Abstract
Aim
Hydrogen sulfide (H2S) is a gaseous signaling molecule that participates in multiple physiological processes in both animals and plants. Mitogen-activated protein kinase (MAPK) is important signaling molecule that links the growth and developmental signals and environment stimuli to cellular responses. In the current study we explored the relationship between H2S and MAPK in drought stress resistance in Arabidopsis.
Methods
The quantitative real-time (qRT)-PCR, root tip bending experiment and stomatal aperture assay were used in this paper.
Results
Drought stress activated both H2S biosynthesis and gene expression of MAPKs. The increase in MAPK expression was depressed in lcd/des1, a double mutant of H2S synthesis. Then we selected MPK4 as our target and used mpk4 mutants for further studies. H2S was able to alleviate the drought stress in wild-type (WT) Arabidopsis but not in mpk4 mutants. Meanwhile, H2S-induced stomatal movement was impaired in mpk4 mutants. We then examined the role of H2S and MPK4 in stomatal movements in response to abscisic acid (ABA) and hydrogen peroxide (H2O2). ABA- and H2O2- mediated stomatal movements were impaired in lcd/des1 and mpk4 mutants, and H2S-induced stomatal closure was impaired in slac1–3 mutants.
Conclusions
Our results suggested that MPK4 is important downstream of H2S in the drought stress response and in stomatal movement, and that the H2S-MPK4 cascade is involved in ABA-mediated stomatal movement to regulate the drought stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen sulfide (H2S) which was long considered a toxic gas because of its unpleasant smell is now known to be a gaseous signaling molecule with important physiological roles in both animals and plants. In Arabidopsis, H2S is enzymatically produced by L-cysteine desulfhydrase (LCD) and desulfhydrase 1 (DES1) via the catalysis of L-cysteine primarily (Álvarez et al. 2010; Papenbrock et al. 2007). In plants, H2S participates in plant growth and development, hormone responses, and biotic and abiotic stress resistance, including drought stress. H2S can improve the ability of plants to resist drought stress by inducing stomatal closure (Jin et al. 2011). Stomata are pores that are each surrounded by two guard cells; stomatal movement results from changes in the turgor of the guard cells in response to fluxes in their ionic composition or other osmotically relevant molecules. Jin et al. reported that H2S is able to mediate the ion fluxes to regulate stomatal movement in Arabidopsis. They used numbers of mutants associated with H2S production enzymes, and detected the fluxes of H+, Ca2+, K+ and Cl− using a non invasive micro-test technique, and found that H2S induced a transmembrane K+ efflux and Ca2+ and Cl− influxes, while not affecting the flow of H+ (Jin et al. 2017). However the underlying mechanism involved in H2S-regulated stomatal movement are still need further study.
Mitogen-activated protein kinase (MAPK) is an essential and conserved component of the signaling transduction pathway that converts growth or environment signals into cellular responses in eukaryotes. MAPK cascade comprises MEKK (MAP3K or MPKKK), MEK (MAP2K or MPKK), MPK (MAPK) which each activate the other in a sequential manner through phosphorylation. Phosphorylated MPK could activate downstream signaling molecules, such as enzymes or transcription factors (Šamajová et al. 2013). MAPK is a large family that contains 60 MEKKs, 10 MEKs, and 20 MPKs in Arabidopsis (Ichimura et al. 2002). Given its many members, MAPK has a role in many physiological processes in plant, including growth differentiation, hormonal responses, and biotic and abiotic stress resistance (Rodriguez et al. 2010). Considering so many functional overlaps between H2S and MAPKs, we studied their relationship in Arabidopsis in previous work and found that H2S has a positive role in MAPK gene expression especially MPK4, and MPK4 is an important downstream component in H2S signaling in cold stress response (Du et al. 2017). However, the relationship between H2S and MAPK in drought stress has not been reported.
Abscisic acid (ABA) is a major phytohormone that participates in seed maturation, plant development, and responses to abiotic stresses. One of the most important functions of ABA is to regulate stomatal movement. The core ABA signaling pathway in stomatal movement has been established. ABA binds with its receptors Pyrabactin Resistance/Pyrabactin resistance-like/Regulatory Component of ABA Receptor (PYR/PYL/RCAR), promoting the bind of PYR/PYL/RCAR with Protein Phosphatase 2C (PP2Cs), leading to the inactivation of PP2Cs. Activated PP2Cs could dephosphorylated hence inactivated SNF1-Related Protein Kinases type 2 (SnRK2s). Therefore, ABA-induced inactivation of PP2Cs results in the activation of SnRK2s. Subsequently, activated SnRK2s could directly activate the S-type anion channels Slow Anion Channel-Associated 1(SLAC1), resulting in the release of anions from the guard cell and inducing stomatal closure. In addition to SLAC1, there are other channels regulated by SnRK2s involved in ABA-mediated stomatal movement such as aluminum-activated anion channels 12 (ALMT12). SnRK2s also regulate the production of hydrogen peroxide (H2O2) and Ca2+ signaling to activate SLAC1 (Danquah et al. 2014; Zelicourt et al. 2016; Jezek and Blatt 2017). H2S-mediated stomatal closure involves in ABA signaling. Jin et al. found that the ability of H2S closing stomata was not influenced in ABA-associated mutants aba3 and abi1, however ABA-induced stomatal closure was impaired in H2S synthesis mutant lcd, indicating that ABA-induced stomatal closure is partially dependent on H2S (Jin et al. 2013). Whereafter, more studies revealed the function of H2S in ABA-mediated stomatal movement. H2S acts downstream of ABI1, a member of the PP2C family (Scuffi et al. 2014), and acts upstream of OST1, a member of the SnRK2 family, to activate the S-type anion channels (Wang et al. 2016). Many members of MAPK are also indicated in the mediation of ABA signaling in guard cells. Guard cell-specific inhibition of MPK3 impaired ABA- and H2O2- regulated stomatal movement (Gudesblat et al. 2007). Double mutants but not single mutants of MPK9 and MPK12 exhibited insensitivity to both ABA- and H2O2- induced stomatal closure and inhibited stomatal opening in Arabidopsis (Jammes et al. 2009). However the role of MPK4 in stomatal movement has not been well elucidated in Arabidopsis.
Therefore in the current study, we examined the relationship of H2S and MAPK especially MPK4 in response to drought stress, focusing on the involvement of H2S and MPK4 in stomatal movement regulated by ABA and H2O2 in Arabidopsis.
Materials and methods
Plant material and growth conditions
lcd/des1 double mutants of Columbia background (Col), generously provided by Shaowu Xue of Huazhong Agriculture University, were formed by hybridization of lcd (SALK-082099) and des1 (SALK-205358C). T-DNA insertion mutant mpk4 of Columbia background and Ds insertion mutant mpk4 of Landsberg background (Ler) were kindly provided by John Mundy of Copenhagen University. These mutants has been described elsewhere (Du et al. 2017). slac1–3 mutants (SALK-099139) of Col background (Vahisalu et al. 2008) were also provided by Shaowu Xue. The primers used in genotyping are listed in Table S1. Seeds were grown in pots containing a soil:perlite:vermiculite (1:1:1 v:v) mixture or on the plates containing 1/2 Murashige and Skoog (1/2 MS) medium after being sterilized with 75% ethyl alcohol and 6% NaClO. The plants were grown under 23 °C, 60% relative humidity, 16/8 h (light/dark) photoperiod and 160 μEmm−2 s−1 light illumination.
PEG treatment
To determine the gene expression level after PEG treatment, 4-week-old seedlings grown in pots containing a soil:perlite:vermiculite (1:1:1 v:v) mixture were used. 20 ml 0.4 g/ml PEG8000 (−1.92 MPa) were applied to the seedlings for indicated time (3 h, 6 h, 9 h), and seedlings of 0 h group were applied with 20 ml diluted water. To perform the root tip bending experiment, the 1/2 MS plates with 0.4 g/ml PEG8000 or not were used in which 20 ml 1/2 MS fluid medium containing 0.4 g/ml PEG8000 or not were add on the normal 1/2 MS plates, and the fluid medium were removed when the plates were used.
Determination of gene expression level
The leaves of 4-week-old seedlings grown in pots containing a soil:perlite:vermiculite (1:1:1 v:v) mixture were used. RNA was extracted using RNAiso Plus (TaKaRa, Shiga, Japan), and cDNA was generated using All-In-One RT MasterMix (abm, Nanjing, China). Quantitative real-time (qRT)-PCR was executed using cDNA as a template and the methods described elsewhere (Shen et al. 2013). The primers used are listed in the Table S1, and UBQ was used as an internal control.
Measurement of endogenous H2S production rate and content
The H2S production rate was measured as described previously with some modifications (Zhao et al. 2001). Briefly, the leaves from 4-week-old seedlings grown in pots containing a soil:perlite:vermiculite (1:1:1 v:v) mixture were homogenized in pre-cold 50 mM phosphate buffer (pH = 7) and centrifuged at 12000 rpm for 10 min. The supernatants were then collected for use in later experiments. The reaction was performed in a 25 ml flask containing reaction mixture (100 mM Tris-HCl pH = 9, 10 mM L-cysteine, 2.5 mM DTT and the supernatant) and a test tube (1.5 ml) containing 0.5 ml 1% zinc acetate as a trapping solution. The flasks were transferred to a table concentrator at 37 °C to initiate the reaction. After 15 min of incubation at 37 °C, the test tubes were removed from flasks and 0.1 ml N,N-dimethyl-p-phenylenediamine sulfate (20 mM in 7.2 M HCl) and 0.1 ml FeCl3 (30 m M in 1.2 M HCl) were added into the test tubes; the test tubes were placed in the dark. The absorbance was measured at 670 nm after 15 min. The leaves from 4-week-old seedlings grown in pots containing a soil:perlite:vermiculite (1:1:1 v:v) mixture were used for H2S content measurement, and the method was described previously (Du et al. 2017).
Root tip bending experiment
7-day-old seedlings grown on 1/2 MS plates were used in a root tip bending study. The seedlings were transferred to 1/2 MS plates with 0.4 g/ml PEG8000 or not. The hook length of roots was observed and measured after 2 days.
Stomatal aperture assay
The leaves from 4-week-old seedlings grown in pots containing a soil:perlite:vermiculite (1:1:1 v:v) mixture were used for stomata observation. To measure the degree of the stomatal closure, the leaves were soaked into epidermal strip buffer (containing 10 mM MES and 50 mM KCl, pH 6.15) and placed under light for 2 h. Then NaHS (H2S donor), ABA, or H2O2 were applied and U0126 (an inhibitor of the MEK2 → MPK4 pathway) were added 30 min prior to NaHS application. After 3 h, the abaxial epidermis was peeled from leaves, and the epidermal strip was used for stomatal aperture observation.
To measure the inhibition of stomatal opening, the leaves were soaked into epidermal strip buffer and placed in the dark for 2 h. Then, NaHS, ABA, or H2O2 were applied and the leaves were placed under a light. After 2 h, the abaxial epidermis was peeled from leaves, and the epidermal strip was used for stomatal aperture observation.
Statistical analysis
The data were expressed as the mean ± standard error (SE). The statistically significant differences were analyzed with SPSS version 17.0. Statistical analyses of H2S production rate, H2S content, the expression level of genes, root bending experiment were performed using one-way analysis of variance (ANOVA) followed by Duncan’s test or students’ t-test as indicated in the figure legends. Statistical analyses of stomatal aperture assay were performed using two-way ANOVA. If no interaction occurred between genotypes and treatments, the one-way ANOVA followed by Duncan’s test was performed, and if interaction occurred, simple effect analysis was performed. Asterisks, plus signs and different letters represented significant differences. At least three independent experiments were performed for every test.
Results
Drought stress induced H2S biosynthesis
To assess the function of H2S in Arabidopsis seedlings under drought stress, we used PEG8000 (0.4 g/ml, −1.92 MPa) to mimic the drought stress and determined the gene expression level of LCD and DES1 (genes encoding H2S production enzymes), the H2S production rate and H2S content in the seedlings exposed to PEG8000 for different lengths of time. The expression levels of LCD and DES1 were increased after 3 h of PEG8000 treatment (Fig. 1a). H2S production rate and H2S content increased after 6 h of PEG8000 treatment (Fig. 1b, c).
a–c The effect of PEG8000 on H2S biosynthesis in Arabidopsis. The expression level of LCD and DES1 (a), H2S production rate (b), and H2S content (c) in WT after PEG8000 treatment. 4-week-old seedlings of WT of Col background treating with 0.4 g/ml PEG8000 for different time (0, 3, 6, 9 h) were used. Data are Mean ± SE and three independent experiments were repeated, the asterisk indicated significant differences compared with each control (one way ANOVA, * P < 0.05). d, e The effect of PEG8000 on the expression of MAPKs in WT (d) and lcd/des1 (e). 4-week-old seedlings of WT and lcd/des1 of Col background treating with 0.4 g/ml PEG8000 for different time (0, 3, 6, 9 h) were used. Data are Mean ± SE and three independent experiments were repeated, the asterisk indicated significant differences compared with each control (one way ANOVA, * P < 0.05)
Drought-induced MAPK gene expression was depressed in lcd/des1 mutants
We then explored the role of MAPK in the drought stress response. We selected MEKK1, MEK1, MEK2, MPK3, MPK4, and MPK6 as targets given that they are the most well-studied MAPK members and are known to participate in the stress response. We determined the expression levels of MAPKs in the WT seedlings subjected to PEG8000 treatment. The gene expression of MAPKs were induced after PEG8000 treatment for different length of time (Fig. 1d). We then determined the role of H2S in PEG8000-induced gene expression of MAPKs by treating homozygous lcd/des1 double mutants (Fig. S1a-b) with PEG8000 for different lengths of time. After PEG8000 treatment, decreases in the expression of MAPKs in lcd/des1 mutants were recorded (Fig. 1e).
H2S alleviating drought stress was impaired in mpk4 mutants
In a previous study, we found that MPK4 is an important downstream component of H2S (Du et al. 2017) and, thus, we chose MPK4 as a target for the following experiments. To further investigate the relationship between MPK4 and H2S in the drought stress response, the root tip bending experiments were performed with WT and mpk4 mutants. We got two kinds of mpk4 mutant, Col background and Ler background. Homozygous mpk4 seedlings of Col background need to be distinguished from heterozygous mpk4 seedlings of Col background because homozygous mpk4 mutants of Col background is too weak to produce seeds (Fig. S2). Homozygous mpk4 of Ler background is able to produce seeds even though the seedlings of homozygous mpk4 is weaker than WT (Fig. S3). So we used homozygous mpk4 of Ler background (Fig. S1c) in root bending experiment. We used NaHS (H2S donor) to fumigate the seedlings of WT and mpk4 mutants and then exposed the seedlings to PEG8000. The hooks of each root were observed and quantified. In WT, the inhibition of root length caused by PEG8000 was significantly rescued by NaHS (Fig. 2a). However, NaHS was unable to alleviate this damage caused by PEG8000 in mpk4 mutants (Fig. 2b).
The alleviation effect of H2S in WT (a) and mpk4 (b) under PEG8000. The 7-day-old seedlings of WT and mpk4 of Ler background grown on 1/2 MS plates were used. The seedlings were fumigated by 5 μM NaHS for 12 h or not, then transferred to the 1/2 MS plates with 0.4 g/ml PEG8000 or not. The hook length of each root were observed and measured 2 d later, 10 hook were measured for each treatment. Data are Mean ± SE and three independent experiments were repeated, different letters indicated significant differences among treatments (one way ANOVA, P < 0.05). Scale bar: 0.5 cm
Stomatal movement in response to H2S was impaired in mpk4 mutants
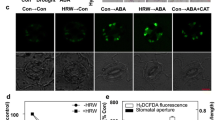
Regulating stomatal closure is an important way that H2S alleviates drought stress (Jin et al. 2011); therefore, we explored the function of MPK4 in stomatal movement regulated by H2S. First, we administered U0126, the inhibitor of MEK2 → MPK4 pathway, to test the function of H2S in closing stomata in WT of Ler background. NaHS treatment and U0126 treatment closed the stomata respectively, whereas administering U0126 before NaHS attenuated the stomatal closing compared with NaHS treatment alone (Fig. 3a). To further verify the function of MPK4, we treated WT and mpk4 mutants of Ler background with NaHS and observed the stomatal apertures. We used mpk4 mutants of Ler background in stomatal aperture assay because mpk4 mutants of Col background is dwarf and its leaves are too small to observe stomata (Fig. S2). As shown in Fig. 3b, NaHS treatment closed the stomata in WT, but did not affect the stomatal aperture in mpk4 mutants. We also studied the process of H2S inhibition stomatal opening. In WT, NaHS inhibited the opening of stomata. Whereas the inhibition of NaHS to stomatal opening displayed small in mpk4 mutants compared with that in WT (Fig. 3c).
The effect of H2S on stomatal movement in WT and mpk4. a The stomatal apeture of WT after U0126 and NaHS treatment. (n = 3 independent experiments, 60 stomatal aperture for each treatment.) The 4-week-old seedlings of WT of Ler background were used. The leaves were treated with 10 μM U0126 for 0.5 h and 50 μM NaHS for 3 h or not before observing the stomatal aperture. b H2S-induced stomatal closure in WT and mpk4. (n = 3 independent experiments, 60 stomatal aperture for each treatment.) c H2S-inhibited stomatal opening in WT and mpk4. (n = 4 independent experiments, 80 stomatal aperture for CK, 120 stomatal aperture for NaHS treatment.) The 4-week-old seedlings of WT and mpk4 of Ler background were used, and stomatal aperture of the leaves were observed after 50 μM NaHS treatment for 3 h (b) or 2 h (c) or not. Data are Mean ± SE, different letters in a indicated significant differences among treatments (one way ANOVA, P < 0.05), the asterisks and plus signs in b and c indicated significant differences compared with each CK and WT treated with NaHS, respectively (two way ANOVA, ** and ++ P < 0.01). The P values in the top right corner of figures indicate the effect of genotypes (WT and mpk4), treatment (CK and NaHS) and their interaction on stomatal aperture. G:genotype, T:treatment
Both H2S and MPK4 were involved in ABA-mediated stomatal movement
H2S is an important component of ABA-dependent stomatal movement (Jin et al. 2013; Scuffi et al. 2014). To understand the mechanism of the H2S and MPK4 response to drought stress, the roles of H2S and MPK4 in ABA-mediated stomatal movement were explored. First, the involvement of H2S in ABA-mediated stomatal closing and opening was confirmed using lcd/des1 mutants. ABA induced stomatal closing (Fig. 4a) and inhibited stomatal opening (Fig. 4b) in WT. However, ABA- induced stomatal closing and inhibited stomatal opening were diminished in the lcd/des1 mutants compared with that in WT. We then examined whether MPK4 participates in the stomatal movement in response to ABA. WT and mpk4 mutants of Ler background were used. As shown in Fig. 4c, ABA was able to close the stomata in WT. But in mpk4 mutants the stomatal closing caused by ABA was impaired compared with that in WT. ABA inhibited the stomatal opening in WT, while this function also was impaired in mpk4 mutants (Fig. 4d).
The effect of ABA on stomatal movement in WT, lcd/des1 and mpk4. a ABA-induced stomatal closure in WT and lcd/des1. (n = 3 independent experiments, 60 stomatal aperture for each treatment.) b ABA-inhibited stomatal opening in WT and lcd/des1. (n = 3 independent experiments, 60 stomatal aperture for CK, 90 stomatal aperture for ABA treatment.) The 4-week-old seedlings of WT and lcd/des1 of Col background were used, and stomatal aperture of the leaves were observed after 20 μM ABA treatment for 3 h (a) or 2 h (b) or not. c ABA-induced stomatal closure in WT and mpk4. (n = 3 independent experiments, 60 stomatal aperture for each treatment.) d ABA-inhibited stomatal opening in WT and mpk4. (n = 3 independent experiments, 60 stomatal aperture for CK, 75 stomatal aperture for ABA treatment.) The 4-week-old seedlings of WT and mpk4 of Ler background were used, and stomatal aperture of the leaves were observed after 20 μM ABA treatment for 3 h (c) or 2 h (d) or not. Data are Mean ± SE, the asterisks and plus signs indicated significant differeces compared with each CK and WT treated with ABA, respectively (two way ANOVA, * and + P < 0.05, ** and ++ P < 0.01). The P values in the top right corner of figures indicated the effect of genotypes (WT and lcd/des1 or mpk4), treatment (CK and ABA) and their interaction on stomatal aperture. G:genotype, T:treatment
Both H2S and MPK4 were involved in H2O2-mediated stomatal movement
H2O2 is involved in ABA-regulated stomatal movement (Danquah et al. 2014). To better understand the mechanism of H2S and MPK4 in ABA signaling, we studied whether both H2S and MPK4 participate in stomatal movement in response to H2O2. As shown in Fig. 5a, b, compared with WT, lcd/des1 mutants were significantly impaired in H2O2- induced stomatal closure and inhibited stomatal opening. mpk4 mutants of Ler background were also significantly impaired in H2O2- induced stomatal closure and inhibited stomatal opening compared with WT (Fig. 5c, d).
The effect of H2O2 on stomatal movement in WT, lcd/des1 and mpk4. a H2O2-induced stomatal closure in WT and lcd/des1. (n = 3 independent experiments, 60 stomatal aperture for CK, 100 stomatal aperture for H2O2 treatment.) b H2O2-inhibited stomatal opening in WT and lcd/des1. (n = 4 independent experiments, 80 stomatal aperture for CK, 96 stomatal aperture for H2O2 treatment.) The 4-week-old seedlings of WT and lcd/des1 of Col background were used, and stomatal aperture of the leaves were observed after 500 μM H2O2 treatment for 3 h (a) or 2 h (b) or not. c H2O2-induced stomatal closure in WT and mpk4. (n = 3 independent experiments, 60 stomatal aperture for CK, 90 stomatal aperture for H2O2 treatment.) d H2O2-inhibited stomatal opening in WT and mpk4 (n = 4 independent experiments, 80 stomatal aperture for CK, 120 stomatal aperture for H2O2 treatment.) The 4-week-old seedlings of WT and mpk4 of Ler background were used, and stomatal aperture of the leaves were observed after 500 μM H2O2 treatment for 3 h (c) or 2 h (d) or not. Data are Mean ± SE, different letters in a indicated significant differences (one way ANOVA, P < 0.05), the asterisks and plus signs in b, c and d indicated significant differeces compared with each CK and WT treated with H2O2, respectively (two way ANOVA, ** and ++ P < 0.01). The P values in the top right corner of figures indicate the effect of genotypes (WT and lcd/des1 or mpk4), treatment (CK and H2O2) and their interaction on stomatal aperture. G:genotype, T:treatment
Stomatal movement in response to H2S was impaired in slac1–3 mutants
SLAC1 is a S-type anion channel that responds to ABA signaling (Vahisalu et al. 2008). Therefore, we studied the effect of H2S and MPK4 on the gene expression of SLAC1 using lcd/des1 mutants and mpk4 mutants (Fig. S1d) of Col background. The expression level of SLAC1 increased in lcd/des1 mutants and did not change significantly in mpk4 mutants compared with that in WT (Fig. 6a, b). We then examined the effect of H2S on stomatal closure in homozygous slac1–3 mutants (Fig. S1e). NaHS was able to induce stomatal closure in WT, however NaHS inducing stomatal closing was impaired in slac1–3 mutants compared with that in WT (Fig. 6c).
The effect of H2S and MPK4 on SLAC1. The expression of SLAC1 in lcd/des1 (a) and mpk4 (b). The 4-week-old seedlings of WT, lcd/des1 and mpk4 of Col background were used. Data are Mean ± SE and three independent experiments were repeated, the asterisk indicated significant differences (Students’ t-test, * P < 0.05). c H2S-induced stomatal closure in WT and slac1–3. (n = 3 independent experiments, 60 stomatal aperture for CK, 90 stomatal aperture for NaHS treatment.) The 4-week-old seedlings of WT and slac1–3 of Col background were used, and stomatal aperture of the leaves were observed after 50 μM NaHS treatment for 3 h or not. Data are Mean ± SE, the asterisks and plus signs indicated significant differeces compared with each CK and WT treated with NaHS, respectively (two way ANOVA, ** and ++ P < 0.01). The P values in the top right corner of figures indicate the effect of genotypes (WT and slac1–3), treatment (CK and NaHS) and their interaction on stomatal aperture. G:genotype, T:treatment
Discussion
H2S and MAPK have positive roles in drought response
The H2S and MAPK pathway is activated by various abiotic stresses (Rodriguez et al. 2010; Šamajová et al. 2013; Shi et al. 2015). Our results showed that H2S production was induced after drought stress (Fig. 1a–c). Likewise, the expression level of MAPKs, including MEKK1, MEK1, MEK2, MPK3, MPK4, and MPK6, also increased after drought stress (Fig. 1d). These results showed that both H2S and MAPK are activated in response to drought stress, and that they have a positive role in the drought response. The kinase activity of MEKK1, MEK1, MPK3, MPK4, and MPK6 increased after drought stress (Rodriguez et al. 2010; Šamajová et al. 2013), and the mRNA abundance of MEKK1 and MPK3 increased after drought treatment (Mizoguchi et al. 1996). Our results showed that the gene expression of not only MEKK1 and MPK3, but also MEK1, MEK2, MPK4, and MPK6 were induced by drought stress, indicating that these MAPKs are regulated not only at the post-translation level, but also at the transcription level during drought stress. However, Ichimura et al. reported that the mRNA abundance of MPK4 and MPK6 was not influenced by drought stress (Ichimura et al. 2000). This discrepancy in results could be caused by the different drought treatments used.
MPK4 is an important downstream component in the alleviation of drought stress by H2S
Given that both H2S and MAPK were activated by drought stress, we explored the involvement of MAPK in the mechanism whereby H2S alleviates drought stress. Figure 1e showed that H2S was indispensable in the MAPK gene expression induced by drought. Our previous study indicated that MPK4 is a critical component downstream of H2S signaling involved in the cold stress response (Du et al. 2017). Therefore, we targeted MPK4 in the following experiment. Figure 2 showed that MPK4 was required in the alleviation of drought stress by H2S. These two results together reveal that MAPKs, MPK4 in particular, are important downstream components in H2S signaling in the drought stress response. On the other hand, H2S also plays important roles in drought-induced expression of other MAPK members including MEKK1, MEK1, MEK2, MPK3, and MPK6 (Fig. 1e). Thus, it is worth studying the functions of these MAPKs in H2S-alleviated drought stress.
Role of MPK4 in H2S-mediated stomatal movement
MPK4 is highly expressed in guard cells in Arabidopsis (Petersen et al. 2000), implying an important function of MPK4 in stomatal movement. Here, we showed that U0126 alone could induce stomatal closure in WT plants (Fig. 3a), indicating the important role of the MEK2 → MPK4 cascade in the process. In addition, stomatal movement in response to H2S was impaired after applying U0126 and in mpk4 mutants (Fig. 3), suggesting that MPK4 has an important role in stomatal movement regulated by H2S. Further studies are required to determine whether other MAPK members, such as MPK3, MPK6, MPK9, and MPK12, also participate in stomatal movement in response to H2S.
A H2S-MPK4 cascade is involved in stomatal movement regulated by ABA and H2O2 in Arabidopsis
H2S is known to participate in the ABA-induced stomatal closure (Jin et al. 2013). Here we used lcd/des1, the double mutants of H2S production enzymes, to confirm that H2S participated in both stomatal closure and inhibition of stomatal opening caused by ABA (Fig. 4a, b).
Few studies have focused on the relationship between H2S and H2O2 in stomatal movement. However, a recent paper showed that H2S was upstream of H2O2 in the process of stomatal closure and that H2S could increase H2O2 production through NADPH oxidases and phospholipase D (Scuffi et al. 2018). By contrast, our results showed that the stomatal movement in response to H2O2 was impaired in the lcd/des1 mutants (Fig. 5a, b), suggesting that H2S is an important downstream component in the mediation of stomatal movement by H2O2. The reason for these discrepancies might be that different materials were used in the two studies (des1 versus lcd/des1 mutants).
There are many studies of the involvement of MAPK members in stomatal movement, such as MPK3, MPK9, and MPK12. However, the role of MPK4 in stomatal movement has remained ambiguous, especially in Arabidopsis. In Nicotiana tabacum, silencing of MPK4 did not alter the ABA sensitivity of guard cells (Marten et al. 2008). However, silencing MPK4 in Nicotiana attenuata impaired both ABA- and H2O2- induced stomatal closure (Hettenhausen et al. 2012). These divergent results suggest the different role of MPK4 in different species. Our results showed that MPK4 is required in stomatal movement in response to both ABA and H2O2 in Arabidopsis (Figs. 4c, d and 5c, d), as also reported for MPK3, MPK9, and MPK12.
The activation of S-type anion channels by H2S requires OST1, a member of the SnR2K family (Wang et al. 2016). H2S actives the production of H2O2 through NADPH oxidases and phospholipase D and acts as upstream of H2O2 in stomatal closure (Scuffi et al. 2018). In the current study, we showed that H2S acts as downstream of H2O2 and upstream of MPK4 in stomatal movement (Figs. 5a, b and 3). However, the precise point of involvement of H2S in ABA signaling in stomatal movement is unclear. We speculate that H2S interacts with the molecules in the pathway of ABA-induced stomatal movement both downstream and upstream, and that there might be more than one target of H2S in ABA-induced stomatal movement (Fig. 7), and further studies are required to determine the underlying mechanisms involved.
A schematic model of the role of H2S in ABA-induced stomatal closure. ABA is absent in guard cells under normal conditions. PYR/PYL/RCAR, the receptors of ABA, remain inactive and PP2Cs bind and inhibit the activity of SnRK2s, which leads to the inactivation of NADPH oxidases and SLAC1or other ion channels and therefore causes the opening of stomata. However, under drought conditions, the ABA signal in guard cells is activated. ABA binds to PYR/PYL/RCAR and inhibits the activity of PP2Cs, leading to the activation of SnRK2s. Therefore, SnRK2s can activate the NADPH oxidases to promote the production of H2O2 or SnRK2s can directly activate SLAC1and other ion channels (not shown in the figure). H2O2 regulates the stomatal movement through MPK4 (Fig. 5c, d). However, whether other MAPK members upstream of MPK4 such as MEK2 are involved in this process remains elusive. On the other hand, drought stress increase the biosynthesis of H2S through LCD and DES1 simultaneously (Fig. 1a–c). The production of H2S also regulated by ABA through increasing the expression level of DES1(Scuffi et al. 2014). H2S plays a crucial role in ABA-mediated stomatal movement. H2S functions downstream of PP2Cs (Scuffi et al. 2014) and upstream of SnRK2s (Wang et al. 2016). H2S is able to enhance the production of H2O2 through NADPH oxidases (Scuffi et al. 2018). H2S serving as downstream of H2O2 and upstream of MPK4 functions in stomatal movement (Figs. 5a, b and 3). H2S-MPK4 cascade can induce stomatal closure in SLAC1- dependent or independent way (Fig. 6c). Arrow-headed lines indicate activation; bar-headed lines indicate inhibition; dotted arrows indicate putative effect
SLAC1 is required in H2S-induced stomatal closure
S-type anion channels are responsible for ABA-mediated stomatal closure. SLAC1 is an important S-type anion channel, and SLAC1 mutants showed very strong insensitivity to ABA-mediated stomatal closure (Vahisalu et al. 2008). H2S can active S-type anion currents via SLAC1 to induce stomatal closure (Wang et al. 2016). Here, we showed that H2S-induced stomatal closure was impaired in slac1–3 mutants (Fig. 6c), providing additional evidence for the role of SLAC1 in H2S-induced stomatal closure, and confirming that H2S is involved in ABA signaling resulting in stomatal movement. However the effect of H2S on stomatal closure was not completely blocked in slcac1–3 mutants (Fig. 6c) indicating that H2S could also close stomata through other ion channels other than SLAC1. Salicylic acid (SA), one of the plant hormones, is able to induce stomatal closure in an ABA-independent pathway (Miura and Tada 2014). It has been reported that H2S and SA are tightly correlated in the Cd stress response (Qiao et al. 2015). It is highly possible that H2S is not only involved in ABA-induced stomatal closure but also in SA-induced stomatal closure. The expression level of SLAC1 was increased in lcd/des1 mutants (Fig. 6a), showing that H2S has a negative effect on SLAC1 gene expression, which might be a compensation effect. The expression level of SLAC1 did not change in mpk4 mutants (Fig. 6b), suggesting that MPK4 does not regulate the SLAC1 at the transcription level. However, further studies are required to determine whether H2S and MPK4 regulate SLAC1 at another level, such as post-transcriptionally or post-translationally.
Overall, our results show that MPK4 is an important downstream molecule involved in H2S-mediated stomatal movement to alleviate drought stress, and that the H2S-MPK4 cascade is involved in ABA signaling pathway resulting in stomatal movement (Fig. 7). However, further work is required to determine the underlying mechanism of H2S regulating stomatal movement through MPK4, and how H2S coordinates the numerous signaling component (H2O2, Ca2+, NO, MAPK, etc) in ABA-mediated stomatal movement.
Abbreviations
- H2S:
-
Hydrogen sulfide
- DES1:
-
Desulfhydrase 1
- LCD:
-
L-cysteine desulfhydrase
- MAPK:
-
Mitogen-activated protein kinase
- ABA:
-
Abscisic acid
- PYR/PYL/RCAR:
-
Pyrabactin Resistance/Pyrabactin resistance-like/Regulatory Component of ABA Receptor
- PP2Cs:
-
Protein Phosphatase 2C
- SnRK2s:
-
SNF1-Related Protein Kinases type 2
- SLAC1:
-
Slow Anion Channel-Associated 1
- H2O2 :
-
Hydrogen peroxide
- 1/2 MS:
-
1/2 Murashige and Skoog
References
Álvarez C, Calo L, Romero LC, García I, Gotor C (2010) An O-acetylserine(thiol)lyase homolog with L-cysteine desulfhydrase activity regulates cysteine homeostasis in Arabidopsis. Plant Physiol 152:656–669. https://doi.org/10.1104/pp.109.147975
Danquah A, Zelicourt AD, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32:40–52. https://doi.org/10.1016/j.biotechadv.2013.09.006
Du X, Jin Z, Liu D, Yang G, Pei Y (2017) Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol Biochem 120:112–119. https://doi.org/10.1016/j.plaphy.2017.09.028
Gudesblat GE, Iusem ND, Morris PC (2007) Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol 173:713–721. https://doi.org/10.1111/j.1469-8137.2006.01953.x
Hettenhausen C, Baldwin IT, Wu J (2012) Silencing MPK4 in Nicotiana attenuata enhances photosynthesis and seed production but compromises abscisic acid-induced stomatal closure and guard cell-mediated resistance to Pseudomonas syringae pv tomato DC3000. Plant Physiol 158:759–776. https://doi.org/10.1104/pp.111.190074
Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozak K (2000) Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J 24:655–665. https://doi.org/10.1046/j.1365-313x.2000.00913.x
Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C, Heberle-Bors E, Ellis BE, Morris PC, Innes RW, Ecker JR, Scheel D, Klessig DF, Machida Y, Mundy J, Ohashi Y, Walker JC (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends In Plant Sci 7:301–308. https://doi.org/10.1016/S1360-1385(02)02302-6
Jammes F, Song C, Shin D, Munemasa S, Takeda K, Gu D, Cho D, Lee S, Giordo R, Sritubtim S, Leonhardt N, Ellis BE, Murata Y, Kwak JM (2009) MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. PNAS 106:20520–20525. https://doi.org/10.1073/pnas.0907205106
Jezek M, Blatt MR (2017) The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol 174:487–519. https://doi.org/10.1104/pp.16.01949
Jin Z, Shen J, Qiao Z, Yang G, Wang R, Pei Y (2011) Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun 414:481–486. https://doi.org/10.1016/j.bbrc.2011.09.090
Jin Z, Xue S, Luo Y, Tian B, Fang H, Li H, Pei Y (2013) Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62:41–46. https://doi.org/10.1016/j.plaphy.2012.10.017
Jin Z, Wang Z, Ma Q, Sun L, Zhang L, Liu Z, Liu D, Hao X, Pei Y (2017) Hydrogen sulfide mediates ion fluxes inducing stomatal closure in response to drought stress in Arabidopsis thaliana. Plant Soil 419:141–152. https://doi.org/10.1007/s11104-017-3335-5
Marten H, Hyun T, Gomi K, Seo S, Hedrich R, Roelfsema MRG (2008) Silencing of NtMPK4 impairs CO2-induced stomatal closure, activation of anion channels and cytosolic Ca2+ signals in Nicotiana tabacum guard cells. Plant J 55:698–708. https://doi.org/10.1111/j.1365-313X.2008.03542.x
Miura K, Tada Y (2014) Regulation of water, salinity, and cold stress responses by salicylic acid. Front Plant Sci 5:4. https://doi.org/10.3389/fpls.2014.00004
Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, Shinozaki K (1996) A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold, and water stress in Arabidopsis thaliana. PNAS 93:765–769. https://doi.org/10.1073/pnas.93.2.765
Papenbrock J, Riemenschneider A, Kamp A, Schulz-Vogt HN, Schmidt A (2007) Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants - from the field to the test tube and back. Plant Biol 9:582–588. https://doi.org/10.1055/s-2007-965424
Petersen M, Brodersen P, Naested H, Andreasson E, Lindhart U, Johansen B, Nielsen HB, Lacy M, Austin MJ, Parker JE, Sharma SB, Klessig DF, Martienssen R, Mattsson O, Jensen AB, Mundy J (2000) Arabidopsis MAP kinase 4 negatively regulates systemic acquired resistance. Cell 103:1111–1120. https://doi.org/10.1016/S0092-8674(00)00213-0
Qiao Z, Jing T, Liu Z, Zhang L, Jin Z, Liu D, Pei Y (2015) H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 393:137–146. https://doi.org/10.1007/s11104-015-2475-8
Rodriguez MCS, Petersen M, Mundy J (2010) Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61:621–649. https://doi.org/10.1146/annurev-arplant-042809-112252
Šamajová O, Plíhal O, Al-Yousif M, Hirt H, Šamaj J (2013) Improvement of stress tolerance in plants by genetic manipulation of mitogen-activated protein kinases. Biotechnol Adv 31:118–128. https://doi.org/10.1016/j.biotechadv.2011.12.002
Scuffi D, Álvarez C, Laspina N, Gotor C, Lamattina L, García-Mata C (2014) Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol 166:2065–2076. https://doi.org/10.1104/pp.114.245373
Scuffi D, Nietzel T, Di Fino LM, Meyer AJ, Lamattina L, Schwarzländer M, Laxalt AM, García-Mata C (2018) Hydrogen sulfide increases production of NADPH oxidase-dependent hydrogen peroxide and phospholipase D-derived phosphatidic acid in guard cell signaling. Plant Physiol 176:2532–2542. https://doi.org/10.1104/pp.17.01636
Shen J, Xing T, Yuan H, Liu Z, Jin Z, Zhang L, Pei Y (2013) Hydrogen sulfide improves drought tolerance in Arabidopsis thaliana by microRNA expressions. PLoS One 8:e77047. https://doi.org/10.1371/journal.pone.0077047
Shi H, Ye T, Han N, Bian H, Liu X, Chan Z (2015) Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Intergr Plant Biol 57:628–640. https://doi.org/10.1111/jipb.12302
Vahisalu T, Kollist H, Wang YF, Nishimura N, Chan WY, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, Schroeder JI, Kangasjärvi J (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452:487–491. https://doi.org/10.1038/nature06608
Wang L, Wan R, Shi Y, Xue S (2016) Hydrogen sulfide activates S-type anion channel via OST1 and Ca2+ modules. Mol Plant 9:489–491. https://doi.org/10.1016/j.molp.2015.11.010
Zelicourt AD, Colcombet J, Hirt H (2016) The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci 21:677–685. https://doi.org/10.1016/j.tplants.2016.04.004
Zhao W, Zhang J, Lu Y, Wang R (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20:6008–6016. https://doi.org/10.1093/emboj/20.21.6008
Acknowledgements
This work was funded by a grant from the National Natural Science Foundation of China (grant numbers 31672140 to Jin Z., 31671605 to Pei Y.) We thank Shaowu Xue of Huazhong Agricultural University and John Mundy of Copenhagen University for providing seeds generously. The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ian Dodd.
Electronic supplementary material
ESM 1
(DOC 42 kb)
Fig. S1
The genotyping of mutants. a-b: the genotyping of lcd/des1. a is genotyping for LCD, and b is genotyping for DES1. c: the genotyping of mpk4 of Ler background. d: the genoptyping of mpk4 of Col background. mpk4 het means mpk4 heterozygote, mpk4 homo means mpk4 homozygote. e: the genotyping of slac1–3. (PNG 723 kb)
Fig. S2
The 4-week-old seedlings of mpk4 of Col background. The homozygous or heterozygous of mpk4 with Col background were indicated in the figure. (PNG 278 kb)
Fig. S3
The 4-week-old seedling of WT (left) and mpk4 (right) of Ler background. (PNG 86 kb)
Rights and permissions
About this article
Cite this article
Du, X., Jin, Z., Zhang, L. et al. H2S is involved in ABA-mediated stomatal movement through MPK4 to alleviate drought stress in Arabidopsis thaliana. Plant Soil 435, 295–307 (2019). https://doi.org/10.1007/s11104-018-3894-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3894-0