Abstract
Plant growth-promoting salt-tolerant bacteria can increase plant resistance to salt stress and correspond to an environmentally friendly approach to alleviate salt stress. With this approach, forty different bacterial species were isolated from salt-affected soil of Kolhapur, Maharashtra, India. Isolates were investigated for the expression of plant growth-promoting traits at five salt stress levels (0, 20, 40, 60, 80 g L−1 NaCl). Among these forty isolates, AA-P11 was the potent strain for maintaining ACC deaminase activity, phosphate solubilization, indole acetic acid, siderophore, ammonia, hydrogen cyanide and exopolysaccharide production under salt stress. Isolate AA-P11 was identified as Enterobacter cloacae strain KBPD. In the pot study, maximum shoot length, root length, fresh and dry weights were recorded in Vigna radiata L. when supplemented with KBPD in the presence of 0, 50, 100 and 150 mM of NaCl after 14 days. Proline content was high in salt-stressed plants but inoculation with E. cloacae KBPD reduced the concentration of proline. Similarly, total chlorophyll content was also high in bacterized salt-stressed plants compared to non-KBPD salt-stressed plants. AA-P11 also showed good rhizospheric competence under salt stress. Hence, the present study suggested that the Enterobacter cloacae strain KBPD has promising potential for alleviating salinity stress and promoting growth of Vigna radiata L. under salt stress conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The total available land for agriculture has been reduced by the increasing worldwide population, industrialization and urbanization. If these global problems are not resolved in time, it will lead to inadequacy of food to feed the world’s population (Glick 2012). Currently 1.5 × 109 ha of cultivated land in the world, i.e. one-fourth of the total agricultural land, suffers from soil salinity (Sparks 2003). Salt stress affects plant growth by making water uptake by roots more difficult and by causing accumulation of high salt concentrations, leading to plant toxicity (Munns and Tester 2008).
Plants, particularly in the horticulture section, are raw material and used by people for food, either as edible products, or for culinary ingredients, for medicinal use or ornamental and aesthetic purposes. They are a genetically very diverse group and play a major role in modern society and economy. Fruits and vegetables are an important component of traditional food, but are also central to healthy diets of the modern urban population (Koc 2015; Mlcek and others 2015; Wojnicka-Poltorak and others 2015). Vigna radiata L. (Mung bean) is the third most important pulse crop of India after chick pea and pigeon pea (Gupta and others 2014). It is important because of its high nutritional value and its ability to improve soil fertility by atmospheric nitrogen fixation (Elahi and others 2004). Soil salinity has reduced the yield of V. radiata L. by 70 % by affecting root and shoot length, chlorophyll content and germination percentage (Saha and others 2010; Sehrawat and others 2013).
When plants are exposed to biotic or abiotic stress conditions, ethylene is synthesized, resulting in retarded root growth and senescence (Arshad and others 2008; Glick 2004; Ma and others 2003; Sheehy and others 1991). Under salinity stress, there is an elevated level of ACC (1-aminocyclopropane-1-carboxylic acid), an immediate precursor of ethylene, which results in increased ethylene concentrations that potentially lead to physiological changes in leaf tissue (Tank and Saraf 2010). Recently several strategies such as genetic engineering (Wang and others 2003) and the use of plant growth-promoting bacteria (PGPB) (Dimkpa and others 2009) have been developed to diminish the toxic effects caused by high salinity on plant growth. It was proven that PGPB could be a better solution to counteract detrimental effects of high salinity on plant growth and support growth of plants by various direct and indirect mechanisms (Glick 1995; Arshad and Frankenberger Jr 2012). PGPB promotes plant growth by increasing nutrient availability including biological nitrogen fixation (Graham and Vance 2000), phosphate solubilization and mineralization (Rodríguez and others 2006), siderophore production (Neilands 1995) and synthesis of plant hormones such as indole, cytokinins or gibberellins (Costacurta and Vanderleyden 1995). Certain PGPB produce the enzyme ACC deaminase which cleaves ACC to form α- ketobutyrate and ammonia and thereby lowers the ethylene level in developing or stressed plants (Jacobson and others 1994; Glick 1995; Glick and others 1998). PGPB with ACC deaminase activity when colonizing the rhizosphere, keeps ethylene levels low, which is helpful to root growth and survival of plants under salinity stress conditions (Glick 1995). Such PGPB might be useful for use in salt-affected soil for survival, growth and development of plants to increase overall crop yield, and help provide food for a growing population.
In the present study, salt-affected soil was used as the source of bacteria, which heightens the probability of having salt stress-acclimatized isolates that can be directly used as effective bioinoculants for salt-stressed soil. Keeping in view the above discussion, the present study was undertaken (i) to isolate and screen bacteria from saline soil for Kolhapur, Maharashtra, India with respect to their salt tolerance, (ii) to check the effect of salt stress on plant growth-promoting traits (ACC deaminase production, phosphate solubilization, nitrogen fixation, production of IAA, siderophore, ammonia, HCN and exopolysaccharide) of screened isolates, (iii) to evaluate the effect of the most promising bacterial inoculants on growth and development of V. radiata L. in the presence and absence of salt stress, (iv) to evaluate the influence of bacterial inoculation on accumulation of proline and chlorophyll in V. radiata L. and (v) to check root colonization potential of potent isolates under non-saline and saline conditions.
Materials and Methods
Chemicals
ACC was purchased from Sigma-Aldrich, India and all the standards like KH2PO4, IAA, l-proline and other media components were purchased from HiMedia, India. All the chemicals used in this study were of analytical grade.
Isolation of Bacteria
Soil samples were collected from different locations such as Alas, Akiwat, Kavthesar and Sethashal in the Kolhapur district with salinity problems. The collected soil samples were analysed for electrical conductance, pH, organic carbon, total nitrogen and available phosphate. Isolation of salt-tolerant bacteria from 4 different soil samples was carried out, in that 10 g of soil was added in 100 ml sterile saline (0.85 %) in 250 ml Erlenmeyer flasks separately. These flasks were kept on a rotary shaker for 30 min (at 120 rpm) at 30 °C. Then a serial dilution technique was used for bacterial isolation and a 0.1 ml aliquot of 10−8 dilution was spread on different media such as nutrient agar, Pikovskaya’s agar, Ashby’s mannitol agar supplemented with 2 % NaCl and incubated at 30 °C for 48 h.
Screening for Salt Tolerance
The isolated plant growth-promoting bacteria were further used to check their intrinsic resistance to salt stress. For this purpose, the isolates were streaked on nutrient agar supplemented with various concentrations of NaCl (40, 60, 80, 100 g L−1). The nutrient agar plate amended with 0.5 g L−1 NaCl was used as a control. The plates were incubated at 30 °C for 48 h and after the incubation period, growth on NaCl-supplemented medium was compared with controls.
Screening for Plant Growth-Promoting Activities
ACC Deaminase Activity
ACC deaminase activity of PGPS was assayed qualitatively by evaluating the ability to grow on DF minimal medium (Dworkin and Foster 1958) with different salt levels (0, 20, 40, 60, 80 g L−1 NaCl) supplemented with 3 mmol−1 ACC as the sole source of nitrogen (Penrose and Glick 2003). Briefly DF minimal medium agar plates were spot inoculated with a loop full of fresh culture. Plates were incubated at 30 °C and development of a colony was considered positive for ACC deaminase production. ACC deaminase activity was assayed quantitatively by using method of Honma and Shimomura (1978) except that the medium was added with different salt levels (0, 20, 40, 60, 80 g L−1 NaCl). ACC deaminase activity was measured by considering the amount of α-ketobutyrate produced when the enzyme acts on the ACC substrate to form ammonia and α-ketobutyrate. ACC deaminase activity was calculated as μmol α-ketobutyrate mg−1 protein h−1.
Phosphate Solubilization
For determination of phosphate solubilization potential, all bacterial isolates were spot inoculated on Pikovskaya’s agar plates (Pikovaskaya 1948). After incubation, the clear zone around the colony indicated solubilization of inorganic phosphate. Phosphate solubilization potential of bacterial isolates in the presence of salt stress was analysed quantitatively using Pikovskaya’s broth medium amended with different concentrations of NaCl. Briefly, 100 ml of Pikovskaya’s liquid medium with salt stress (0, 20, 40, 60, 80 g L−1 NaCl) was inoculated with 1 ml aliquot of seed culture and incubated under shaking conditions at 30 °C for 7 days. Sterile uninoculated medium served as control. After 24 h, the culture was harvested by centrifugation at 6000 rpm for 15 min. The amount of soluble phosphate in the supernatant was quantified using the Fiske and Subbarow method (1925). The amount of soluble phosphate was detected from the standard curve of KH2PO4.
Nitrogen Fixation
The nitrogen fixation ability of salt-tolerant isolates was determined using the method described by Gothwal and others (2008). After incubation, the formation of a blue colour zone around the culture colony indicated nitrogen fixation.
IAA Production
Salt-tolerant bacterial isolates were tested for IAA production and quantified using the method of Gordon and Weber (1951) with slight modification. One ml aliquot of seed culture was inoculated in 250 ml Erlenmeyer flasks containing 100 ml nutrient broth supplemented with a graded series of NaCl (0, 20, 40, 60, 80 g L−1 NaCl) with 0.2 % of tryptophan and incubated at 30 °C for 6 days on a rotary shaker. After 24 h, the amount of IAA produced was determined by using Salkowski reagent (1 ml 0.5 M FeCl3 in 50 ml of 35 % HClO4). Absorbance of the resulting pink colour was measured at 540 nm using a UV–Vis spectrophotometer. The concentration of IAA was determined from a standard curve of IAA (10–100 µg ml−1).
Siderophore Production
Isolates were tested for siderophore production by the chrome azurol S agar (CAS) method described by Schwyn and Neilands (1987). All the isolates were spot inoculated on chrome azurol S agar plates with salt stress (0, 20, 40, 60, 80 g L−1 NaCl), and the plates were incubated at 30 °C for 6–7 days. After the incubation period, the development of a yellow–orange halo zone around the colony was considered positive for siderophore production.
Ammonia Production
A freshly grown culture of PGPS was inoculated separately into 5 ml peptone water containing salt (0, 20, 40, 60, 80 g L−1 NaCl) and incubated at 30 °C for 48 h. After the bacterial growth, Nessler’s reagent (0.3 ml) was added to each tube. Development of brown to yellow colour indicates a positive test for ammonia production (Marques and others 2010) and uninoculated medium was used as a blank.
Hydrogen Cyanide Production
Isolates were qualitatively checked for cyanide production using King’s medium (King and others 1954). Each bacterial isolate was spread on to King’s agar plates with different salt concentrations (0, 20, 40, 60, 80 g L−1 NaCl). The Petri plates were covered with a lid containing a piece of filter paper soaked in 1 % picric acid and moistened with a few drops of 10 % NaCO3 (Lorck 1948), plates were sealed with parafilm and incubated at 30 °C. Control plates were prepared without inoculation. Change in filter paper colour from yellow to brown was considered positive for HCN production.
Exopolysaccharide Production
Production of exopolysaccharide by the isolates was assayed qualitatively according to Nicolaus and others (1999). Bacterial strains were grown in 100 mL of medium containing 1 g yeast extract; 0.75 g casamino acids; 0.3 g trisodium citrate; 0.2 g KCl; 2 g MgSO47H2O; 0.036 mg MnCl24H2O; 5 g FeSO47H2O in 250 mL Erlenmeyer flasks with salt stress (0, 20, 40, 60, 80 g L−1 NaCl) at 30 °C for 5 days under shaking conditions (150 rpm). The supernatant was collected by centrifuging at 8000 rpm for 15 min at 4 °C. Cold absolute ethanol was then added dropwise under stirring, and the formation of a precipitate was considered positive for the production of exopolysaccharide.
Biochemical Characterization and Identification of Isolate
The ability of the salt-tolerant isolate to utilize different carbohydrates was tested using a carbohydrate utilization test kit (KB 009, HiMedia) as per instructions. Gram staining and a IMViC (Indole, Methyl Red, Voges Proskauer, Citrate utilization) test were performed following standard protocol (Prescott and others 2002). Isolate AA-P11 was identified by 16S rDNA gene sequence analysis. The evolutionary history was inferred using the neighbour-joining method (Saitou and Nei 1987). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the maximum composite likelihood method (Tamura and others 2004) and were in the units of the number of base substitutions per site. The analysis involved 20 nucleotide sequences. All positions containing gaps and missing data were eliminated. There were a total of 1233 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 (Tamura and others 2013). The partial 16S rDNA gene sequences were deposited in the Gene Bank data base under accession number KU759932.
Preparation of Bacterial Inoculum and Seed Treatment
On the basis of plant growth-promoting properties, Enterobacter cloacae strain KBPD was evaluated for its ability to alleviate salt stress in Vigna radiata L. plants. Preparation of the bioinoculum and seed treatment was performed according to Penrose and Glick (2003) with some modifications. An in vivo pot study was carried out in the month of September in which the average temperature was in the range of 30–35 °C and average humidity was more than 30 %. The pot study was carried out in triplicate. Soil used for the experiment was sterilized by autoclaving. Seeds were surface-sterilized by treating with 70 % ethanol for 1 min followed by four times washing with sterilized distilled water and treated with Enterobacter cloacae strain KBPD by soaking in broth inoculated with the culture and then seeded into the pots. For imposing salt stress, plants were watered with 0, 50, 100 and 150 mM salt solution at 48 h intervals separately in each pot. After 14 days of sowing, all the plantlets were uprooted and vegetative parameters like shoot length, root length, fresh and dry weight were analysed.
Estimation of Chlorophyll in Plants
The fresh leaves of control plants and salt-stressed plants were collected randomly after 14 days of plantation, washed thoroughly and blotted. Then, 1 g of leaf tissue was crushed in acetone (80 % v/v) and the final volume was adjusted to 20 ml and stored overnight under refrigeration. This extract was then centrifuged and filtered through glass wool. The absorbance of the supernatant was recorded at 663 and 645 nm using a spectrophotometer and the total chlorophyll content was determined as the mean of all readings (Arnon 1949).
Estimation of Proline in Plants
Proline content was estimated according to Bates and others (1973). Briefly, 0.5 g fresh leaves were homogenized with 3 % sulfosalicylic acid and immediately centrifuged at 11,500 rpm for 5 min. One millilitre of supernatant was taken for analysis. Absorbance was measured at 520 nm and the calibration curve was determined using pure l-proline as standard.
Root Colonization Assay
To quantify root colonization by KBPD, root samples with adhering soil were dipped in a sterile saline solution (0.85 % NaCl) and were kept on a shaker for 1 h. Serial dilutions of bacterized roots were prepared in sterile saline and spread on nutrient agar plates. The plates were incubated at 30 °C for 24 h. The experiment was replicated three times, and the average number of bacterial colony-forming units was calculated and presented as mean cfu g−1 root biomass.
Statistical Analysis
Results were developed as an average of three or more determinations. Analysis of variance was carried out for all data at p < 0.05 using Graph Pad software (GraphPadIn Stat version 3.00, GraphPad Software, San Diego, CA, USA).
Results
Isolation of Plant Growth-Promoting Bacteria and Their Salt Tolerance
The soil samples collected from four different places were analysed for physicochemical properties and results are summarized in Supplementary Table 1. All soil samples had an alkaline pH with more or less the same electrical conductance. From that soil sample, forty bacterial isolates were selected based on their distinct morphology and salt tolerance (2 %). Out of forty isolates 27 isolates were grown on nutrient agar, seven isolates on Pikovskaya’s agar and 6 isolates on Ashby’s mannitol agar. Among the 40 isolates, twenty isolates showed salt tolerance up to 4 %, whereas isolate AA-P1, AA-P8, AA-P10, AA-P11, AA-P12 and AA-P13 were capable of tolerating up to 8 % salt concentration (Supplementary Table 2).
In Vitro Plant Growth-Promoting Activities of Salt-Tolerant Plant Growth-Promoting Bacteria
ACC Deaminase Activity
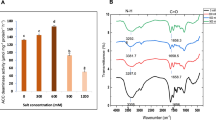
All PGPS were screened for ACC deaminase activity and only three isolates (AA-P4, AA-P11 and AA-P20) showed growth on DF minimal medium under non-saline as well as saline conditions. The production of ACC deaminase was affected differentially by various NaCl concentrations in the growth media. AA-P11 showed growth on DF minimal medium under non- saline as well as saline conditions up to 6 % salt level; further increase in salt stress inhibited the growth of the organism. Quantitative assay showed that 2 % salt stress slightly induced the activity of ACC deaminase of the AA-P11 isolate, whereas at 4 and 6 % salt stress there was reduction in activity. Salt stress showed a negative effect on the activity of ACC deaminase in tested bacteria (Fig. 1).
Phosphate Solubilization
All isolates were screened for phosphate solubilization. Six isolates showed a clear zone in Pikovskaya’s agar plates. Quantitative phosphate solubilization was carried out for 7 days in continuous culture medium. Out of six isolates, AA-P11 showed maximum phosphate solubilization of 3692 ± 2.64 µg/ml after 96 h of incubation, even in the presence of 60 g L−1 NaCl, which is highest as compared to the amount of phosphate solubilized, 1602 ± 2.64 µg/ml, in the absence of salt stress under an identical incubation period. This reveals the phosphate solubilization potential of AA-P11 in the presence of salinity stress (Supplementary Fig. 1). In addition, phosphate solubilization by AA-P11 was increased with increasing salt stress up to 6 % but a further increase showed decreased phosphate solubilization. The remaining isolates showed phosphate solubilization under non-saline conditions but their potential for phosphate solubilization decreased with increasing salt stress (Supplementary Fig. 1).
Nitrogen Fixation
From all isolates only three isolates were able to fix atmospheric nitrogen. Among the three isolates, AA-P16 fixed the highest amount of atmospheric nitrogen.
Indole Acetic Acid Production
In the study of IAA production, five isolates were able to produce IAA under non-saline conditions as well as at different salt concentrations. Among these five isolates, AA-P11 was the most tolerant to salinity with respect to IAA production. The IAA production potential of AA-P11 increased with increased salt stress (up to 6 %). AA-P11 produced the maximum IAA of 67.11 ± 1.05 µg/ml after 96 h of incubation, even in the presence of 60 L−1 NaCl, which was very high as compared to the maximum amount of IAA produced, 22.16 ± 1.57 µg/ml, in the absence of salt stress after 48 h of incubation (Supplementary Fig. 2). However, other salt-tolerant isolates showed decreased IAA production with increasing salinity stress (Supplementary Fig. 2).
Siderophore, Ammonia and HCN Production
Out of all the PGPS, twelve, five and seventeen isolates showed siderophore, ammonia and cyanide production, respectively under control conditions; however, their production ability was reduced at 2 % NaCl and completely inhibited at 4 % NaCl stress (Supplementary Table 3). On the other side, isolate AA-P11 showed siderophore and ammonia production up to 6 % of salt stress and complete inhibition at 8 % salt stress but cyanide production continued up to the 8 % salt stress level. However, less ammonia and siderophore were produced at 6 % and cyanide production at 8 % salinity as compared to non-saline conditions (Table 1).
Exopolysaccharide Production
Among all PGPS, three isolates showed exopolysaccharide production but their production level was negatively affected by increasing salt stress. AA-P11 showed exopolysaccharide production under control as well as salt stress conditions and that production levels were not affected by salt concentrations (Table 1). In contrast, the remaining isolates were unable to produce exopolysaccharide under normal or saline conditions (Supplementary Table 3).
Identification of Potent Isolate
AA-P11 was the most potent plant growth-promoting salt-tolerant isolate among them all. The biochemical profile of the isolates is summarized in Supplementary Table 4. The isolate was identified as Enterobacter cloacae strain KBPD by 16S rDNA sequence analysis. The phylogenetic tree of Enterobacter cloacae strain KBPD in relation with GenBank data is illustrated in Fig. 2.
Effect of Bacterial Inoculation on Growth of Vigna radiata L. and Content of Photosynthetic Pigments Under Salt Stress
Vigna radiata L. was exposed to different levels of salt stress (50, 100 and 150 mM) for two weeks and growth was impaired significantly with increasing salt stress compared with control. Salt stress leads to 35.36, 50.60, 66.58 % reduction in shoot and 48.25, 61.86, 58.75 reduction in root length of V. radiata L. under 50,100 and 150 mM of salt stress, respectively. Salt-stressed plants bacterized with PGPS Enterobacter cloacae strain KBPD showed significant improvement in root, shoot length and root, shoot biomass than non-bacterized salt stress plants. Under the presence of salt stress, the average shoot lengths of KBPD treatments were 13.33 ± 0.2, 10.2733 ± 0.26 and 7.5433 ± 0.20 cm (Fig. 3a) and root lengths 4.2333 ± 0.12, 3.6366 ± 0.05 and 4.39 ± 0.14 cm (Fig. 3b), whereas non-bacterized salt-stressed plants only had an average shoot lengths of 10.333 ± 0.30, 8.42 ± 0.37 and 5.26 ± 0.24 cm and root lengths of 3.17 ± 0.15, 2.3366 ± 0.26 and 2.5266 ± 0.13 cm in the presence of 50, 100, 150 mM of salt stress, respectively.
Similarly, the individual fresh weights of salt-stressed plants inoculated with KBPD were 0.72 ± 0.01, 0.6306 ± 0.01 and 0.6333 ± 0.02 gm (Fig. 4) and dry weights 0.093 ± 0.002, 0.0756 ± 0.003 and 0.0826 ± 0.001 gm (Fig. 5), whereas non-bacterized salt stress plants showed fresh weights of 0.6236 ± 0.005, 0.53 ± 0.01 and 0.5166 ± 0.01 gm and dry weights of 0.082 ± 0.002, 0.0843 ± 0.002 and 0.064 ± 0.001 gm in the presence of 50, 100 and 150 mM of salt stress, respectively. The isolate was able to increase the chlorophyll content in V. radiata L. at 50, 100 and 150 mM of salt stress, by 19.31, 15.42 and 43.20 % with respect to the non-bacterized salt-stressed seedlings (Fig. 6).
Effect of Enterobacter cloacae Strain KBPD on Proline Content of Vigna radiata L
Our findings indicated that salt-stressed plants synthesized proline to a greater extent (Fig. 7). However, plants bacterized with KBPD showed decreased contents of proline in salt stress of 50, 100 and 150 mM by 38.56, 45.78 and 28.46 % respectively.
Root colonization Assay
The root colonization assay showed increased numbers of bacteria on the root surface of Vigna radiata L. plants under normal as well as salt stress after inoculation with KBPD strains (Table 2). The highest bacterial cfu g−1 root biomass was observed with the studied strain under non-saline conditions and it decreased with increasing salt stress except at the 150 mM level. At this salinity level, reduction in the initial population (control) was also less than observed for other salt stress conditions (50, 100 mM).
Discussion
Plant growth-promoting bacteria have direct and indirect mechanisms that help them stimulate growth and development of plants in the presence of biotic as well as abiotic stressors. Salt stress is one of the major stresses affecting growth, development and productivity of plants (Allakhverdiev and others 2000). Previous study has shown that ACC deaminase-containing rhizobacteria can lessen the negative impact of ethylene stress on root growth by degrading ACC into ammonia and α-ketobutyrate (Ali and others 2004; Glick and others 1998; Mayak and others 2004; Shaharoona and others 2011). The present study showed amelioration of salt stress in Vigna radiata L. by bacterization of seeds and further colonization of roots by ACC deaminase-producing PGPS Enterobacter cloacae strain KBPD isolated from salt-affected soil of Kolhapur, Maharashtra, India. Enterobacter cloacae is a Gram-negative organism known to have plant growth-promoting attributes which has been previously reported (Jha and others 2012; Khalifa and others 2016).
Among all isolates, strain KBPD exhibited the ability to grow on nutrient agar medium containing NaCl concentrations as high as 8 %. These results are in accordance with those obtained on Enterobacter spp. which could tolerate 7 % levels of NaCl stress (Deepa and others 2010). The existence of organisms in salinity-stressed soil may be responsible for its salt-tolerance quality. This ability to tolerate salt is adventitious to PGPB populating the soil affected by salinity. KBPD showed variation in salinity tolerance characteristics. The isolate showed tolerance up to the 8 % salt stress level when grown on nutrient agar but did not show the same salt-tolerance profile when grown in chrome azurol s agar and DF minimal medium containing ACC as the sole nitrogen source. The basic composition of the culture medium might be the reason for such a response of the isolate to salinity. These results corroborate with results of previous studies (Yaish and others 2015).
In the present study, ACC was used as the sole source of nitrogen by KBPD, AA-P4 and AA-P20 in the presence and the absence of salt stress. At the salinity level of 2 % AA-P4 and AA-P20 strains showed an unexpected decrease in degradation of ACC, the precursor of ethylene stress. The consistent high ACC deaminase activity of KBPD thus indicates its high power for promoting plant growth over a range of salt stress that would result in ethylene stress accumulation (Zahir and others 2009). This result was similar to results of previous studies (Ahmad and others 2011; Nadeem and others 2010; Tank and Saraf 2010) regarding ACC deaminase activity under salinity stress.
Phosphate is a limiting factor for growth of plants under salinity stress (Pick and others 1990). Differences in phosphate solubilization ability among bacteria were observed. KBPD showed phosphate solubilization in non-saline plus saline conditions. The maximum value of phosphate solubilization was 3692 ± 2.64 µg/ml. The isolate showed a 2.3-fold increase in phosphate solubilization in salt stress (6 % salt stress) as compared to control. This observation indicates superior performance of KBPD, an isolate of salt-affected soil, in releasing phosphorous from TCP even under salt stress conditions and may also contribute to improved nutrition of plants growing in salinity-stressed soil. The presence of such phosphate-solubilizing bacteria provides plants with accessible phosphate and leads to increased phosphate content of plants (Kucey and others 1989; Mehana and Wahid 2002; Zaidi and others 2004).
Plant hormones play an important role in regulation of growth and development. Auxin is one of the extensively studied hormones regulating cell division, cell elongation, cell differentiation and pattern formation in plants (Berleth and Sachs 2001). Root exudates are natural sources of l-tryptophan, which may enhance auxin biosynthesis in the rhizosphere by PGPB (Kravchenko and others 1991; Martens and FrankenbergerJr 1994). In addition to phosphate solubilization, the KBPD strain also showed the highest IAA production of 67.11 ± 1.05 µg/ml. In the present study, the IAA production ability of some of the tested isolates decreased with increasing salt stress; whereas 6 % salt stress induced IAA production in KBPD by 3-fold compared to control. This result is further supported from the work of Nakbanpote and others (2014), who found that IAA production by Pseudomonas spp. increased under salinity stress, whereas Serratia spp. showed reduced IAA production.
Some of these isolated salt-tolerant bacterial strains were able to produce siderophore, ammonia and cyanide (Supplementary Table 3). Plants consume macro- plus micronutrients during their routine growth and development. However, in the presence of salt stress, physiological processes demand more energy, which increases the nutrient needs of the plant. This causes the plants to invest more energy (Yaish and others 2015). The strain KBPD exhibited the ability to produce siderophore, ammonia and cyanide, which may act as sources of nutrients to the plants exposed to salt stress. Other researchers have also reported that indigenous rhizobacteria commonly possess a variety of plant growth-promoting traits such as ACC deaminase activity, IAA production, phosphate solubilization and siderophore and cyanide production, alone or in combination (Kumar and others 2011; Sharma and others 2011; Timmusk and others 2011). The 8 % salt tolerance in addition to plant growth-promoting traits under non-saline and saline conditions leads to the selection of AA-P11 as a bioinoculant, to study its effect on growth of Vigna radiata L. (Table 1).
Pot trials were carried out by using Vigna radiata L. in the presence of 50, 100, 150 mM of salt stress. Plants were exposed to salt stress and when bacterized with PGPS Enterobacter cloacae strain KBPD showed an increase in root length, shoot length, fresh and dry weight. In agreement with our results, Mayak and others (2004) observed increased tomato plant biomass under 120 and 207 mM NaCl stress inoculated with Achromobacter piechaudii ARV8. Similarly, Singh and others (2015) observed that inoculation of wheat (Triticum aestivum L.) with ACC deaminase-producing Klebsiella spp. SBP-8 under salt stress results in increased root length, shoot length, fresh and dry weight.
Salt-stressed bacterized plant showed increased chlorophyll content, as compared with non-bacterized salt-stressed plants, which ultimately indicates improvement in photosynthetic activity. Similarly, Cheng and others (2012) observed the same results on Brassica napus by inoculation of ACC deaminase-producing Pseudomonas putida UW4. Inoculation with P. fluorescens, B. megaterium and V. paradoxs also improved the chlorophyll contents in leaves of cucumber grown under salt-stressed conditions (Nadeem and others 2016).
Accumulation of proline content was observed in salt-stressed plants; however, inoculation with KBPD caused a reduction in proline content of salt-stressed roots and shoots. Similar results were shown by Hamdia and others (2004) and Nadeem and others (2007). The significant contribution of the isolate in reducing salt stress was also indicated by the decreased level of proline in bacterized plants exposed to salt stress. Ashraf and others (2004) found that inoculation with exopolysaccharide-producing bacteria could restrict Na+ influx into roots. EPS production by plant growth-promoting strains helps in binding cations, including Na+, and thus decreases the content of Na+ available for uptake by plants. This is especially beneficial for alleviating salt stress in plants (Geddie and Sutherland 1993). In addition to other plant growth-promoting characteristics, the ability of the strain KBPD to produce exopolysaccharides makes it a powerful candidate for use as a bioinoculant for salt-stressed soil, which was evaluated by the colonization assay. Production of exopolysaccharides may be responsible for the adhesion of the isolate to plant roots which showed rhizospheric competence of KBPD even in a salt stress environment.
The approach of screening isolates for salt tolerance firstly followed by screening of plant growth-promoting salt-tolerant bacteria for plant growth-promoting traits at different salt stress levels omitted other isolates that were not salt-tolerant as well as isolates that failed to show plant growth-promoting traits under saline conditions. The KBPD isolate showed maximum growth under salinity and this was found to be a suitable salt concentration for expression of plant growth-promoting traits. Salinity significantly reduced seedling growth (shoot length, root length, shoot biomass, root biomass) of V. radiata L. Plants inoculated with the PGPS Enterobacter cloacae strain KBPD showed improvement in plant growth by reducing the deleterious effects of salinity, increasing root length, shoot length and fresh, dry biomass, chlorophyll content and by reducing proline content. These results suggest that the ACC deaminase-producing PGPS has the ability to diminish the detrimental effect of salt stress on growth of V. radiata L. plants.
Conclusion
In this study, we have shown that the plant growth-promoting salt-tolerant bacteria Enterobacter cloacae strain KBPD is able to facilitate plant growth under abiotic stress (salinity) conditions. The experiments confirm that inoculation of Vigna radiata L. with the ACC deaminase-producing Enterobacter cloacae strain KBPD protects plants against adverse effects of salt stress. Our findings mainly focus on the inoculation of bacteria that could be able to ameliorate the salinity stress by combination of several aspects including promotion of plant growth through synthesis of plant hormones like IAA, and efficient ACC deaminase activity to reduce stress-induced ethylene in plants. The excellent performance of KBPD in promoting plant growth by having plant growth-promoting traits under salt stress and by good rhizospheric competence holds great potential for use as an ideal bioinoculant especially for salinity-affected soil.
References
Ahmad M, Zahir ZA, Asghar HN, Asghar M (2011) Inducing salt tolerance in mung bean through coinoculation with rhizobia and plant-growth-promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 57(7):578–589
Ali S, Charles TC, Glick BR (2014) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem 80:160–167
Allakhverdiev SI, Sakamoto A, Nishiyama Y, Inaba M, Murata N (2000) Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol 123(3):1047–1056
Arnon DI (1949) Copper enzymes in isolated chloroplasts.Polyphenoloxidase in Beta vulgaris. Plant Physiol 24(1):1
Arshad M, Frankenberger WT Jr (2012) Ethylene: agricultural sources and applications. Springer, New York
Arshad M, Shaharoona B, Mahmood T (2008) Inoculation with Pseudomonas spp. containing ACC-deaminase partially eliminates the effects of drought stress on growth, yield, and ripening of pea (Pisum sativum L.). Pedosphere 18(5):611–620
Ashraf M, Hasnain S, Berge O, Mahmood T (2004) Inoculating wheat seedlings with exopolysaccharide-producing bacteria restricts sodium uptake and stimulates plant growth under salt stress. Biol Fertil Soils 40(3):157–162
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39(1):205–207
Berleth T, Sachs T (2001) Plant morphogenesis: long-distance coordination and local patterning. Curr Opin Plant Biol 4(1):57–62
Cheng Z, Woody OZ, McConkey BJ, Glick BR (2012) Combined effects of the plant growth-promoting bacterium Pseudomonas putida UW4 and salinity stress on the Brassica napus proteome. Appl Soil Ecol 61:255–263
Costacurta A, Vanderleyden J (1995) Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol 21(1):1–18
Deepa CK, Dastager SG, Pandey A (2010) Isolation and characterization of plant growth promoting bacteria from non-rhizospheric soil and their effect on cowpea (Vigna unguiculata (L.)Walp.) seedling growth. World J Microbiol Biotechnol 26(7):1233–1240
Dimkpa C, Weinand T, Asch F (2009) Plant–rhizobacteria interactions alleviate abiotic stress conditions. Plant Cell Environ 32(12):1682–1694
Dworkin M, Foster JW (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75(5):592
Elahi NN, Mustafa S, Mirza JI (2004) Growth and nodulation of mungbean (Vigna radiata [L.]Wilczek) as affected by sodium chloride. Growth 15(2):139–143
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66(2):375–400
Geddie JL, Sutherland IW (1993) Uptake of metals by bacterial polysaccharides. J Appl Bacteriol 74(4):467–472
Glick BR (1995) The enhancement of plant growth by free-living bacteria. Can J Microbiol 41(2):109–117
Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv Appl Microbiol 56:291–312
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012:1–15
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190(1):63–68
Gordon SA, Weber RP (1951) Colorimetric estimation of indole acetic acid. Plant Physiol 26(1):192
Gothwal RK, Nigam VK, Mohan MK, Sasmal D, Ghosh P (2008) Screening of nitrogen fixers from rhizospheric bacterial isolates associated with important desert plants. Appl Ecol Environ Res 6(2):101–109
Graham PH, Vance CP (2000) Nitrogen fixation in perspective: an overview of research and extension needs. Field Crops Res 65(2):93–106
Gupta S, Gupta R, Sharma S (2014) Impact of pesticides on plant growth promotion of Vigna radiata and non-target microbes: comparison between chemical-and bio-pesticides. Ecotoxicology 23(6):1015–1021
Hamdia MAES, Shaddad MAK, Doaa MM (2004) Mechanisms of salt tolerance and interactive effects of Azospirillum brasilense inoculation on maize cultivars grown under salt stress conditions. Plant Growth Regul 44(2):165–174
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42(10):1825–1831
Jacobson CB, Pasternak JJ, Glick BR (1994) Partial purification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can J Microbiol 40(12):1019–1025
Jha CK, Annapurna K, Saraf M (2012) Isolation of rhizobacteria from Jatropha curcas and characterization of produced ACC deaminase. J Basic Microbiol 52(3):285–295
Khalifa AY, Alsyeeh AM, Almalki MA, Saleh FA (2016) Characterization of the plant growth promoting bacterium, Enterobacter cloacae MSR1, isolated from roots of non-nodulating Medicago sativa. Saudi J Biol Sci 23(1):79–86
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med 44(2):301–307
Koc A (2015) Effect of plant growth-promoting bacteria and arbuscular mycorrhizal fungi on lipid peroxidation and total phenolics of strawberry (Fragaria × ananassa’San Andreas’) under salt stress. Turk J Agric For 39(6):992–998
Kravchenko LV, Borovkov AV, Pshikvil Z (1991) The possibility of auxin biosynthesis in wheat rhizosphere by associated nitrogen-fixing bacteria. Microbiology 60:927–931
Kucey RMN, Janzen HH, Leggett ME (1989) Microbially mediated increases in plant-available phosphorus. Adv Agron 42(199228):60525–60528
Kumar K, Amaresan N, Bhagat S, Madhuri K, Srivastava RC (2011) Isolation and characterization of rhizobacteria associated with coastal agricultural ecosystem of rhizosphere soils of cultivated vegetable crops. World J Microbiol Biotechnol 27(7):1625–1632
Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiol Plant 1(2):142–146
Ma W, Guinel FC, Glick BR (2003) Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-1-carboxylate deaminase promotes nodulation of pea plants. Appl Environ Microbiol 69(8):4396–4402
Marques AP, Pires C, Moreira H, Rangel AO, Castro PM (2010) Assessment of the plant growth promotion abilities of six bacterial isolates using Zea mays as indicator plant. Soil Biol Biochem 42(8):1229–1235
Martens DA, FrankenbergerJr WT (1994) Assimilation of exogenous 2′-14C-indole-3-acetic acid and 3′-14C-tryptophan exposed to the roots of three wheat varieties. Plant Soil 166(2):281–290
Mayak S, Tirosh T, Glick BR (2004) Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem 42(6):565–572
Mehana TA, Wahid OA (2002) Associative effect of phosphate dissolving fungi, Rhizobium and phosphate fertilizer on some soil properties, yield components and the phosphorus and nitrogen concentration and uptake by Vicia faba L. under field conditions. Pak J Biol Sci 5:1226–1231
Mlcek J, Valsikova M, Druzbikova H, Ryant P, Jurikova T, Sochor J, Borkovcova M (2015) The antioxidant capacity and macroelement content of several onion cultivars. Turk J Agric For 39(6):999–1004
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nadeem SM, Zahir ZA, Naveed M, Arshad M (2007) Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol 53(10):1141–1149
Nadeem SM, Zahir ZA, Naveed M, Asghar HN, Arshad M (2010) Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci Soc Am J 74(2):533–542
Nadeem SM, Ahmad M, Naveed M, Imran M, Zahir ZA, Crowley DE (2016) Relationship between in vitro characterization and comparative efficacy of plant growth-promoting rhizobacteria for improving cucumber salt tolerance. Arch Microbiol 198:1–9
Nakbanpote W, Panitlurtumpai N, Sangdee A, Sakulpone N, Sirisom P, Pimthong A (2014) Salt-tolerant and plant growth-promoting bacteria isolated from Zn/Cd contaminated soil: identification and effect on rice under saline conditions. J Plant Interact 9(1):379–387
Neilands JB (1995) Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 270(45):26723–26726
Nicolaus B, Lama L, Esposito E, Manca MC, Improta R, Bellitti MR, Gambacorta A (1999) Haloarculaspp able to biosynthesize exo-and endopolymers. J Ind Microbiol Biotechnol 23(6):489–496
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118(1):10–15
Pick U, Chitlaru E, Weiss M (1990) Polyphosphate-hydrolysis-a protective mechanism against alkaline stress? FEBS Lett 274(1):15–18
Pikovaskaya RI (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Microbiology 17:362–370
Prescott LM, Harley JP, Klein DA (2002) Laboratory exercises in microbiology. McGraw-Hill, New York
Rodríguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287(1–2):15–21
Saha P, Chatterjee P, Biswas AK (2010) NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian J Exp Biol 48(6):593
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Sehrawat N, Bhat KV, Sairam RK, Toomoka N, Kaga A, Shu Y, Jaiwal PK (2013) Diversity analysis and confirmation of intra-specific hybrids for salt tolerance in mungbean (Vigna radiata L. Wilczek). IJIB 14:65–73
Shaharoona B, Imran M, Arshad M, Khalid A (2011) Manipulation of ethylene synthesis in roots through bacterial ACC deaminase for improving nodulation in legumes. Crit Rev Plant Sci 30(3):279–291
Sharma M, Mishra V, Rau N, Sharma RS (2011) Functionally diverse rhizobacteria of Saccharum munja (a native wild grass) colonizing abandoned morrum mine in Aravalli hills (Delhi). Plant Soil 341(1–2):447–459
Sheehy RE, Honma M, Yamada M, Sasaki T, Martineau B, Hiatt WR (1991) Isolation, sequence, and expression in Escherichia coli of the Pseudomonas sp. strain ACP gene encoding 1-aminocyclopropane-1-carboxylate deaminase. J Bacteriol 173(17):5260–5265
Singh RP, Jha P, Jha PN (2015) The plant-growth-promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J Plant Physiol 184:57–67
Sparks DL (2003) Environmental soil chemistry. Academic press, Boston
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101(30):11030–11035
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Tank N, Saraf M (2010) Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J Plant Interact 5(1):51–58
Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E (2011) Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. Plos One 6(3):e17968
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218(1):1–14
Wojnicka-Półtorak A, Celiński K, Chudzińska E, Prus-Głowacki W, Niemtur S (2015) Genetic resources of Pinus cembra L. Marginal populations from the Tatra Mountains: implications for conservation. Biochem Genet 53(1–3):49–61
Yaish MW, Antony I, Glick BR (2015) Isolation and characterization of endophytic plant growth-promoting bacteria from date palm tree (Phoenix dactylifera L.) and their potential role in salinity tolerance. Antonie Van Leeuwenhoek 107(6):1519–1532
Zahir ZA, Ghani U, Naveed M, Nadeem SM, Asghar HN (2009) Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for improving growth and yield of wheat (Triticum aestivum L.) under salt-stressed conditions. Arch Microbiol 191(5):415–424
Zaidi PH, Rafique S, Rai PK, Singh NN, Srinivasan G (2004) Tolerance to excess moisture in maize (Zea mays L.): susceptible crop stages and identification of tolerant genotypes. Field Crops Res 90(2):189–202
Acknowledgments
Prashant K. Bhagwat is thankful to UGC, India for awarding BSR meritorious Fellowship for doctoral research. Corresponding author wish to thank UGC-MRP with sanction Grant No. F. No. 41-1282/2012(SR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhise, K.K., Bhagwat, P.K. & Dandge, P.B. Plant Growth-Promoting Characteristics of Salt Tolerant Enterobacter cloacae Strain KBPD and Its Efficacy in Amelioration of Salt Stress in Vigna radiata L.. J Plant Growth Regul 36, 215–226 (2017). https://doi.org/10.1007/s00344-016-9631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-016-9631-0