Abstract
Objectives
A method named computed tomography angiography-derived fractional flow reserve (FFRCT) is an alternative method for detecting hemodynamically significant coronary stenosis. We carried out a meta-analysis to derive reliable assessment of the diagnostic performances of FFRCT and compare the diagnostic accuracy with CCTA using FFR as reference.
Methods
We searched PubMed, EMBASE, The Cochrane Library, and Web of science for relevant articles published from January 2008 until May 2019 using the following search terms: FFRCT, noninvasive FFR, non-invasive FFR, noninvasive fractional flow reserve, non-invasive fractional flow reserve, and CCTA. Pooled estimates of sensitivity and specificity with the corresponding 95% confidence intervals (CIs) and the summary receiver operating characteristic curve (sROC) were determined.
Results
Sixteen studies published between 2011 and 2019 were included with a total of 1852 patients and 2731 vessels. The pooled sensitivity and specificity for FFRCT at the per-patient level was 89% (95% CI, 85–92%) and 71% (95% CI, 61–80%), respectively, while on the per-vessel basis was 85% (95% CI, 82–88%) and 82% (95% CI, 75–87%), respectively. No apparent difference in the sensitivity at per-patient and per-vessel level between FFRCT and CCTA was observed (0.89 versus 0.93 at per-patient; 0.85 versus 0.88 at per-vessel). However, the specificity of FFRCT was higher than CCTA (0.71 versus 0.32 at per-patient analysis; 0.82 versus 0.46 at per-vessel analysis).
Conclusions
FFRCT obtained a high diagnostic performance and is a viable alternative to FFR for detecting coronary ischemic lesions.
Key Points
• Noninvasive FFRCThas higher specificity for anatomical and physiological assessment of coronary artery stenosis compared with CCTA.
• Noninvasive FFRCTis a viable alternative to invasive FFR for the detection and exclusion of coronary lesions that cause ischemia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is responsible for 17% of all death worldwide [1]. The prevalence of CAD is still increasing worldwide making CAD the most common cause of cardiovascular disease mortality [2]. Fractional flow reserve (FFR), a pressure wire-based index that is used during coronary angiography to assess the potential coronary stenosis, is considered as the reference standard for evaluating the severity of stenosis in CAD and one of the key parameters for revascularization therapy [3, 4]. Compared with the strategy directed by angiography, FFR guided blood transport reconstruction is capable of improving event-free survival in a cost-saving and long-lived way [5, 6]. However, as an invasive method, the implementation of FFR needs expensive devices and has potential procedure-related complications such as non-fatal myocardial infarction, cerebrovascular accidents, and even death, which should not be ignored by clinicians. Coronary computed tomography angiography (CCTA) as a non-invasive method for detecting CAD is widely used in patients with a low-to-intermediate pre-test risk [7]. Unfortunately, coronary stenosis assessed by CCTA is often overestimated, and only a few proportions of severe stenosis identified by CCTA could explain myocardial ischemia [8, 9]. Recently, computed tomography angiography-derived fractional flow reserve (FFRCT) has been recommended for evaluating functional severity by utilizing computational fluid dynamics to calculate coronary blood pressure [10]. This method can calculate the blood flow and pressure field of the coronary artery according to the anatomical image data without invasive operation or hyperemia process [11, 12]. In addition, FFRCT has showed high diagnostic performance in the presence of coronary artery calcification [13]. However, the diagnostic accuracy of FFRCT in the assessment of lesion-specific myocardial ischemia is not yet clear. The purpose of this meta-analysis is to determine the diagnostic performance of FFRCT to assess the functional significance of coronary stenosis in patients with suspected or known CAD using invasive FFR as the reference standard.
Methods
Data sources and searches
The analysis was performed according to the PRISMA guidelines [14]. We have performed a computerized literature search of the PubMed, EMBASE, The Cochrane Library, and Web of science for relevant articles published from January 2008 until May 2019 using the following keyword search terms: FFRCT, noninvasive FFR, non-invasive FFR, noninvasive fractional flow reserve, non-invasive fractional flow reserve, and CCTA. No restrictions were applied to the language.
Study selection
We included a study if (1) study population comprised ≥ 30 patients with suspected or known CAD clinically; (2) data were presented at patient- and/or vessel-level; (3) invasive FFR was performed for coronary lesions in all patients; (4) the FFR threshold to diagnose ischemia was ≤ 0.80; (5) either the absolute number of true positives (TP), false positives (FP), false negatives (FN), and true negatives (TN), or sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) could be retrieved from the published full text.
Studies were excluded based on the following criteria: (1) studies were not on humans (studies in vitro or animal systems); (2) studies did not report the diagnostic results associated with the determination of the results of interest (FFR ≤ 0.80); (3) the literature related to reviews, prognostic studies, comments, and case reports.
The title and abstract were examined and the full text of potentially qualified studies was double-checked by two independent reviewers (BY Z and SL W). A third reviewer (MJ L) was consulted to resolve any uncertainty regarding eligibility if there was a discrepancy.
Data extraction and quality assessment
Two reviewers (BY Z and SL W) independently extracted the data. Disagreements were resolved through discussion, and a third reviewer (MJ L) was involved to achieve a consensus when necessary. The quality of included studies was assessed using the standards for reporting of diagnostic accuracy (STARD) tool. The tool is structured as a list of 30 questions, each should be answered “yes,” “no,” or “unclear,” as previously described in detail by Bossuyt et al [15].
Data synthesis and analysis
The diagnostic performance analysis was carried out both at the per-patient and per-vessel levels. The major calculated outcome data were sensitivity and specificity. The pooled corresponding 95% CIs of sensitivity and specificity were also calculated.
According to whether there is statistical heterogeneity exist, random effect or fixed effect model was used to collect data using weighted averages based on the sample size of each study at a per-patient and per-vessel level [16]. Data with heterogeneity were pooled using a random effect model (DerSimonian-Laird model), while data without heterogeneity were pooled using a fixed effect model (Mantel-Haenszel model). Potential heterogeneity, which means variation between studies, was defined as an I2 statistic value of more than 50% [17]. The summary receiver operating characteristic curve (SROC) was fitted using estimates of sensitivity and specificity from the studies included in the meta-analysis and the pooled area under the curve (AUC) was also calculated [18]. Theoretically, AUCs between 0.75 and 0.92 represent a good degree of diagnostic accuracy, while AUCs of 0.93–0.96 are considered much better [19].
Publication bias was assessed visually using a scatter plot of the inverse of the square root of the effective sample size (1/ESS1/2) versus the diagnostic log odds ratio (lnDOR), which exhibits a symmetrical funnel shape when publication bias is absent. Formal testing for publication bias was conducted using a regression of lnDOR against 1/ESS1/2, and weighting by ESS. A p < 0.05 for the slope coefficient indicates significant asymmetry [20].
The sensitivity analysis was conducted by leaving out each reference and reanalyzing the data to test if there are any studies significantly influenced the results.
To compare test performance, probability modifying plots of pre-test and post-test probabilities were synthesized. Furthermore, the clinical or patient relevant utility of CCTA and FFRCT was evaluated using the positive/negative likelihood ratio (LR) to calculate the post-test probability based on Bayes’ theorem [21]. When heterogeneity exits, meta-regress analysis will be performed to identify possible sources of heterogeneity. The analysis was performed by using Stata14.0 (Cochrane collaboration) and Meta-Disc1.4.
Results
Literature search
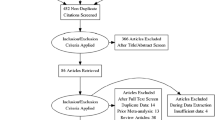
The initial search obtained 248 potentially related publications. After exclusions based on title, abstract, and text, 16 studies were finally included in the present meta-analysis [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. The detailed progress of study selection is described in the flow chart in Fig. 1.
Characteristics of the included studies
A total of 1852 patients and 2731 vessels were analyzed. Studies were published between 2011 and 2019. The sample size of each study ranged from 32 to 254 patients (32 to 484 vessels). Study populations were typical patients undergoing evaluation for suspected or known CAD. Baseline characteristics such as the study design, body mass index, number of participants, and vessels are listed in Table 1 and relevant parameters during the experiment such as stressor for FFR and administration of β-blockers are listed in Table 2; characteristics about intervention history, FFR threshold, equipment parameters, and high dangerous elements are displayed in Table 3.
Data synthesis
For a per-patient basis, seven studies reporting the relevant values for evaluating diagnostic performance of FFRCT were included in the analysis [24,25,26,27, 34,35,36]. The sensitivity and specificity of FFRCT at patient-level ranged from 76 to 94% and 54 to 84% with a pooled sensitivity and specificity of 89% (95% CI 85–92%) and 71% (95% CI 61–80%) using a random effect model (Fig. 2), respectively.
Forest plots illustrating detailed sensitivity and specificity at per-patient level. Diagnostic performance of CCTA for diagnosis of ischemia: pooled sensitivity is 93% (95% CI 85–97%) and pooled specificity is 32% (95% CI 26–39%); diagnostic performance of FFRCT for diagnosis of ischemia: pooled sensitivity is 89% (95% CI 85–92%) and pooled specificity is 71% (95% CI 61–80%)
For a per-vessel basis, 13 studies reporting the necessary values for evaluating diagnostic performance of FFRCT were included in the analysis [23,24,25,26,27,28,29,30, 32,33,34,35,36], while 12 studies reported the necessary values for evaluating diagnostic performance of CCTA [22,23,24, 26,27,28,29,30,31, 33,34,35]. The sensitivity of FFRCT for the included studies ranged from 76 to 100% and specificity ranged from 73 to 96% with a pooled sensitivity of 85% (95% CI 82–88%) using a fixed-effects model and pooled specificity of 82% (95% CI 75–87%) using a random-effects model (Fig. 3).
Forest plots illustrating detailed sensitivity and specificity at per-vessel level. Diagnostic performance of CCTA for diagnosis of ischemia at a per-vessel level: pooled sensitivity is 88% (95% CI 81–92%) and pooled specificity is 46% (95% CI 37–56%); diagnostic performance of FFRCT for diagnosis of ischemia: pooled sensitivity is 85% (95% CI 82–88%) and pooled specificity is 82% (95% CI 75–87%)
Compared with CCTA alone, noninvasive FFRCT was more specific and discriminative for detecting hemodynamic coronary stenosis when invasive FFR was used as a reference standard (Table 4). The pooled sensitivity of FFRCT and CCTA were quite similar (0.89 versus 0.93 at per-patient, p = 0.44; 0.85 versus 0.88 at per-vessel, p = 0.87). However, the specificity of FFRCT was higher than that of CCTA (0.71 versus 0.32 at per-patient analysis, p < 0.001; 0.82 versus 0.46 at per-vessel analysis, p < 0.001).
The AUCs of FFRCT at the per-patient level and the per-vessel level were 0.90 and 0.91, respectively, which are higher than those of CCTA (0.76 at per-patient level and 0.73 at per-vessel level) (Fig. 4).
Summary receiver operating characteristic (ROC) curve, plotting the true positive rate (sensitivity) against the false-positive rate (1—specificity) of FFRCT and CCTA at vessel level and patient level. Each symbol represents an individual study in the meta-analysis, with the size of the symbol proportional to the sample size of the study. The Q* statistic represents the point where sensitivity and specificity are equal. AUC indicates area under the summary receiver operating characteristic curve. The AUCs (area under the SROC) of FFRCT at the per-patient level and the per-vessel level were 0.90 and 0.91, which are 0.76 at per-patient level and 0.73 at per-vessel level of CCTA
Quality of the included studies
Our inter-rater reliability for assessing quality items was perfect (kappa = 0.89). Quality assessment using the Standards for Reporting of Diagnostic Accuracy (STARD) tool (Suppl. material 1) showed that 16 studies were recorded as “NOT” in item #20 and item #23. One study was recorded as “NOT” in item #17. All studies get “Yes” in the other items, indicating the included studies have high quality.
Publication bias
The publication bias was assessed using Deek’s funnel plot asymmetry test. Each of the four plots resembled a symmetrical funnel shape. The p value for Deek’s funnel plot asymmetry test was 0.05. Therefore, there is no significant publication bias exist (Fig. 5).
Meta-regress analysis
Heterogeneity between studies were assessed using the inconsistency index (I2). Meta-regress analysis was applied when I2 ≥ 50%. Multivariable meta-regress analysis at the per-patient and per-vessel level showed that study design, sample size, age, gender, proportion of diabetes, proportion of smoking, hypertension, hyperlipidemia, heart rate, CT scanner type, and time period between FFRCT and FFR were not the sources of heterogeneity.
Sensitivity analysis and probability modifying plots
The sensitivity analysis, in order to investigate the influence of each individual study on the overall meta-analysis summary estimate conducted at both the patient and vessel levels, demonstrated that no study significantly influenced the pooled sensitivity and specificity (Suppl. material 2).
Probability modifying plots were plotted with pre-test versus post-test probabilities (Fig. 6). At both per-vessel and per-patient levels, when the disease was estimated to be pre-test positive, the higher value of post-test probability was obtained with FFRCT strategy than CCTA. It indicated that FFRCT showed better performance on identifying true positive patients. On the other hand, when pre-test estimate was negative, FFRCT and CCTA produced similar value of post-test probability, indicating similar performance on identifying true negative patients.
To be precise, the per-patient analysis revealed a positive LR of 3.08 and a negative LR of 0.16, when the per-vessel analysis revealed a positive LR of 4.64 and a negative LR of 0.18 for FFRCT. As for CCTA, the per-patient analysis revealed a positive LR of 1.37 and a negative LR of 0.23, when the per-vessel analysis revealed a positive LR of 1.64 and a negative LR of 0.26. Based on Bayes’ theorem, at per-patient level, FFRCT could increase the post-test probability of CAD > 64% with a pre-test probability of > 37% (CCTA is > 45%) and can decrease post-test probability of CAD < 30% with a pre-test probability of < 73% (CCTA is < 38%). At per-vessel level, FFRCT could increase the post-test probability of CAD > 73% with a pre-test probability of > 37% (CCTA is > 49%) and can decrease post-test probability of CAD < 33% with a pre-test probability of < 73% (CCTA is < 41%).
Probability modifying plots at per-vessel level and at per-patient level. FFRCT could increase the post-test probability of CAD > 64% with a pre-test probability of > 37%, while CCTA is > 45%, and can decrease post-test probability of CAD < 30% with a pre-test probability of < 73%, while CCTA is < 38% at per-patient level. And at per-vessel level, FFRCT could increase the post-test probability of CAD > 73% with a pre-test probability of > 37%, while CCTA > 49%, and can decrease post-test probability of CAD < 33% with a pre-test probability of < 73%, while CCTA < 41%
Discussion
We found that a noninvasive form of FFR derived from CCTA (FFRCT) exhibited high diagnostic accuracy for the detection of hemodynamic relevance of stenoses in patients with known or suspected CAD by using invasive FFR as a standard reference. These findings remained consistent regardless of whether it was examined at a per-patient or per-vessel level when FFR cutoff value of 0.8 was used as reference standard.
The high sensitivities indicated that FFRCT and CCTA have the ability to measure the proportion of actual myocardial ischemia [38]. CCTA is a validated method for the patients with low or mild pre-test probability and the long-term prognostic value was confirmed [39]. However, the limitation of CCTA remains the only anatomical assessment of coronary stenoses in the absence of evaluation of their functional hemodynamic significance, while functional hemodynamic for those stenoses graded as an intermediate at the anatomical assessment are rather important [40]. FFRCT, which found to be better for the noninvasive screening of CAD patients with diameter stenosis than CCTA [41], can noninvasively obtain pressure and blood flow information by using vessel specific fractional flow reserve data derived from CCTA. The calculation of FFR requires the knowledge of the pressure profile inside a coronary artery before and after the stenosis. This makes FFRCT exhibits more accuracy than CCTA in detecting coronary ischemic lesions. Besides, as mentioned above, the low specificity of CCTA indicated that only a minority severe stenosis identified by CCTA have been confirmed to cause ischemia. The higher specificity of FFRCT overcomes the shortcoming of CCTA that tends to overestimate coronary stenosis. FFRCT served as a combined anatomic and functional assessment can accurately identify patients who have lesion-causing ischemia. In addition, FFRCT has higher AUC versus that of CCTA, which means a superior degree of diagnostic accuracy. FFRCT was a feasible and safe alternative to invasive coronary angiography (ICA), which enables estimation of FFR value without the need of additional invasive procedure, extra administration of medication, radiation exposure, or modification of acquisition protocols. It can provide information both on the anatomic and functional significance of a coronary lesion in a relatively safe and economical manner and make a cost–benefit balance in terms of clinical management and patient’s care. The accuracy and computational time of FFRCT crucially depends on highly accurate image input data, which in turn depends on CCTA protocol variations [42]. FFRCT acted as a new noninvasive method could be an alternative to ICA in helping guide patient care [43]. In conclusion, our findings suggest that FFRCT may be a good diagnostic tool for screening of hemodynamic relevance of stenoses in patients with known or suspected CAD.
Recently, five analyses have discussed the potential effectiveness of the utilization of FFRCT to guide clinical decision-making [44,45,46,47,48]. Their studies demonstrated similar results that FFRCT significantly improves specificity without noticeably altering the sensitivity of CCTA with invasive FFR as a reference standard for the detection of hemodynamically relevant stenosis. Cook et al [44] performed a systematic review including 908 vessels from 536 patients in 5 studies. They reported that the overall per-vessel diagnostic accuracy of FFR-CT was 81.9% (95% CI, 79.4–84.4%) in association of FFRCT with invasive FFR in different levels. In Baumann et al’s [45] meta-analysis, a total of 765 patients and 1306 vessels were included. They found that FFRCT significantly improves specificity without noticeably altering the sensitivity of CCTA (sensitivity 83.7% vs 84.6%, specificity74.7% vs 49.7% on per-lesion basis; sensitivity 89.2% vs 70.2%, specificity 90.2 % vs 35.4% on per-patient basis) with invasive FFR as a reference standard. And the intermediate stenosis subgroup exhibited the same result which indicated FFRCT may become particularly relevant for the difficult evaluation of intermediate stenosis to guide the indication for revascularization. Celeng et al [46] included studies that compared the diagnostic performance of coronary computed tomography angiography (CCTA), CT myocardial perfusion (CTP), fractional flow reserve CT (FFRCT), the transluminal attenuation gradient (TAG), and their combined use with CCTA. After analyzing 1069 patients of 18 articles, they found that FFRCT demonstrated a substantial improvement in the identification of hemodynamically significant CAD compared with CCTA. At vessel and patient level, pooled specificity of FFRCT was 0.78 and 0.76 respectively which was substantially higher than that of CCTA (0.61, 0.48). The SROCs also showed good diagnostic performance for FFRCT compared with CCTA. In our meta-analysis, we updated relevant literature published in 2017–2019 and performed a similar analysis but much higher specificity and diagnosis accuracy of FFRCT because of the larger sample size (1852 vs 1069) and the higher quality literatures included in our meta-analysis. Gonzalez JA and his colleagues [47] compared the pooled diagnostic performance of FFRCT with conventional CCTA by using FFR as the gold standard. Eighteen studies with a total 1535 patients were included in the meta-analysis. The sensitivity and specificity of CCTA at the patient level is 0.92 (0.88–0.98) and 0.43 (0.38–0.47), respectively. Comparing with CCTA, their findings suggested that FFRCT had similar sensitivity value (90% vs. 92%), while higher specificity (72% vs. 43%). However, the inclusion criteria of the previous studies covered relatively wide range including some studies with low quality were included, which may result in lacking of credible reliability. The total number of vessels in the analysis of per-vessel level was not mentioned in their study, thus resulting in the lack of data integrity. In addition, the summary receiver operating characteristic curve (sROC) and the area under curve (AUC) was not performed in previous studies [47]. Regress analysis was not analyzed even though the results have heterogeneity [47], which hinders the conduciveness to find sources of heterogeneity. In a study with 2216 patients and 2798 vessels, Deng et al [48] reported the pooled sensitivity and specificity of FFRCT at the per-patient level were 90% and 73%, respectively, while at the per-vessel level were 82% and 79%, respectively. Although the inclusion criteria of our study were not all the same, the pooled specificity and sensitivity were similar, which aggrandizes more universalism and stringency to our finding. However, insufficient document feature extraction of their study may add difficulty to heterogeneity analysis. We used STARD as quality assessment tool, which is much more detailed than QUADAS and can assess literatures more rigorously.
Comparing with FFRCT, all other noninvasive methods used to detect hemodynamic relevance of stenoses including computed tomography perfusion (CTP), single-photon emission computed tomography (SPECT), and perfusion cardiovascular magnetic resonance (perfusion-CMR) have disadvantages to some extent. Firstly, CTP, especially dynamic CTP, has the risk of radiation exposure [49], which is potentially a problem in patients with high BMI and fast heart rates. Secondly, although belonging to noninvasive examination, SPECT has high ionization radiation but lower spatial resolution, poor attenuation correction, and limited usage of tracers [50]. Thirdly, perfusion-CMR imaging does not suffer from attenuation artifacts compared to nuclear techniques and provides the highest spatial resolution. But CMR stress perfusion is time consuming and MR compatible monitor is required which is the shortcoming of this technique [51].
Limitations
Several points regarding limitations of this analysis are worth mentioning. Firstly, the evidence considered in this review exhibited methodological limitations. (1) The heterogeneity existed in our meta-analysis, but we cannot explore the source, making it harder to transpose the findings to the clinical setting and make a definitive conclusion. (2) All of our involved studies used clinically relevant cutoff of 0.80; however, in some studies [52], a cutoff of 0.75 was used to assess the prognostic value of FFR for revascularization. Due to lack of trials, our study could not determine the diagnostic accuracy of FFRCT < 0.75. Besides, a 50% stenosis threshold by CCTA is associated with high sensitivity but poor specificity for hemodynamically significant coronary artery disease [53]. Maybe a 70% threshold performs better in this situation. These remains future experiments to explore a suitable threshold. Secondly, there are some technological limitations hindering the clinical application of FFRCT. The image quality may be reduced by various factors including image noise, motion artifacts, and beam-hardening artifacts from metallic devices or from coronary calcifications. And the diagnostic performance of FFRCT may be affected by the adherence of physiological and protocol-dependent factors such as heart rate control, blood pressure, contrast enhancement methods, and the use of pre-scan nitroglycerin [54]. More importantly, although FFRCT-testing processing times resulting from software are expected to be improved, current FFRCT evaluation also demands a considerable amount of time (2–6 h) and some discrepancy between modeling FFRCT and directly measured FFR is expected to be present due to the need for post-processing and the slow analysis procedure [13]. Recently, the evaluation of virtual functional assessment index (vFAI) measured from CCTA-based coronary anatomical models [55] has been allowed to determine the hemodynamic relevance of a given coronary lesion with a few minutes long computation time. It can also be used as an adjunct technique to diagnose hemodynamic abnormalities.
Conclusions
In summary, the present data shows that noninvasive FFRCT derived from standard CCTA image data exhibited high diagnostic performance in patients with known or suspected CAD for the detection of hemodynamically significant coronary stenosis. However, it still requires more data to explain how to bring this new technology into the real clinical practice to guide the decision-making in the coming years.
Abbreviations
- AUC:
-
Area under the SROC
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomography angiography
- CIs:
-
Confidence intervals
- CMR:
-
Cardiovascular magnetic resonance
- CTP:
-
Computed tomography perfusion
- FFR:
-
Fractional flow reserve
- FFRCT:
-
Computed tomography angiography-derived fractional flow reserve
- FN:
-
False negative
- FP:
-
False positive
- I2 :
-
Inconsistency index
- ICA:
-
Invasive coronary angiography
- LR−:
-
Negative likelihood ratio
- LR+:
-
Positive likelihood ratio
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- SPECT:
-
Single-photon emission computed tomography
- SROC:
-
Summary receiver operating characteristic curve
- TN:
-
True negative
- TP:
-
True positive
References
Laslett LJ, Alagona P Jr, Clark BA 3rd et al (2012) The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol 60:S1eS49
Mozaffarian D, Benjamin EJ, Go AS et al (2016) Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133:e38iseas
De Bruyne B, Pijls NH, Kalesan B et al (2012) Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med 367:991–1001
Task Force Members, Montalescot G, Sechtem U et al (2013) 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34:2949–3003
Matsumura M, Johnson NP, Fearon WF et al (2017) Accuracy of fractional flow reserve measurements in clinical practice: observations from a core laboratory analysis. JACC Cardiovasc Interv 10:1392–1401
Xu B, Whitbourn R, Wilson A et al (2014) Clinical impact of fractional flow reserve in a real-world cohort of patients. Int J Cardiol 172:251–252
Min JK, Feignoux J, Treutenaere J, Laperche T, Sablayrolles J (2010) The prognostic value of multidetector coronary CT angiography for the prediction of major adverse cardiovascular events: a multicenter observational cohort study. Int J Cardiovasc Imaging 26:721–728
Andrew M, John H (2015) The challenge of coronary calcium on coronary computed tomographic angiography (CCTA) scans: effect on interpretation and possible solutions. Int J Cardiovasc Imaging 31:145–157
Arbab-Zadeh A, Hoe J (2011) Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography methods, caveats, and implications. JACC Cardiovasc Imaging 4:191–202
Taylor CA, Fonte TA, Min JK (2013) Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve. J Am Coll Cardiol 61:2233–2241
Gaur S, Ovrehus KA, Dey D et al (2016) Coronary plaque quantification and fractional flow reserve by coronary computed tomography angiography identify ischaemia-causing lesions. Eur Heart J 37:1220–1227
Kim KH, Doh JH, Koo BK et al (2014) A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv 7:72–78
Norgaard BL, Leipsic J, Koo BK et al (2016) Coronary computed tomography angiography derived fractional flow reserve and plaque stress. Curr Cardiovasc Imaging Rep 9:2
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the Prisma statement. Ann Intern Med 4(264-9):W64
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies 1. Radiology 272:826–832
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58(10):982–990
Higgins JP, Thompson SG, Deek JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327:557in60
Irwig L, Tosteson AN, Gatsonis C et al (1994) Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med 120:667es f
Jones CM, Athanasiou T (2005) Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg 79:16–20
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58:882–893
Jaeschke R, Guyatt GH, Sackett DL (1994) Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence-Based Medicine Working Group. JAMA 271(9):703–707
Ko BS, Wong DT, Cameron JD et al (2014) 320-row CT coronary angiography predicts freedom from revascularisation and acts as a gatekeeper to defer invasive angiography in stable coronary artery disease: a fractional flow reserve-correlated study. Eur Radiol 24:738–747
Wardziak L, Kruk M, Pleban W et al (2019) Coronary CTA enhanced with CTA based FFR analysis provides higher diagnostic value than invasive coronary angiography in patients with intermediate coronary stenosis. J Cardiovasc Comput Tomogr 13(1):62–67
Koo BK, Erglis A, Doh JH et al (2011) Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol 58:1989–1997
Min JK, Leipsic J, Pencina MJ et al (2012) Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA 308:1237–1245
Norgaard BL, Leipsic J, Gaur S et al (2014) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol 63:1145–1155
Renker M, Schoepf UJ, Wang R et al (2014) Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol 114:1303–1308
Wang R, Renker M, Schoepf UJ et al (2015) Diagnostic value of quantitative stenosis predictors with coronary CT angiography compared to invasive fractional flow reserve. Eur J Radiol 84:1509–1515
Coenen A, Lubbers MM, Kurata A et al (2015) Fractional flow reserve computed from non-invasive CT angiography data: diagnostic performance of an on-site clinician operated computational fluid dynamics algorithm 1. Radiology 274:674–683
Min JK, Koo BK, Erglis A et al (2012) Usefulness of noninvasive fractional flow reserve computed from coronary computed tomographic angiograms for intermediate stenoses confirmed by quantitative coronary angiography. Am J Cardiol 110:971–976
Wong DT, Ko BS, Cameron JD et al (2013) Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: a comparison with fractional flow reserve. J Am Coll Cardiol 61:1271–1279
Tesche C, De Cecco CN, Caruso D et al (2016) Coronary CT angiography derived morphological and functional quantitative plaque markers correlated with invasive fractional flow reserve for detecting hemodynamically significant stenosis. J Cardiovasc Comput Tomogr 10:199–206
Ko BS, Cameron JD, Munnur RK et al (2017) Noninvasive CT-derived FFR based on structural and fluid analysis: a comparison with invasive FFR for detection of functionally significant stenosis. JACC Cardiovasc Imaging 10:663–673
Kruk M, Wardziak L, Demkow M et al (2016) Workstation-based calculation of CTA-based FFR for intermediate stenosis. JACC Cardiovasc Imaging 9:690–699
Chung JH, Lee KE, Nam CW et al (2017) Diagnostic performance of a novel method for fractional flow reserve computed from noninvasive computed tomography angiography (NOVEL-FLOW Study). Am J Cardiol 120(3):362–368
Rother J, Moshage M, Dey D et al (2018) Comparison of invasively measured FFR with FFR derived from coronary CT angiography for detection of lesion-specific ischemia: results from a PC-based prototype algorithm. J Cardiovasc Comput Tomogr 12(2):101–107
Sand NPR, Veien KT, Nielsen SS et al (2018) Prospective comparison of FFR derived from coronary CT angiography with SPECT perfusion imaging in stable coronary artery disease: the ReASSESS study. JACC Cardiovasc Imaging 11(11):1640–1650
von Knebel Doeberitz PL, De Cecco CN, Schoepf UJ et al (2019) Coronary CT angiography-derived plaque quantification with artificial intelligence CT fractional flow reserve for the identification of lesion-specific ischemia. Eur Radiol 29(5):2378–2387
Abdulla J, Abildstrom SZ, Gotzsche O, Christensen E, Kober L, Torp-Pedersen C (2007) 64-multislice detector computed tomography coronary angiography as potential alternative to conventional coronary angiography: a systematic review and meta-analysis. Eur Heart J 28(24):3042–3050
Schuijf JD, Achenbach S, de Feyter PJ, Bax JJ (2011) Current applications and limitations of coronary computed tomography angiography in stable coronary artery disease. Heart 97(4):330–337
Yu M, Lu Z, Shen C et al (2019) The best predictor of ischemic coronary stenosis: subtended myocardial volume, machine learning-based FFRCT, or high-risk plaque features? Eur Radiol 29(7):3647–3657
Norgaard BL, Gaur S, Leipsic J et al (2015) Influence of coronary calcification on the diagnostic performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT Trial. JACC Cardiovasc Imaging 8(9):1045–1055
Liu X, Wang Y, Zhang H et al (2019) Evaluation of fractional flow reserve in patients with stable angina: can CT compete with angiography? Eur Radiol 29(7):3669–3677
Cook CM, Petraco R, Shun-Shin MJ et al (2017) Diagnostic accuracy of computed tomography–derived fractional flow reserve. JAMA Cardiol 2(7):803
Baumann S, Renker M, Hetjens S et al (2016) Comparison of coronary computed tomography angiography-derived vs invasive fractional flow reserve assessment: meta-analysis with subgroup evaluation of intermediate stenosis. Acad Radiol 23(11):1402–1411
Celeng C, Leiner T, Maurovich-Horvat P et al (2018) Anatomical and functional computed tomography for diagnosing hemodynamically significant coronary artery disease: a metaanalysis. JACC Cardiovasc Imaging 12:1316–1325
Gonzalez JA, Lipinski MJ, Flors L, Shaw PW, Kramer CM, Salerno M (2015) Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am J Cardiol 116:1469–1478
Deng SB, Jing XD, Wang J et al (2015) Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in coronary artery disease: a systematic review and meta-analysis. Int J Cardiol 184:703–709
Caruso D, Eid M, Schoepf UJ et al (2016) Dynamic CT myocardial perfusion imaging. Eur J Radiol 85:1893–1899
Melikian N, De Bondt P, Tonino P et al (2010) Fractional flow reserve and myocardial perfusion imaging in patients with angiographic multivessel coronary artery disease. JACC Cardiovasc Interv 3:307–314
Heydari B, Jerosch-Herold M, Kwong RY (2011) Assessment of myocardial ischemia with cardiovascular magnetic resonance. Prog Cardiovasc Dis 54:191–203
Bech GJ, De Bruyne B, Pijls NH et al (2001) Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation 103(24):2928–2934
Tesche C, Vliegenthart R, Duguay TM et al (2017) Coronary computed tomographic angiography-derived fractional flow reserve for therapeutic decision making. Am J Cardiol 120(12):2121–2127
Leipsic J, Yang TH, Thompson A et al (2014) CT angiography (CTA) and diagnostic performance of noninvasive fractional flow reserve: results from the determination of fractional flow reserve by anatomic CTA (DeFACTO) Study. AJR Am J Roentgenol 202:989–994
Siogkas PK, Anagnostopoulos CD, Liga R et al (2019) Noninvasive CT-based hemodynamic assessment of coronary lesions derived from fast computational analysis: a comparison against fractional flow reserve. Eur Radiol 29(4):2117–2126
Funding
This study has received funding by Research Grant of National Natural Science Foundation of China (81571647, 81971588, 81620108015, 81771811), and Capital Clinical Special Program (Z191100006619021).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Minjie Lu.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1747 kb)
Rights and permissions
About this article
Cite this article
Zhuang, B., Wang, S., Zhao, S. et al. Computed tomography angiography-derived fractional flow reserve (CT-FFR) for the detection of myocardial ischemia with invasive fractional flow reserve as reference: systematic review and meta-analysis. Eur Radiol 30, 712–725 (2020). https://doi.org/10.1007/s00330-019-06470-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06470-8