Abstract
Purpose
Fractional flow reserve based on coronary computed tomographic angiography (CCTA; FFRCT) can evaluate functional severity in coronary artery disease (CAD). This study investigated the diagnostic value of FFRCT for determining CAD severity.
Materials and methods
Medline, Cochrane, EMBASE, and Google Scholar databases were searched until June 16, 2016 using the following search terms: fractional flow reserve, coronary computed tomography angiography, myocardial ischemia. Randomized controlled trials, two-arm prospective studies, and retrospective studies were included in the analysis.
Results
Twenty-one studies were included with a total of 2216 subjects and 2798 vessels. FFRCT, sensitivity per-vessel and per-patient were ≥82% and specificity was ≥73% for diagnosis of ischemia. FFRCT had better diagnostic accuracy and discrimination than CCTA.
Conclusion
This study indicates that FFRCT may be a good tool for screening and diagnosing of myocardial ischemia in patients with CAD.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Invasive coronary angiography and non-invasive coronary computed tomographic angiography (CCTA) are established methods for detecting coronary artery disease (CAD) [1–3]. The main use of CCTA is in the diagnosis or exclusion of obstructive CAD [4]. For this use, CCTA has high sensitivity (>90%) and high negative predictive value (>98%) [4]. Overall, clinical situations where CCTA is considered of benefit to perform is when CAD is suspected in patients at intermediate risk who have undergone stress tests that are not conclusive or in whom a stress test cannot be performed [4]. CCTA is a safe technique and can be useful in clinical management of patients [5]. Improvement in scanners, image acquisition, and reconstruction has allowed CCTA to consistently be performed in a radiation range of 1–5 mSV [6]. Despite recent advancements, CCTA remains strictly an anatomic test, and, hence, similar to conventional invasive angiography, it lacks the necessary information to guide revascularization decisions, which requires objective evidence of ischemia [6].
However, both conventional invasive angiography and CCTA are limited. Invasive coronary angiography is limited by that fact that the correlation between angiographic and physiological stenosis is poor [7, 8]. A limitation of CCTA is that in the presence of CAD, it can overestimate the severity of stenosis compared with invasive coronary angiography [3]. In addition, CAD functional severity only weakly correlates with CCTA severity. This latter limitation is of particular importance in that determination of functional severity is a key factor used in decision-making with regard to the management of CAD [3].
Fractional flow reserve (FFR), which assesses the ratio of flow across a stenosis to putative flow in the absence of stenosis, is considered the reference standard for evaluating the severity of stenosis in CAD and is one of the key parameters for revascularization therapy [9–11]. FFR, as measured by conventional invasive angiography, is considered the gold standard for discriminating lesion-specific ischemia [6]. Prior studies have shown that an FFR ratio of 0.80 identifies patients with coronary lesions who will benefit from coronary revascularization [10, 12]. FFR is limited by its invasiveness and costs, and, hence, it is not commonly used in clinical practice for coronary revascularization decision-making [6].
Recently, a method that utilizes computational fluid dynamics to calculate coronary blood flow, pressure, and FFR based on routinely acquired CCTA at rest (FFRCT) has been introduced for evaluating functional severity [13–15]. FFRCT combines functional and anatomical information to facilitate determination of appropriate treatments, and has the benefit of being non-invasive [1]. FFRCT may be a useful diagnostic tool for distinguishing patients who will benefit from revascularization therapy from those who will not [1]. The diagnostic performance of FFRCT as been tested in three prospective multicenter trials [1, 14, 15]. All three trial found FFRCT was superior compared with invasive FFR [1, 14, 15]. FFRCT has also demonstrated high diagnostic performance in the presence of coronary calcification [6]. However, the diagnostic accuracy of FFRCT in the evaluation of lesion-specific myocardial ischemia is not clear, particularly in patients with intermediate coronary stenosis. No current guidelines provide recommendations regarding the use of FFRCT testing and interpretation [6]. The purpose of this meta-analysis was to further evaluate the diagnostic value of FFRCT compared with invasive FFR for diagnosing myocardial ischemia in patients with CAD. The study also compared the diagnostic accuracy of FFRCT with CCTA.
Materials and methods
Search strategy
This analysis was performed in accordance with the PRISMA guidelines. Medline, Cochrane, EMBASE, and Google Scholar databases were searched until June 16, 2016 using the following search terms: fractional flow reserve, coronary computed tomography angiography, myocardial ischemia. Randomized controlled trials (RCTs), two-arm prospective studies, and retrospective studies were included in the analysis. Eligible studies evaluated adult patients with suspected coronary artery disease who underwent clinically indicated invasive coronary angiography or CCTA, and reported quantitatively outcomes of interest. Letters, comments, editorials, case reports, proceedings, and personal communications were excluded. Potential references were hand-searched by two independent reviewers to evaluate if they met the inclusion/exclusion criteria. A third reviewer was consulted, when necessary, to resolve any uncertainty regarding eligibility.
Data extraction and quality assessment
The following information/data was extracted from the included studies: the name of the first author, year of publication, study design, number of participants and vessels, participants’ age and gender, previous CAD, comorbidities, smoking history, and the major outcomes.
The quality of the included studies was evaluated using Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) [16].
Outcome measures
The outcomes were sensitivity, specificity, positive and negative predictive values of FFR for diagnosis of ischemia, and areas under the curve (AUCs) of per-patient and per-vessel performance of FFRCT ≤0.80 and computed tomography (CT) stenosis ≥50% compared with invasive FFR for diagnosing ischemia.
Statistical analysis
Accuracy data were used to construct 2 × 2 tables of non-invasive FFR from CCTA for diagnosis of ischemia. The true-positive, false-positive, true-negative, and false-negative values were abstracted and recorded. Ischemia was defined by an FFRCT ≤0.80 or a CCTA with stenosis ≥50%. Diagnostic performance of FFRCT and CCTA stenosis was assessed with invasive FFR as the reference standard. Pooled measures for diagnostic performance, such as sensitivity (true positive/true-positive + false negative), specificity (true negative/true negative + false positive), diagnostic odds ratios (diagnostic ORs) with their corresponding 95% confidence intervals (95% CIs), and area under the receiver-operating characteristic (AUC ROC) curves were calculated. The sensitivity and specificity “per-vessel” was based on the number of vessel diagnosed, and “per-patient” was based on the number of patients evaluated. The diagnostic ORs combine sensitivity and specificity into one measure for diagnostic performance. A diagnostic OR of 1 means the test has no ability to discriminate. In the context of this study, higher diagnostic OR represents better diagnostic accuracy of FFRCT for determining ischemia. Inverse variance weights were used for pooling and significant heterogeneity, which was expected because of the emerging nature of this field, was addressed by the application of random effects (DerSimonian–Laird approach) modeling. All statistical assessments were two-sided and a P value <0.05 was considered to indicate statistical significance. All analyses were performed using Meta-Disc version 1.4 [17].
Results
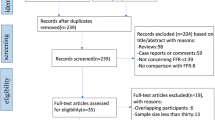
Of the 175 studies initially identified, 133 were excluded for being duplicates or irrelevant (Fig. 1). Forty-two underwent full text review of which 21 were excluded for not reporting complete outcomes, reporting data on duplicate study populations, not reporting outcomes of interest, or investigating other types of interventions.
Twenty-one studies were included in the analysis with a total of 2216 subjects and 2798 vessels [1, 2, 14, 15, 18–34]. The number of patients in the studies ranged from 37 to 475 and the number of vessels in the studies ranged from 37 to 484 (Table 1). The age range of patients was similar across studies with the mean age being about 60 years. The majority of patients were male. In general, over half the patients across the studies had hypertension (range 37–81%) and less than a third had diabetes (range 10–68% The incidence of dyslipidemia varied among the studies (range 19–95%). The percentage of patients who smoked was from 14 to 46% (Table 1). Less than a quarter of the patients had prior myocardial infarction (range 2–19%) or percutaneous coronary intervention (range 3–23%; Table 2). The cut-off threshold for both invasive FFR and FFRCT ranged from 0.75 to 0.80, and the cut-off threshold for CCTA ranged from 50 to 65% (Table 2).
Sensitivity, specificity, diagnostic odd ratios (ORs), and area under the receiver-operating characteristic curve (AUC) for FFRCT
A total of eight studies [1, 2, 14, 15, 18, 25, 29, 31] were included in the meta-analysis of diagnostic performance of FFRCT for ischemia on a per vessel basis (Fig. 2). Thirteen studies were excluded from the analysis as they did not report true-positive, false-positive, true-negative, and false-negative values. The range of sensitivities among the studies was from 74 to 100% and the pooled sensitivity was 0.82 (95% CI 0.79–0.86; Fig. 2a). The range of specificities varied from 63 to 96% and the pooled specificity was 0.79 (95% CI 0.76–0.82; Fig. 2b). For all studies, the pooled diagnostic OR was 25.18 (95% CI 11.82–53.66; Fig. 2c) and the areas under the summary ROC curves were 0.8957 (with a standard error of 0.017; Fig. 2d).
Only four studies reported diagnostic performance of FFRCT for ischemia on a per-patient basis, and were included in the analysis [1, 14, 15, 29] (Fig. 3). The range of sensitivities among the studies was from 86 to 94% and the pooled sensitivity was 0.90 (95% CI 0.86–0.93; Fig. 3a). The range of specificities among the studies was from 54 to 91% and the pooled specificity was 0.73 (95% CI 0.68–0.77; Fig. 3b). In addition, the pooled diagnostic OR was 31.41 (95% CI 10.94–90.19; Fig. 3c) and the areas under the summary ROC curves were 0.9574 (with a standard error of 0.025; Fig. 3d).
Sensitivity, specificity, diagnostic odd ratios (ORs), and area under the receiver-operating characteristic curve (AUC) for CCTA stenosis
Sixteen studies reported true-positive, false-positive, true-negative, and false-negative values and were included in the meta-analysis of diagnostic performance of CCTA for ischemia on a per-vessel basis (Fig. 4) [1, 2, 14, 19–24, 26, 27, 29, 30, 32–34]. The range of sensitivities among the studies varied from 53 to 95% and the pooled sensitivity was 0.87 (95% CI 0.85–0.89; Fig. 4a). The range of specificities among the studies was from 34 to 88% and the pooled specificity was 0.63 (95% CI 0.60–0.65; Fig. 4b). For all studies, the pooled diagnostic OR was 12.25 (95% CI 7.84–19.14; Fig. 4c) and the areas under the summary ROC curves were 0.8543 (with a standard error of 0.030; Fig. 4d).
For a per-patient basis, eight studies reported the necessary values for evaluating diagnostic performance of CCTA and were included in the analysis [1, 14, 15, 20, 23, 28, 29, 34] (Fig. 5). The range of sensitivities among the studies varied from 83 to 100% and the pooled sensitivity was 0.91 (95% CI 0.88–0.93; Fig. 5a). The range of specificities among the studies varied from 24 to 92% and the pooled specificity was 0.61 (95% CI 0.58–0.65; Fig. 5b). The pooled diagnostic OR was 15.91 (95% CI 4.95–51.09; Fig. 5c) and the area under the summary ROC curves was 0.9087 (with a standard error of 0.060; Fig. 5d).
In summary, this analysis found that noninvasive FFRCT was associated with improved diagnostic specificity and discrimination compared with CCTA alone for the diagnosis of ischemia when invasive FFR was served as the referent standard (diagnostic OR, 25.18 vs. 12.25 for per-vessel basis and diagnostic OR, 31.41 vs. 15.91 for per-patient basis).
Quality assessment
The included studies were evaluated using QUADAS-2 criteria. All studies had low risk of bias with regard to patient selection, index test, reference standard and flow and timing (Table 3). The risk of bias was low for applicability. These findings indicate the studies were of good quality.
Discussion
The aim of this analysis was to investigate the diagnostic value of noninvasive FFRCT and CCTA compared with invasive FFR for the diagnosis of myocardial ischemia in patients with CAD. No apparent difference in the pooled sensitivity per vessel and per patients between FFRCT and CCTA was observed. However, the pooled specificity of FFRCT per vessel (0.79) and per patients (0.73) was relatively higher than the pooled specificity per vessel (0.63) and per patients (0.61) of CCTA. Therefore, noninvasive FFRCT was associated with improved diagnostic specificity and discrimination compared with CCTA alone for the diagnosis of ischemia when FFR served as the reference standard (diagnostic OR 25.18 vs. 12.25 for per vessel basis, diagnostic OR 31.41 vs. 15.91 for per patient basis).
One prior meta-analysis assessed the diagnostic performance of FFRCT compared with FFR [35]. Their analysis included 706 patients and 1165 vessels of lesions. The pooled sensitivity and specificity at the per-patient level were 90 and 72%, respectively, and at the per-vessel level, were 83 and 78%, which are quite similar to the values we found, and support the findings that FFRCT has high diagnostic accuracy compared with invasively measured FFR for the detection of ischemia-causing stenosis in stable patients with known or suspected CAD.
Another prior meta-analysis compared the diagnostic performance of CCTA, computed tomography perfusion (CTP), and FFRCT for evaluating the functional significance of coronary stenosis in patients with suspected or known CAD using FFR as the gold standard technique [36]. The meta-analysis included 18 studies with a total of 1535 patients. They found that CCTA had a high sensitivity and negative predictive value (92 and 87%, respectively) for ruling out stenosis as defined by FFR on a per-patient basis. CTP and FFRCT had similar sensitivities (94 and 90%, respectively) and negative predictive values (92 and 90%) on a per-patient basis. Their findings indicate that sensitivity and negative predictive value are not improved with CTP or FFRCT compared with CCTA. However, CTP and FFRCT had higher estimates for specificity (77 and 72%, respectively) and positive predictive value on a per-patient analysis compared with CCTA. This is similar to our analysis which indicated FFRCT has similar sensitivity but higher specificity compared with CCTA alone.
Two other meta-analyses evaluated pooled data from three prospective, international, multicenter studies that evaluated the diagnostic performance of FFRCT using invasive FFR as a reference standard [37, 38]. Together, the three included studies had a total of 609 patients and 1050 vessels. The two meta-analyses found the pooled sensitivity was 89%, specificity 71%, the positive predictive value 70%, and negative predictive value 90% at the per-patient level and were 83, 78, 61, and 92%, respectively, at the per-vessel level. Both meta-analyses also found FFRCT demonstrated significantly better accuracy compared with CCTA in detecting lesion-specific ischemia. FFRCT remained highly specific and sensitive in patients with intermediate coronary stenosis with respect to ischemia diagnosis [37]. The meta-analysis of Li et al. found the diagnostic OR at the per-patient level was 21.2 and at the per-vessel level was 19.2. Consistent with our findings, these two meta-analyses conclude the FFRCT is a viable alternative to invasive FFR [37, 38].
Another meta-analysis evaluated the diagnostic performance of multiple methods, including single-photon emission computed tomography (SPECT), stress echocardiography (SE), invasive coronary angiography (ICA), CCTA, FFRCT, and cardiac magnetic resonance imaging (MRI) [39]. They found MRI had a high performance for diagnosing hemodynamically significant CAD both for per patient and per vessel when compared with FFR as the reference standard, and also that FFRCT yielded high diagnostic sensitivity (90% per patient and 83% per vessel) and specificity (71% per patient and 78% per vessel). FFRCT diagnostic performance was overall greater than that of CCTA (per-patient sensitivity and specificity were 90 and 39%, respectively, and per-vessel, they were 91 and 58%). FFRCT also had greater diagnosis performance compared with ICA and SPECT.
In general, our study and the prior meta-analyses summarized above indicate that FFRCT has diagnostic benefit compared with invasive FFR in evaluating stenosis in patients with CAD and may have greater benefit than CCTA, particularly in differentiating a true positive from a false positive. This conclusion is supported by the other studies that found FFRCT provided increased diagnostic performance compared with CCTA alone, using invasive FFR as a reference, in discriminating hemodynamically significant coronary stenosis [40–42]. Compared with prior meta-analysis, our meta-analysis included a larger sample size comparing the diagnostic performance of FFRCT and CCTA and incorporated recent clinical studies.
Although FFRCT shows good diagnostic performance, there are currently several limitations to this technology. Significant CT imaging artifacts, such as low contrast, motion, and blooming due to coronary calcification may negatively impact the performance of CCTA and, consequently, FFRCT [6]. Currently, FFRCT testing and analysis can take significant time (2–6 h) due to limitations of computer software and the fact that computer processing is typically done offsite [6]. The fact the analysis is slow and done offsite has limited its use, although new computational approaches are being developed to change this current situation [6]. In addition, studies are necessary to compare the cost-effectiveness of FFRCT with other diagnostic modalities.
There were several limitations to the study that should be considered when interpreting the results. The patient populations were heterogeneous and only a limited number of vessels were evaluated by FFRCT, which may have affected the results particularly since FFRCT is specific for detecting epicardial vascular lesions. In addition, the definition of significant functional stenosis differed across the studies, ranging from 30 to 95%. Moreover, some studies did not evaluate lesions that were less than 50% or greater than 75%. Different cutoffs for performing a procedure were also used among the studies. The findings were also limited by the methods (hardware and software) used to obtain images for FFRCT analysis which differed across studies. These differences may have impacted the quality of images obtained which would have influenced interpretation of the findings, and consequently, final diagnostic performance. Higher-quality images may have necessitated higher radiation and contrast doses; however, the ability of studies to do this was limited by the potential of an increased risks of adverse effects in certain patient populations, such as those of reproductive age or with renal failure. The quality of images was also limited by the body mass index (BMI) and the heart rate of patients, which may have resulted in sampling bias. The findings of this analysis were limited by the small number of studies included in each meta-analysis. These small numbers also precluded us from evaluating if publication bias may have influenced our results.
Conclusion
In conclusion, our findings suggest that FFRCT may be a good diagnostic tool for screening for myocardial ischemia in patients with CAD. Additional studies are required to further explore these findings.
References
Norgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (analysis of coronary blood flow using CT angiography: next steps). J Am Coll Cardiol. 2014;63:1145–55.
Coenen A, Lubbers MM, Kurata A, Kono A, Dedic A, Chelu RG, et al. Fractional flow reserve computed from noninvasive CT angiography data: diagnostic performance of an on-site clinician-operated computational fluid dynamics algorithm. Radiology. 2015;274:674–83.
Min JK, Shaw LJ, Berman DS. The present state of coronary computed tomography angiography a process in evolution. J Am Coll Cardiol. 2010;55:957–65.
di Cesare E, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, et al. Clinical indications for cardiac computed tomography. From the Working Group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM). Radiol Med. 2012;117:901–38.
Cademartiri F, Di Cesare E, Francone M, Ballerini G, Ligabue G, Maffei E, et al. Italian registry of cardiac computed tomography. Radiol Med. 2015;120:919–29.
Norgaard BL, Leipsic J, Koo BK, Zarins CK, Jensen JM, Sand NP, et al. Coronary computed tomography angiography derived fractional flow reserve and plaque stress. Curr Cardiovasc Imaging Rep. 2016;9:2. doi:10.1007/s12410-015-9366-5.
Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–21.
De Bruyne B, Hersbach F, Pijls NH, Bartunek J, Bech JW, Heyndrickx GR, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation. 2001;104:2401–6.
Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, et al. Measurement of fractional flow reserve to assess the functional severity of coronary-artery stenoses. N Engl J Med. 1996;334:1703–8.
De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001.
Task Force M, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003.
Muller O, Mangiacapra F, Ntalianis A, Verhamme KM, Trana C, Hamilos M, et al. Long-term follow-up after fractional flow reserve-guided treatment strategy in patients with an isolated proximal left anterior descending coronary artery stenosis. JACC Cardiovasc Interv. 2011;4:1175–82.
Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233–41.
Koo BK, Erglis A, Doh JH, Daniels DV, Jegere S, Kim HS, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive fractional flow reserve computed from coronary computed tomographic angiograms. Results from the prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. 2011;58:1989–97.
Min JK, Leipsic J, Pencina MJ, Berman DS, Koo BK, van Mieghem C, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT angiography. JAMA. 2012;308:1237–45.
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36.
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi:10.1186/1471-2288-6-31.
Tesche C, De Cecco CN, Caruso D, Baumann S, Renker M, Mangold S, et al. Coronary CT angiography derived morphological and functional quantitative plaque markers correlated with invasive fractional flow reserve for detecting hemodynamically significant stenosis. J Cardiovasc Comput Tomogr. 2016;10:199–206.
Meijboom WB, Van Mieghem CA, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. 2008;52:636–43.
Rossi A, Dharampal A, Wragg A, Davies LC, van Geuns RJ, Anagnostopoulos C, et al. Diagnostic performance of hyperaemic myocardial blood flow index obtained by dynamic computed tomography: does it predict functionally significant coronary lesions? Eur Heart J Cardiovasc Imaging. 2014;15:85–94.
Choo KS, Hwangbo L, Kim JH, Park YH, Kim JS, Kim J, et al. Adenosine-stress low-dose single-scan CT myocardial perfusion imaging using a 128-slice dual-source CT: a comparison with fractional flow reserve. Acta Radiol. 2013;54:389–95.
Kristensen TS, Engstrom T, Kelbaek H, von der Recke P, Nielsen MB, Kofoed KF. Correlation between coronary computed tomographic angiography and fractional flow reserve. Int J Cardiol. 2010;144:200–5.
Stuijfzand WJ, Danad I, Raijmakers PG, Marcu CB, Heymans MW, van Kuijk CC, et al. Additional value of transluminal attenuation gradient in CT angiography to predict hemodynamic significance of coronary artery stenosis. JACC Cardiovasc Imaging. 2014;7:374–86.
Voros S, Rinehart S, Vazquez-Figueroa JG, Kalynych A, Karmpaliotis D, Qian Z, et al. Prospective, head-to-head comparison of quantitative coronary angiography, quantitative computed tomography angiography, and intravascular ultrasound for the prediction of hemodynamic significance in intermediate and severe lesions, using fractional flow reserve as reference standard (from the ATLANTA I and II Study). Am J Cardiol. 2014;113:23–9.
Dai N, Lv HJ, Xiang YF, Fan B, Li WM, Xu YW. Three-dimensional modeling and numerical analysis of fractional flow reserve in human coronary arteries. Postepy Kardiol Interwencyjnej. 2016;12:25–31.
Wong DT, Ko BS, Cameron JD, Nerlekar N, Leung MC, Malaiapan Y, et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: a comparison with fractional flow reserve. J Am Coll Cardiol. 2013;61:1271–9.
Osawa K, Miyoshi T, Miki T, Koyama Y, Sato S, Kanazawa S, et al. Diagnostic performance of first-pass myocardial perfusion imaging without stress with computed tomography (CT) compared with coronary CT angiography alone, with fractional flow reserve as the reference standard. PLoS One. 2016;11:e0149170.
Neglia D, Rovai D, Caselli C, Pietila M, Teresinska A, Aguade-Bruix S, et al. Detection of significant coronary artery disease by noninvasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;. doi:10.1161/CIRCIMAGING.114.002179.
Renker M, Schoepf UJ, Wang R, Meinel FG, Rier JD, Bayer RR 2nd, et al. Comparison of diagnostic value of a novel noninvasive coronary computed tomography angiography method versus standard coronary angiography for assessing fractional flow reserve. Am J Cardiol. 2014;114:1303–8.
Choi JH, Koo BK, Yoon YE, Min JK, Song YB, Hahn JY, et al. Diagnostic performance of intracoronary gradient-based methods by coronary computed tomography angiography for the evaluation of physiologically significant coronary artery stenoses: a validation study with fractional flow reserve. Eur Heart J Cardiovasc Imaging. 2012;13:1001–7.
Kim KH, Doh JH, Koo BK, Min JK, Erglis A, Yang HM, et al. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv. 2014;7:72–8.
Ko BS, Wong DT, Cameron JD, Leong DP, Leung M, Meredith IT, et al. 320-row CT coronary angiography predicts freedom from revascularisation and acts as a gatekeeper to defer invasive angiography in stable coronary artery disease: a fractional flow reserve-correlated study. Eur Radiol. 2014;24:738–47.
Opolski MP, Kepka C, Achenbach S, Pregowski J, Kruk M, Staruch AD, et al. Advanced computed tomographic anatomical and morphometric plaque analysis for prediction of fractional flow reserve in intermediate coronary lesions. Eur J Radiol. 2014;83:135–41.
Schaap J, Kauling RM, Boekholdt SM, Nieman K, Meijboom WB, Post MC, et al. Incremental diagnostic accuracy of hybrid SPECT/CT coronary angiography in a population with an intermediate to high pre-test likelihood of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2013;14:642–9.
Deng SB, Jing XD, Wang J, Huang C, Xia S, Du JL, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in coronary artery disease: A systematic review and meta-analysis. Int J Cardiol. 2015;184:703–9.
Gonzalez JA, Lipinski MJ, Flors L, Shaw PW, Kramer CM, Salerno M. Meta-analysis of diagnostic performance of coronary computed tomography angiography, computed tomography perfusion, and computed tomography-fractional flow reserve in functional myocardial ischemia assessment versus invasive fractional flow reserve. Am J Cardiol. 2015;116:1469–78.
Xu R, Li C, Qian J, Ge J. Computed tomography-derived fractional flow reserve in the detection of lesion-specific ischemia: an integrated analysis of 3 pivotal trials. Medicine (Baltimore). 2015;94:e1963. doi:10.1097/MD.0000000000001963.
Li S, Tang X, Peng L, Luo Y, Dong R, Liu J. The diagnostic performance of CT-derived fractional flow reserve for evaluation of myocardial ischaemia confirmed by invasive fractional flow reserve: a meta-analysis. Clin Radiol. 2015;70:476–86.
Danad I, Szymonifka J, Twisk JW, Norgaard BL, Zarins CK, Knaapen P, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J. 2016;. doi:10.1093/eurheartj/ehw095.
Wang R, Renker M, Schoepf UJ, Wichmann JL, Fuller SR, Rier JD, et al. Diagnostic value of quantitative stenosis predictors with coronary CT angiography compared to invasive fractional flow reserve. Eur J Radiol. 2015;84:1509–15.
Nakanishi R, Matsumoto S, Alani A, Li D, Kitslaar PH, Broersen A, et al. Diagnostic performance of transluminal attenuation gradient and fractional flow reserve by coronary computed tomographic angiography (FFR(CT)) compared to invasive FFR: a sub-group analysis from the DISCOVER-FLOW and DeFACTO studies. Int J Cardiovasc Imaging. 2015;31:1251–9.
Baumann S, Wang R, Schoepf UJ, Steinberg DH, Spearman JV, Bayer RR 2nd, et al. Coronary CT angiography-derived fractional flow reserve correlated with invasive fractional flow reserve measurements—initial experience with a novel physician-driven algorithm. Eur Radiol. 2015;25:1201–7.
Acknowledgements
The authors thank very much Dr. Jianping Lu and Dr. Minjie Wang from Changhai Hospital of Shanghai, Radiology Department, for the great help with the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
About this article
Cite this article
Ding, A., Qiu, G., Lin, W. et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in ischemia-causing coronary stenosis: a meta-analysis. Jpn J Radiol 34, 795–808 (2016). https://doi.org/10.1007/s11604-016-0589-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-016-0589-4