floral meristem initiation (Fig. 1d). Thus both LD and SD buds were floral competent by 56 days after budbreak, and the inflorescence primordia were well developed in LD buds by 77 days after budbreak. The SD treatment slowed but did not stop floral development, suggesting that grapevine is a facultative LD plant and that it can continue floral development under other photoperiod conditions.
Inflorescence primordia development in V. riparia LD and SD buds. a Shoot meristem in primary bud on day 1 of photoperiod treatment. b Inflorescence/tendril meristem in 21 SD bud. c Inflorescence/tendril meristem development in 42 SD bud. d Inflorescence primordia differentiation in 42 LD bud. Letters identify meristem parts: br bract, im inflorescence or tendril meristem, ip inflorescence primordium, lp leaf primordia
Differentially expressed flower related genes during bud development in response to photoperiod
Manual curation of the grape genome indicated that there were 167 probesets on the Affymetrix Vitis vinifera (Grape) Genome Array for 132 orthologues of flowering and floral related genes identified in the grape genome (Grimplet et al. 2009). A significant differential Photoperiod × Time effect was observed for 63 of these probesets, which represent 60 unigenes. The percent of probesets significantly differentially expressed at each time point varied and was greatest at both the early and later stages of photoperiod treatment and bud development (Fig. 2). Maximum differential expression occurred on day 1 of the photoperiod treatments, when 29 probesets were up regulated and 10 probesets were down regulated in SD buds relative to LD buds. The number of probesets significantly differentially expressed decreased through day 14 and then increased from day 21 to day 42, during which time more probesets were down regulated than up regulated in SD buds relative to LD buds.
Differential expression of flowering related genes in SD buds. Sixty-three probesets of flowering related genes that were significantly differentially expressed in SD buds relative to LD buds were used to calculate the percentage of genes that were up regulated, down regulated, or did not change at each day of photoperiod treatment
The significantly differential gene expression was partitioned into two phases based on inflorescence development in the bud (Table 1). The first phase was an early photoperiod response or early signaling phase (days 1, 3, 7 and 14) that corresponds to a primary bud that has well developed leaf primordia and may have some uncommitted primordia. The second phase was a late photoperiod response or floral morphogenesis phase (days 21, 28 and 42) that corresponds to SD buds that contain inflorescence primordia (Fig. 1c) and to LD buds that contain more developed inflorescence primordia with floral meristem initiation. Gene expression related to these two phases fell into three response types: those expressed in the early (27 genes), late (8 genes), or both (25 genes) phases of inflorescence development and photoperiod response.
In the early signaling phase, sixteen genes were significantly differentially expressed only at one time point, with thirteen of these occurring on day one (Table 1). Nine were up-regulated in SD buds relative to LD buds and included abscisic acid (ABA) and cytokinin signaling, transcription factors and other circadian rhythm and flower transition genes. Others (six genes) transiently down regulated on a single day during the early signaling phase included auxin and gibberellin signaling, transcription factors and other meristem formation and patterning genes.
A group of genes (26) showed differential expression in both the early signaling and the later floral morphogenesis phase (Table 1). Some genes exhibited the same expression trend in both phases, while other genes’ expression patterns switched upon transitioning to the late phase. There were six genes up regulated in SD buds relative to LD buds in both phases, including hormonal signaling, fruit ripening related, circadian rhythm and flowering transition related genes. Genes that were down regulated in SD buds relative to LD buds in both phases were predominantly related to flowering transition, light harvesting, and meristem formation and patterning. Eight genes exhibited a switch in their expression patterns upon transitioning to the floral morphogenesis phase. Genes that were up regulated in the early signaling phase and down regulated in the later floral morphogenesis phase in SD buds relative to LD buds were circadian rhythm, flowering transition and meristem formation and patterning genes. Genes that were first down regulated and then up regulated in SD buds relative to LD buds were predominantly circadian rhythm related.
Finally, eight genes were identified that were expressed only in the late photoperiod response or floral morphogenesis phase, when inflorescence primordia were present in both LD and SD buds (Table 1). All but one of these genes were significantly differentially expressed at more than one timepoint in the late phase and all were down regulated in SD buds relative to LD buds. These genes were predominantly transcription factors (6) and were either flower transition related or meristem formation and patterning related.
Discussion
Relationships between floral transition and photomorphogenesis and photoperiod response
The floral development in this study’s differential photoperiods corresponded with earlier reports that grapevine floral initiation occured in varying photoperiod conditions (Srinivasan and Mullins 1980); however, inflorescence initiation and floral development were delayed by SD. Our results verified that the grapevine is a facultative LD plant and indicated that many genes were photoperiod responsive. In contrast to obligate SD flowering response plants, floral initiation in grapevines is LD facultative, whereas growth cessation and dormancy induction in grapevines may be SD obligate or SD facultative, depending on the genotype (Fennell and Hoover 1991). This makes the grapevine a unique model for studying the potential roles that floral pathway genes may play in the initiation of dormancy transitions.
The differential photoperiod treatments and resulting delay in floral meristem initiation in SD buds in the present study allowed predictions to be made for floral pathway genes that have a potential role in the initiation of dormancy transition in the grapevine. The timing of significant differential expression along with expression trends (up or down regulated in SD buds relative to LD buds) relative to the progression of inflorescence meristem development allowed us to identify genes that were: (1) predominantly related to floral transition and floral meristem development, (2) SD responsive with the potential to contribute to a cascade towards bud maturation and dormancy initiation, and (3) potentially involved in both floral transition and development and dormancy initiation (dual role). Genes predominantly related to floral transition and floral meristem development were identified by their association with stages of grape floral meristem development and their consistent significant expression pattern in LD buds that corresponded with their previously reported mode of action in flowering pathways (predominantly up regulated in LD relative to SD unless noted otherwise). Genes with the potential to contribute to a cascade towards bud maturation/dormancy initiation were identified by their functional role in Arabidopsis flowering transitions, but were found to be up regulated only in SD buds (which had delayed floral meristem development). Genes with a potential dual role were identified by differential expression trends, previously identified functional roles in flowering, and unexpected expression relative to the floral development stage in grape buds. These categories provided a filter for the identification of candidate genes that could play a role in dormancy transitions; however, further work will be needed to discriminate a specific functional relationship with bud dormancy transitioning.
Flowering transition and floral meristem development specific gene expression
Flowering transition and floral meristem development were promoted by LD photoperiod treatment, and the corresponding gene expression was evident in this study. During the flowering transition phase several genes were down regulated in SD relative to LD only in the early signaling phase. These included the meristem formation and patterning genes BREVIPEDICELLUS 1 (BP1), DIVARICATA (DIV), SEPALLATA1 (SEP1), RADIALIS (RAD), and ARABIDOPSIS RAD-LIKE 1 (ATRL1). RAD (Fig. 3a) is a MYB transcription factor implicated in development of floral symmetry/asymmetry (Corley et al. 2005). BP1 is a gene involved in inflorescence architecture and is also known as KNOTTED-LIKE FROM ARABIDOPSIS THALIANA (KNAT1), which has a role in controlling cell fate in shoot meristems (Frugis et al. 2001). Signaling genes (BES1-INTERACTING MYC-LIKE PROTEIN 1 (BIM1), CULLIN 1C (CUL1), GIBBERELLIC ACID INSENSITIVE (GAI)) were also down regulated in SD buds in this early signaling phase. BIM1 (Table 1; Fig. 3b) is involved in brassinosteroid mediated signaling (Yin et al. 2005), interacts with auxin signaling genes, and has an implicated role in embryonic development and patterning (Chandler et al. 2009). CUL1 is an auxin regulation gene reported to be involved in ubiquitination and proteasomal degradation of target proteins (Moon et al. 2007). These signaling genes are implicated as floral pathway specific in this study as they were down regulated in SD buds relative to LD buds, and there was a subsequent delay in SD bud floral meristem development (Fig. 1c, d).
Expression of signaling and flowering related genes during bud development in V. riparia and Vitis spp. ‘Seyval’ LD and SD buds. Differential photoperiod treatments were imposed 35 days after budbreak. X-axis is number of days of photoperiod treatment. Y-axis is the mean log2-transformed values of Affymetrix Vitis vinifera (Grape) Genome array expression data
The delay in development in SD buds was also associated with down regulation of light harvesting, signaling, transport, floral transition and meristem formation and patterning genes. Down regulation in SD buds of photosynthetic related genes CHLOROPHYLL A/B BINDING (CAB) (Fig. 3c) and GOLDEN2-LIKE 1 (GLK1) (Fig. 3d), which is involved in chloroplast organization and maintenance of photosynthetic apparatus (Waters et al. 2008), suggested that there was greater photosynthate availability in the LD buds. There was a corresponding down regulation in SD buds of transport genes such as MULTIDRUG RESISTANCE 1 (AtMDR1) (Fig. 3e), which is involved in auxin transport (Noh et al. 2001), and SQUAMOSA PROMOTER BINDING-LIKE 2 (SPL2), a multi function family that is involved in controlling other transcription factor families that regulate membrane protein transport, metabolism of glucose and other metabolic functions (Fig. 3f) (Wang et al. 2009). A similar trend occurred in signal transduction as GENERAL REGULATORY FACTOR 7 (GRF7) (Fig. 3g), which codes for a 14-3-3 protein (Rosenquist et al. 2001), and HEME ACTIVATED PROTEIN 5C (HAP5C) (Fig. 3h), which has been implicated in early flowering (Ben-Naim et al. 2006), were down regulated in SD buds relative to LD buds. HAP5C has a suggested role in mediating the effect of CONSTANS (CO) on flowering time (Wenkel et al. 2006). CO was not differentially expressed in this study; however, CONSTANS-LIKE 2 (COL-2) (Fig. 3i) and CONSTANS-LIKE 16 (COL-16) were down regulated in SD relative to LD buds in this period.
Down regulation of genes associated with cell division or meristem organization could have contributed to slowed floral meristem development in the SD buds. In this study this included CYCLIN DELTA 3 (CYCD3) (Table 1), which encodes a cyclin D-type protein involved in regulation of cell proliferation (Dahl et al. 1995; Menges et al. 2006), SHOOT GRAVITROPISM 7/SHORT ROOT (SGR7/SHR) (Fig. 3j), which is involved in the radial organization of root and shoot axial organs, CHINESE FOR ‘UGLY’ (TSO1) (Fig. 3k), which has a regulatory role in floral meristem cell division (Andersen et al. 2007; Song et al. 2000; Hauser et al. 2000), and CLAVATA3 (CLV3) (Fig. 3l), which regulates the amount of undifferentiated cells in both the shoot and floral meristem (Clark et al. 1995). These genes were significantly differentially expressed at multiple time points from early signaling to floral morphogenesis phase, and their down regulation in SD buds relative to LD buds along with the greater floral meristem development in LD implicated these as predominantly floral pathway related. Clark et al. (1995) postulated that the role of CLV was to restrict undifferentiated proliferative cells in the shoot meristem center. This results in the meristem flank cells differentiating into floral meristems, whose proliferation is subsequently restricted then stopped by CLV’s action. It is reasonable therefore that CLV3 expression was significantly lower in SD grape buds than in LD buds in the floral morphogenesis phase as floral meristem development occured in the LD buds.
Cascade towards bud maturation/dormancy initiation
There were no apparent differences in the bud meristems during the early signaling phase of photoperiod treatment; however, several floral and hormone signaling genes were up regulated in SD buds relative to LD buds. These genes may have a role in SD signaling perception or contributing to coordination of dormancy transitioning. Some flower transition and clock related genes appeared to contribute to early signaling of SD in this study. Included were genes CLP-SIMILAR PROTEIN3 (CLPS3) and MULTIFUNCTIONAL PROTEIN 2 (MFP2) (Fig. 3m), which have been shown to be involved in vegetative to reproductive transitioning (Xing et al. 2008; Richmond and Bleecker 1999), SEUSS 3B PROTEIN (SEU3B), which is thought to participate in regulation of auxin response genes and to be involved in floral meristem patterning (Pfluger and Zambryski 2004), and floral morphogenesis genes HUA ENHANCER 4 (HEN4) and ENHANCER OF AG-41 (HUA1) (Fig. 3n), which have a suggested role in AGAMOUS RNA processing (Cheng et al. 2003; Li et al. 2001).
Hormone signaling potentially related to decreased energy metabolism was suggested by the down regulation of CAB and GLK1 in SD buds and the up regulation of ETHYLENE-INSENSITIVE3 (EIN3), which has transcription factor activity in sugar mediated signaling (Fig. 3o) (Chao et al. 1997; Solano et al. 1998). EIN3 codes for a trans-activation factor nuclear protein that acts in ethylene signal transduction. It was noted by Ruttink et al. (2007) that an ethylene spike preceeded ABA mediated bud maturation and dormancy induction in poplar. Potentially related to these processes is the up regulation of ARABIDOPSIS THALIANA HOMEOBOX 7 (ATHB7) in both the early and later time points of photoperiod treatment (Fig. 3p; Table 1). ATHB7 is thought to be regulated in an ABA-dependent manner, reducing elongation (Soderman et al. 2000). Thus ATHB7 may play a role in the cascade contributing to bud maturation and dormancy initiation. Achard et al. (2007) showed that activated ethylene signaling reduced gibberellic acid (GA) levels and increased DELLA (GAI) accumulation. Accumulation of GAI subsequently delayed flowering by repressing LEAFY (LFY) and SUPRESSOR OF OVER-EXPRESSION OF CONSTANS 1 (SOC1). Achard et al. (2007) observed that ethylene acted on GAI through the ethylene response pathway downstream of EIN3 activity. The observed coordinated down regulation of photosynthesis related genes and up regulation of EIN3 therefore may be involved in SD promotion of dormancy transitioning. In the current study, EIN3 was up regulated in SD buds transiently on days 1 and 7 while GAI was down regulated in SD buds on day 3 (Table 1). This observed expression pattern could be the result of a negative feedback loop (Achard et al. 2007) whereby accumulation of GAI increases the abundance of GA-biosynthesis gene transcripts, which then could downregulate GAI and thus enable floral initiation to progress even under SD photoperiod.
Dual role in floral pathway and SD dormancy induction
Floral development promoters and inhibitors were also up regulated in SD buds (which showed delayed floral meristem development) relative to LD buds, suggesting both a daylength signaling and contribution to dormancy transitioning in the SD buds. FRIGIDA-like (FRI-like), VERNALIZATION INSENSITIVE 3 (VIN3), VERNALIZATION INDEPENDENCE 4 (VIP4), and METHYL-CPG-BINDING DOMAIN 9 (MBD9) were up regulated in SD buds, and these are known to interact with FLOWERING LOCUS C-LIKE MADS-BOX PROTEIN (FLC). FRI-like has been identified as playing a role in Arabidopsis vernalization requirement, as expression of a FRI protein promotes expression of FLC, which delays or represses flowering (Schmitz et al. 2005). VIN3 (Fig. 3q) has been shown to repress FLC, thus promoting early flower transition (Bond et al. 2009), whereas MBD9 and VIP4 activate FLC and inhibit flower development (Zhang and Nocker 2002). The conflicting interactions of VIN3, VIP4 and MBD9 with FLC (Fig. 3r), and expression of all of these genes in the early signaling phase point to potential dual roles of these genes in photoperiod response. In contrast another gene, INDETERMINATE 1 (Id1) (Fig. 3s), which has a well established role in floral development (Colasanti et al. 1998; Colasanti and Sundaresan 2000; Kozaki et al. 2004), is up regulated only in the early signaling phase when floral development of LD and SD buds is similar. The differential expression of VIN3, VIP4 and MBD9 suggested they could have a potential role in SD perception and mediation of bud development, however, a direct relationship between daylength and development was not readily apparent.
A dual role in floral development and promotion of dormancy transitioning was observed for several flowering transition or clock related genes. PSUEDO-RESPONSE REGULATOR 7 (PRR7) and PSUEDO-RESPONSE REGULATOR 9 (PRR9) are positive regulators of flowering time, and it is interesting to note that PRR9 is up regulated in SD buds while PRR7 is up regulated in the early signaling phase in SD buds and down regulated in the floral morphogenesis phase (up regulated in LD buds). This suggests a dual role similar to the findings in leafy spurge, where several circadian-regulated PRRs were all up regulated throughout dormancy induction, maintenance and release (Horvath et al. 2008). Similarly, RECEPTOR-LIKE KINASE IN FLOWERS 3 (RKF3) (receptor kinase involved in flowering) is up regulated in SD buds early and up regulated along with RECEPTOR-LIKE KINASE IN FLOWERS 1 (RKF1) (Fig. 3t) in LD buds later, which suggests a dual role in SD signaling as well as floral development.
Meristem formation and patterning related genes ARABIDOPSIS SKP1-LIKE 1 (ASK1) (Fig. 3u), ARABIDOPSIS SKP1-LIKE 2 (ASK2), and ARABIDOPSIS SHAGGY RELATED PROTEIN KINASE GAMMA (ASK-gamma) may also have a dual role, possibly in signaling changes in day length and also in cell fate determination (Dornelas et al. 2000). Similarly, APETELA 2 (AP2) (Fig. 3v), which has been shown to regulate flower meristem and organ identity (Bowman et al. 1989, 1991; Irish and Sussex 1990; Jofuku et al. 1994; Komaki et al.1988; Kunst et al. 1989), was up regulated in early signaling phase in SD buds and again with bud maturation on days 28 and 42 when SD buds, although delayed in comparison with LD buds, had inflorescence primordia present. The early SD response and up regulation in the later phase suggested that these genes may have a dual role in SD perception and floral morphogenesis or dormancy transition.
Differential expression of PHYTOCHROME A and B, circadian clock, and flower transition genes have been noted during dormancy transitions, and it has been hypothesized that the flowering pathway genes FT, EARLY FLOWERING 4 (ELF4), GIGANTEA (GI), and SOC1 may play a key role in signaling processes regulating dormancy induction (Horvath et al. 2008; Ruonala et al. 2008; Ruttink et al. 2007). In this study, PHYTOCHROME A and B and FT were not significantly differentially expressed between the age matched LD and SD buds, in part due to the fact that these genes are typically expressed in the leaf and this study examined buds. However, several genes, including ELF4 (Fig. 3w), GI (Fig. 3x), SOC1 (Fig. 3y), ELONGATED HYPOCOTYL 5 (HY5), ATHB7 and PRR7, were differentially expressed in relation to both development and photoperiod. The varying expression trends of these genes in response to differential photoperiod treatment in grapevine buds and in relation to the timing of floral meristem development suggest that they may also play a dual role in floral development and dormancy induction.
Identification of SD specific regulation of a fruit ripening gene and CO related genes was also noted in the early and late photoperiod phases. A fruit ripening related gene DDTFR8 (Giovannoni et al. 1999) was up regulated in SD buds on day 1 and also on days 21, 28 and 42 (Fig. 3z) during the period of late bud development and maturation when inflorescence initiation and floral development also occurs. It is likely that this gene has multiple roles in grapevine and may be important in flowering transition and floral development as well as fruit development and ripening. A group of CO related genes [COL-2, COL-13, COL-16, and CONSTANS INTERACTING PROTEIN (CIP6)] were differentially expressed in LD and SD buds. CO regulates flowering time by positively regulating the expression of two floral integrators, FT and SOC1 (An et al. 2004; Yoo et al. 2005). In this study, COL-2 and COL-16 were down regulated during flower transition and floral morphogenesis in SD buds, which had delayed development in comparison to LD buds. This expression pattern suggested that COL-2 and COL-16 may be more related to floral development than dormancy transition pathways. In contrast, COL-13 and CIP6 were up regulated in response to SD, and COL-13 was expressed both during the early and late photoperiod treatment time points when floral morphogenesis was delayed in SD buds, but the buds were transitioning to dormancy.
Finally the expression pattern of REGULATOR OF THE ATPASE OF THE VACUOLAR MEMBRANE 2 (RAV2) (Table 1) may or may not be related to floral morphogenesis or initiation of dormancy transition. RAV2 has been suggested to be a touch sensitive gene and a negative regulator of shoot growth and development in Arabidopsis (Kagaya and Hattori 2009). In this study its expression is significantly down regulated in SD relative to LD buds at 28 and 42 days of differential photoperiod treatment, which at first seemed counter to the developmental processes observed. However, at these later time points the LD buds were rapidly developing floral meristems rather than increasing nodes and leaf primordia number, which could explain the RAV2 expression trend in LD buds.
Model integrating floral and dormancy development
A model is suggested herein that encompasses existing literature and the gene expression trends relative to bud development noted in this study (Fig. 4). Floral development processes in grapevine may occur in two stages as a result of the balance changing between positive and negative regulators. The first stage could determine the transition to floral initiation in the bud, from uncommitted primordium to inflorescence/tendril meristem. The second stage could be the development of floral meristems and floral development from the inflorescence meristem. Genes involved in photoperiod signaling, sugar mediated signaling, and hormonal pathways were differentially expressed in response to day length change. Photoresponse genes include PRR7 and PRR9. GI, which promotes CO, also promotes LATE ELONGATED HYPOCOTYL (LHY) upregulation of TOC1 in a “three-loop model of the circadian clock” in A. thaliana (Lagercrantz 2009), where LHY in turn suppresses GI and promotes PRR7 and PRR9. PRR7 and PRR9 have been shown to suppress LHY (Lagercrantz 2009) and have been reported to upregulate CO through suppression of CDF1, a repressor of CO (Jarillo et al. 2008). COL-2 could also be upregulated in a similar fashion (Fig. 4) by PRR7 and PRR9. COL-2, which has been reported to be down regulated with growth cessation and dormancy (Holefors et al. 2009), showed a similar trend in grapevine buds.
Model integrating the influence of photoperiod on pathways leading to floral development and dormancy induction. A line ending in an arrow head indicates that the gene it originated from is a promoter of the gene or process it points to. A line ending in a perpendicular line indicates that the gene it originated from is a repressor of the gene or process it abuts. A question mark (?) in the model identifies gene activities that are assumed and could not be confirmed by any study in the literature. CO is in parentheses because a probeset for it was not present on the Affymetrix microarray. FT is in parenthesis because it was present on the microarray but was not differentially expressed in this study. This is most likely because FT is activated in leaves and moves to the shoot apical meristem to trigger flowering (Corbesier et al. 2007)
Floral development appeared to be delayed by SD because of activation of suppressors in the vernalization pathway (FRI-like and FLC). Genes such as MBD9 and VIP4, which increase FLC activity (Zhang and Nocker 2002) and independently suppress flowering, were also upregulated soon after exposure to SD. However, expression of promoters of flowering such as Id1, CLPS3 and VIN3, which suppress FLC (Bond et al. 2009), and downregulation of ELF4 and LHY could have kept buds progressing through the floral initiation pathway in SD. ELF4 and LHY were upregulated during the floral morphogenesis phase of this study (days 28–42), further delaying floral development. HY5, which is reported to be involved in photo-morphogenesis and also acts as an integrator between light responses and hormonal responses (Chattopadhyay et al. 1998), was also differentially expressed.
GA and auxin are known to inhibit dormancy while ABA is known to promote it (Saniewski et al. 2000). In the hormonal pathways, genes such as GAI, which was down regulated, could have promoted floral development in SD. EIN3, a transcription factor in the ethylene-signaling cascade, is regulated by sugars (Yanagisawa et al. 2003). Glucose enhances the degradation of EIN3, while ethylene appears to promote EIN3 stability (Rolland and Sheen 2005). There could also be cross talk between ethylene signaling and GA pathways. As previously indicated, ethylene signaling reduces bioactive GA levels, resulting in GAI accumulation, which delays floral transition (Achard et al. 2007). Upregulation of EIN3 in SD buds could also play a role in growth cessation (Binder et al. 2004; Ruttink et al. 2007). ATHB7, a promoter of flowering under SD (Soderman et al. 2000) which is activated by ABA, was also upregulated in SD in the present study and suggests that apart from GAs, ABAs could also be involved in floral development grape buds in SD. HAP5C upregulation in LD shows that the CO-HAP5 complex previously suggested to mediate flowering (Cai et al. 2007) could play a role in floral development in LD in the present study. Auxins could be a major hormonal pathway involved in flowering in LD as AtMDR1 and CUL1C, which are involved in auxin transport, were upregulated in LD. SEP1, implicated in flowering transition and meristem and organ identity, was upregulated in LD in the early period of observation and SPL2, RAV2 and RKF1, which are also involved in flowering transition and floral meristem formation, were upregulated during the period of floral meristem development in LD. More meristem formation and floral organ formation genes such as CLV3, RAV2, RKF and TSO1 were upregulated in LD buds relative to SD buds in the floral morphogenesis phase (days 28–42). This suggests rapid development of floral meristems in this phase after flowering transition in LD. Flowering transition genes seemed to be the major upregulated genes in SD during this phase, which agrees with the delay in development observed in the histology studies.
Conclusion
Grapevines are facultative LD plants with respect to floral initiation in the latent bud; however, they may be either SD obligate or facultative for latent bud dormancy transition. As changing daylength is a seasonal cue common to the initiation of both flowering and dormancy transitions in many plants, a regulatory role for flower transition genes has been frequently suggested. In this study, SD delayed inflorescence development in the grape bud, providing distinct developmental phases that could be related to trends in gene expression patterns. Analysis of 60 significantly differentially expressed flowering related genes during 42 days of grapevine bud development in differential photoperiod treatments (SD and LD) suggested three potential roles: floral transition and floral meristem development related, cascade towards bud maturation/dormancy initiation, or a potential dual role in floral initiation and SD response. A large number of genes were implicated in perception of day length change. Candidate genes with a strong potential to play a role in dormancy transition are the flowering transition or circadian rhythm genes AP2, BT1, COL-13, EIN3, ELF4, DDTFR8, GAI and HY5. While these or related genes have been implicated by other researchers, further analysis will be needed to provide a definitive role in dormancy transitioning.
References
Achard P, Baghour M, Chapple A, Hedden P, Van der Straeten D, Genschik P, Moritz T, Harberd NP (2007) The plant stress hormone ethylene controls floral transition via DELLA dependent regulation of floral meristem-identity genes. PNAS 104:6484–6489
An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, Coupland G (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131:3615–3626
Andersen SU, Algreen-Petersen RG, Hoedl M, Jurkiewicz A, Cvitanich C, Braunschweig U, Schauser L, Oh SA, Twell D, Jensen EØ (2007) The conserved cysteine-rich domain of a tesmin/TSO1-like protein binds zinc in vitro and TSO1 is required for both male and female fertility in Arabidopsis thaliana. J Exp Bot 58:3657–3670
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B 57:289–300
Ben-Naim O, Eshed R, Parnis A, Teper-Bamnolker P, Shalit A, Coupland G, Samach A, Lifschitz E (2006) The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J 46:462–476
Binder BM, Mortimore LA, Stepanova AN, Ecker JR, Bleecker AB (2004) Short-term growth responses to ethylene in Arabidopsis seedlings are EIN3/EIL1 independent. Plant Phys 136:2921–2927
Böhlenius H, Huang T, Charbonnel-Campaa L, Brunner AM, Jansson S, Strauss SH, Nilsson O (2006) CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 312:1040–1043
Bond DM, Dennis ES, Pogson BJ, Finnegan EJ (2009) Histone acetylation, VERNALIZATION INSENSITIVE 3, FLOWERING LOCUS C, and the vernalization response. Mol Plant 2:724–737
Boss PK, Thomas MR (2002) Association of dwarfism and floral induction with a grape “green revolution” mutation. Nature 416:847–850
Boss PK, Sreekantan L, Thomas MR (2006) A grapevine TFL1 homologue can delay flowering and alter floral development when overexpressed in heterologous species. Funct Plant Biol 33:31–41
Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1:37–52
Bowman JL, Smyth DR, Meyerowitz EM (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development 112:1–20
Butrosse MS (1968) Some effects of light intensity and temperature on dry weight and shoot growth of grapevines. Ann Bot 32:753–765
Butrosse MS (1970) Fruitfulness in grapevines: the response of different cultivars to light, temperature and day length. Vitis 9:21–125
Butrosse MS (1974) Climatic factors and fruitfulness in grapevines. Hortic Abstr 44:319–325
Cai X, Ballif J, Endo S, Davis E, Liang M, Chen D, DeWald D, Kreps J, Zhu T, Wu Y (2007) A putative CCAAT-binding transcription factor is a regulator of flowering timing in Arabidopsis. Plant Physiol 145:98–105
Calonje M, Cubas P, Martinez-Zapater JM, Carmona MJ (2004) Floral meristem identity genes are expressed during tendril development in grapevine. Plant Physiol 135:1491–1501
Carmona MJ, Cubas P, Martinez-Zapater JM (2002) VFL, the grapevine FLORICAULA/LEAFY ortholog, is expressed in meristematic regions independently of their fate. Plant Physiol 130:68–77
Chandler JW, Cole M, Flier A, Werr W (2009) BIM1, a bHLH protein involved in brassinosteroid signaling, controls Arabidopsis embryonic patterning via interaction with DORNRÖSCHEN and DORNRÖSCHEN-LIKE. Plant Mol Biol 69:57–68
Chang S, Puryear J, Cairney J (1993) A simple efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR (1997) Activation of the ethylene gas response pathway in Arabidopsis by nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89:1133–1144
Chatelet VL, Fernandez L, Sreekantan L, Lacombe T, Martinez-Zapater JM, Thomas MR, Torregrosa L (2007) Characterization of Vitis vinifera L. somatic variants exhibiting abnormal flower development patterns. J Exp Bot 58:4107–4118
Chattopadhyay S, Ang L, Puente P, Deng X, Wei N (1998) Arabidopsis bZIP protein HY5 directly interacts with light-responsive promoters in mediating light control of gene expression. Plant Cell 10:673–683
Cheng Y, Kato N, Wang W, Li J, Chen X (2003) Two RNA binding proteins, HEN4 and HUA1, act in the processing of AGAMOUS Pre-mRNA in Arabidopsis thaliana. Dev Cell 4:53–66
Chouard P (1960) Vernalization and its relations to dormancy. Annu Rev Plant Physiol Plant Mol Biol 11:191–238
Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121:2057–2067
Colasanti J, Sundaresan V (2000) ‘Florigen’ enters the molecular age: long-distance signals that cause plants to flower. Trends Biochem Sci 25:236–240
Colasanti J, Yuan Z, Sundaresan V (1998) The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93:593–603
Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona F, Gissot L, Turnbull C, Coupland G (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316:1030–1033
Corley SB, Carpenter R, Copsey L, Coen E (2005) Floral asymmetry involves an interplay between TCP and MYB transcription factors in Antirrhinum. PNAS 102:5068–5073
Cramer GR, Ergul A, Grimplet J, Tillett R, Tattersall E, Bohlman M, Vincent D, Sonderegger J, Evans J, Osborne C, Quilici D, Schlauch K, Schooley D, Cushman J (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics 7:111–134
Dahl M, Meskiene I, Bögre L, Ha DT, Swoboda I, Hubmann R, Hirt H, Heberle-Bors E (1995) The D-type alfalfa cyclin gene cycMs4 complements G1 cyclin-deficient yeast and is induced in the G1 phase of the cell cycle. Plant Cell 7:1847–1857
Dornelas MC, Van Lammeren AA, Kreis M (2000) Arabidopsis thaliana SHAGGY-related protein kinases (AtSK11 and 12) function in perianth and gynoecium development. Plant J 21:419–429
Fennell A, Hoover E (1991) Influence of photoperiod on growth, bud dormancy, and cold acclimation in V. labruscana and V. riparia. J Am Soc Hortic Sci 116:270–273
Fennell A, Sreekantan L, Mathiason K, Grimplet J, Dickerson J, Cramer G, Schlauch K (2009) Transcriptomic profiling and molecular mapping of dormancy induction response in grapevine. Plant & Animal Genomes XVII conference, San Diego CA, January 10–14, p 665
Fernandez L, Torregrosa L, Terrier N, Sreekantan L, Grimplet J, Davies C, Thomas MR, Romieu C, Ageorges A (2007) Identification of genes associated with flesh morphogenesis during grapevine fruit development. Plant Mol Biol 63:307–323
Frugis G, Giannino D, Mele G, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, Onckelen HV, Mariotti D (2001) Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol 126:1370–1380
Gautier L, Cope L, Bolstad B, Irizarry RA (2004) Affy—analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20:307–315
Giovannoni J, Yen H, Shelton B, Miller S, Vrebalov J, Kannan P, Tieman D, Hackett R, Grierson D, Klee H (1999) Genetic mapping of ripening and ethylene-related loci in tomato. TAG 98:1005–1013
Grimplet J, Cramer GR, Dickerson JA, Van Hemert J, Mathiason K, Fennell A (2009) VitisNet: Omics integration through grapevine molecular networks. PLoS ONE 4(12):e8365. doi:10.1371/journal.pone.0008365
Hauser BA, He JQ, Park SO, Gasser CS (2000) TSO1 is a novel protein that modulates cytokinesis and cell expansion in Arabidopsis. Development 127:2219–2226
Holefors A, Opseth L, Rosnes AKR, Ripel L, Snipen L, Fossdal CG, Olsen JE (2009) Identification of PaCOL1 and PaCOL2, two CONSTANS-like genes showing decreased transcript levels preceding short day induced growth cessation in Norway spruce. Plant Physiol Biochem 47:105–115
Horvath DP, Anderson JV, Chao WS, Foley ME (2003) Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci 8:534–540
Horvath DP, Chao WS, Suttle JC, Thimmapuram J, Anderson JV (2008) Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). BMC Genomics 9:536
Irish VF, Sussex IM (1990) Function of the apetala-1 gene during Arabidopsis floral development. Plant Cell 2:741–753
Irizarry RA, Hobbs R, Collin R, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Jarillo JA, del Olmo I, Gómez-Zambrano A, Lázaro A, López-González L, Miguel E, Narro-Diego L, Sáez D, Piñeiro M (2008) Review. Photoperiodic control of flowering time. Span J Agric Res 6:221–244
Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK (1994) Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell 6:1211–1225
Johansen DA (1940) Plant microtechnique. McGraw-Hill, New York
Joly D, Perrin M, Gertz C, Kronenberger J, Demangeat G, Masson E (2004) Expression analysis of flowering genes from seedling-stage to vineyard life of grapevine cv, Riesling. Plant Sci 166:1427–1436
Kagaya Y, Hattori T (2009) Arabidopsis transcription factors, RAV1 and RAV2, are regulated by touch-related stimuli in a dose-dependent and biphasic manner. Genes Genet Syst 84:95–99
Kobayashi A, Yukinaga H, Nii N (1965) Studies on the thermal conditions of grapes. IV. Effect of day and night temperatures on the growth of Delaware. J Jpn Soc Hortic Sci 34:77–84
Kobayashi A, Sugiura A, Watanabe H, Yamamura H (1966) On the effects of day length on the growth and flower bud formation of grapes. Mem Res Inst Food Sci Kyoto Univ 27:15–27
Komaki MK, Okada K, Nishino E, Shimura Y (1988) Isolation and characterization of novel mutants of Arabidopsis thaliana defective in flower development. Development 104:195–203
Kozaki A, Hake S, Colasanti J (2004) The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res 32:1710–1720
Kunst L, Klenz JE, Martinez-Zapater J, Haughn GW (1989) AP2 gene determines the identity of perianth organs in flowers of Arabidopsis thaliana. Plant Cell 1:1195–1208
Lagercrantz U (2009) At the end of the day: a common molecular mechanism for photoperiod responses in plants? J Exp Bot 60:2501–2515
Li J, Jia D, Chen X (2001) HUA1, a regulator of stamen and carpel identities in Arabidopsis, codes for a nuclear RNA binding protein. Plant Cell 13:2269–2281
Mathiason K, He D, Grimplet J, Venkateswari J, Galbraith DW, Or E, Fennell A (2009) Transcript profiling in Vitis riparia during chilling requirement fulfillment reveals coordination of gene expression patterns with optimized bud break. Functl Integr Genomics 9:81–96
Menges M, Samland AK, Planchais S, Murray JA (2006) The D-type cyclin CYCD3:1 is limiting for the G1-to-S-phase transition in Arabidopsis. Plant Cell 18:893–906
Moon J, Zhao Y, Dai X, Zhang W, Gray WM, Huq E, Estelle M (2007) A new CULLIN 1 mutant has altered responses to hormones and light in Arabidopsis. Plant Physiol 143:684–696
Noh B, Murphy AS, Spalding EP (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13:2441–2454
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2002–2007
Pfluger J, Zambryski P (2004) The role of SEUSS in auxin response and floral organ patterning. Development 131:4697–4707
Richmond TA, Bleecker AB (1999) A defect in β-oxidation causes abnormal inflorescence development in Arabidopsis. Plant Cell 11:1911–1923
Rohde A, Bhalerao RP (2007) Plant dormancy in the perennial context. Trends Plant Sci 12:217–223
Rolland F, Sheen J (2005) Sugar sensing and signaling networks in plants. Nutrient sensing through the plasma membrane of eukaryotic cells. Biochem Soc Trans 33:269–271
Rosenquist M, Alsterfjord M, Larsson C, Sommarin M (2001) Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol 127:142–149
Ruonala R, Rinne PLH, Kangasjarvi J, Schoot CV (2008) CENL1 expression in the rib meristem affects stem elongation and the transition to dormancy in Populus. Plant Cell 20:59–74
Ruttink T, Arend M, Morreel K, Storme V, Rombauts S, Fromm J, Bhalerao RP, Boerjan W, Rohde A (2007) A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell 19:2370–2390
Saniewski M, Kawa-Miszczak L, Wegrzynowicz-Lesiak E (2000) Role of ABA, gibberellins and auxin in dormancy and dormancy release of tulip bulbs. In: Viémont J, Crabbé J (eds) Dormancy in plants: from whole plant behaviour to cellular control. CAB International publishing, New York, pp 227–245
Schmitz RJ, Hong L, Michaels S, Amasino RM (2005) FRIGIDA-ESSENTIAL 1 interacts genetically with FRIGIDA and FRIGIDA-LIKE 1 to promote the winter—annual habit of Arabidopsis thaliana. Development 132:5471–5478
Soderman E, Hjellstrom M and Engstrom P (2000) High level expression of ATHB7 in transgenic Arabidopsis causes a suppression of elongation growth consistent with a role of ATHB7 in the drought stress response. Abstract conference proceedings 11th international conference on Arabidopsis research, 2000 TAIR accession publication: 1546918
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12:3703–3714
Song JY, Leung T, Ehler LK, Wang C, Liu Z (2000) Regulation of meristem organization and cell division by TSO1, an Arabidopsis gene with cysteine-rich repeats. Development 127:2207–2217
Sreekantan L, Thomas MR (2005) Genes involved in grapevine flowering. Proceedings Australian society of viticulture and oenology conference: transforming flowers to fruit, Mildura, July 4–6
Sreekantan L, Thomas MR (2006) VvFT and VvMADS8, the grapevine homologues of the floral integrators FT and SOC1, have unique expression patterns in grapevine and hasten flowering in Arabidopsis. Funct Plant Biol 33:1129–1139
Sreekantan L, Torregrosa L, Fernandez L, Thomas MR (2006) VvMADS9, a class B MADS-box gene involved in grapevine flowering, shows different expression patterns in mutants with abnormal petal and stamen structures. Funct Plant Biol 33:877–886
Srinivasan C, Mullins MG (1979) Flowering in Vitis: conversion of tendrils in inflorescences and bunches of grapes. Planta 145:187–192
Srinivasan C, Mullins MG (1980) Effects of temperature and growth regulators on formation of anlagen, tendrils and inflorescences in Vitis vinifera L. Ann Bot 45:439–446
Srinivasan C, Mullins MG (1981) Physiology of flowering in grapevines—a review. Am J Enol Vitic 32:47–63
Sugiura A, Utsunomiya N, Kobayashi A (1975) Effects of day-length and temperature on growth and bunch differentiation of grapevines. Jpn J Hortic Sci 43:387–392
Wang Y, Hu Z, Yang Y, Chen X, Chen G (2009) Function annotation of an SBP-box gene in Arabidopsis based on analysis of co-expression networks and promoters. Int J Mol Sci 10:116–132
Waters MT, Moylan EC, Langdale JA (2008) GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J 56:432–444
Wenkel S, Turck F, Singer K, Gissot L, Gourriere JL, Samach A, Coupland G (2006) CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis. Plant Cell 18:2971–2984
Xing D, Zhao H, Li QQ (2008) Arabidopsis CLP1-SIMILAR PROTEIN3, an ortholog of human polyadenylation factor CLP1, functions in gametophyte, embryo, and postembryonic development. Plant Physiol 148:2059–2069
Yanagisawa S, Yoo SD, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425:521–525
Yin Y, Vafeados D, Tao Y, Yoshida S, Asami T, Chory J (2005) A new class of transcription factors mediates brassinosteroid-regulated gene expression in Arabidopsis. Cell 120:249–259
Yoo SK, Chung KS, Joonki K, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol 139:770–778
Zhang H, Nocker S (2002) The VERNALIZATION INDEPENDENCE 4 gene encodes a novel regulator of FLOWERING LOCUS C. Plant J 31:663–673
Acknowledgments
This work was funded by the National Science Foundation (NSF) Plant Genome Program DBI0604755 and South Dakota State University Agricultural Experiment Station. The South Dakota State University Functional Genomics Core Facility, supported in part by the NSF funding EPSCoR0091948, was used to conduct histological analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2010_9611_MOESM1_ESM.tif
Online Resource 1. Supplemental Figure 1. Validation of microarray expression data by real-time PCR. LD to SD ratios for every timepoint (i.e. day 1 LD/day 1 SD) were calculated for both real-time PCR and microarray expression values and log2 transformed. A linear regression resulted in individual regression coefficients (R) of 0.977, 0.828, 0.947, 0.751, and 0.842 for EARLY LIGHT-INDUCABLE PROTEIN (ELIP1), Histone H3, stress enhanced protein 2 (SEP2), phosphoenolpyruvate carboxykinase (PEPCK), and indoleacetic acid-induced protein 6 (IAA6), respectively. (TIFF 13 kb)
Rights and permissions
About this article
Cite this article
Sreekantan, L., Mathiason, K., Grimplet, J. et al. Differential floral development and gene expression in grapevines during long and short photoperiods suggests a role for floral genes in dormancy transitioning. Plant Mol Biol 73, 191–205 (2010). https://doi.org/10.1007/s11103-010-9611-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-010-9611-x