Abstract
Objectives
To compare the reproducibility of cardiovascular magnetic resonance feature-tracking (CMR-FT) packages to assess global left ventricular (LV) myocardial strain.
Methods
In 45 subjects (i.e. 15 controls, 15 acute myocardial infarction, 15 dilated cardiomyopathy patients), we determined inter-vendor, inter-observer (two readers) and intra-observer reproducibility of peak systolic global radial, circumferential and longitudinal strain (GRS, GCS and GLS, respectively) comparing four commercially available software packages. Differences between vendors were assessed with analysis of variance (ANOVA), between observers and readings with intraclass correlation coefficient (ICC) and coefficient of variation (CV).
Results
The normalised end-diastolic volume was 91, 77 and 119 ml/m2 (median, Q1, Q3) and ejection fraction was 41 ± 14%, range 12-67%. Global longitudinal strain (GLS), global circumferential strain (GCS) and global radial strain (GRS) values were 13.9% ± 5.4% (3.9-23.8%), 12.2% ± 5.8% (1.0-25.1%) and 32.0% ± 14.7 (3.6-67.8%), respectively. ANOVA showed significant differences between vendors for GRS (p < 0.001) and GLS (p = 0.018), not for GCS (p = 0.379). No significant bias was found for both intra- and inter-observer variability. The ICC for inter- and intra-observer reproducibility ranged 0.828-0.991 and 0.902-0.997, respectively. The CV, however, ranged considerably, i.e. 4.0-28.8% and 2.8- 27.7% for inter- and intra-observer reproducibility, respectively. In particular, for GRS differences in CV values between vendors were large, i.e. 5.2-28.8% and 2.8-27.7%, for inter- and intra-observer reproducibility, respectively.
Conclusions
In a cohort of subjects with a wide range of cardiac performances, GRS and GLS values are not interchangeable between vendors. Moreover, although intra- and inter-observer reproducibility amongst vendors is excellent, some vendors encounter problems to reproducibly measure global radial strain.
Key Points
• Different software packages are currently available for myocardial strain assessment using routinely acquired cine CMR images.
• Global myocardial strain values are not interchangeable between vendors for global longitudinal and global radial strain.
• Inter- and intra-observer reproducibility for global strain assessment is excellent. However, some vendors encounter problems to reproducibly measure global radial strain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Assessment of ventricular performance has become a key parameter to appraise cardiac disease severity, to evaluate therapeutic response and to risk stratify patients [1]. Whereas ejection fraction (EF) is a well-established and generally accepted parameter to appreciate global ventricular systolic performance, it fails to provide data with regard to regional contractility and lacks sensitivity to depict subtle alternations in ventricular function [2]. Alternatively, myocardial strain, defined as the percentage change of myocardial dimension in a specific direction, is a reliable parameter to assess myocardial performance and has shown to be an early marker of systolic dysfunction [3, 4].
Although speckle tracking echocardiography is the reference to assess myocardial strain, in recent years cardiovascular magnetic resonance (CMR) feature tracking (FT) has become an appealing technique for myocardial strain assessment as well [5,6,7]. Cine steady-state free-precession images, which are part of a standard study CMR protocol, can be used for CMR-FT based myocardial strain analysis and quantification. An advantage of this approach is that the technique does not require additional CMR sequences, such as myocardial tagging, but can be applied to standard cine steady-state free-precession exams, thereby leaving the potential to retrospectively analyse strain in patients in whom no specific strain image acquisition sequence was performed [8]. Currently, several vendors provide dedicated CMR-FT analysis software. Although several papers have been published providing data with regard to intra- and inter-observer reproducibility of the different software packages, to our knowledge only two studies so far have focused on the inter-vendor agreement in selective populations, i.e. healthy volunteers undergoing dobutamine stress CMR, and segmental RV strain analysis in patients with overt or preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy [9,10,11,12,13,14,15,16]. Therefore, it was our aim to evaluate intra-/inter-observer, and inter-vendor agreement of four commercially available software packages including subjects with a wide variation in cardiac performance. For the present study, we used TomTec, Medis, Circle cvi42 and Segment Medviso. For the purposes of this study, the different software tools are referred to as “TomTec”, “Circle”, “Medis” and “Medviso”. TomTec, Medis and Circle rely on optical flow technology to discriminate ventricular boundaries, while Medviso uses a non-rigid, elastic algorithm for image registration and segmentation [9, 17,18,19].
Methods
Study population
From the CMR database, we selected a cohort of 45 patients (all CMR studies were performed in 2016 or later). A first group (n = 15) yielded normal CMR findings, i.e. LV ejection fraction ≥ 50%, end-diastolic LV wall thickness ≤12 mm and no myocardial enhancement at late gadolinium enhancement imaging (control group). A second group consisted of 15 patients with a reperfused acute ST-elevation myocardial infarction (infarct group). A third group consisted of 15 patients with a dilated cardiomyopathy (DCM group) (see Table 1). More information with regard to the specifics of the three groups can be found in the Electronic supplementary material (ESM). Only CMR studies were selected yielding a good to excellent image quality, as judged by an experienced reader >10 years of CMR experience. All subjects were in sinus rhythm at the time of the CMR exam. The study protocol conformed to the Declaration of Helsinki and the study was approved by the ethical committee of our hospital (S51789). Because of the retrospective nature of this study, patient informed consent was waived.
Cardiovascular magnetic resonance acquisition
All CMR studies were performed on a 1.5-T unit (Ingenia; Philips Healthcare, Erlangen, Germany) by using commercially available CMR imaging software, electrocardiographic triggering, and a cardiac-dedicated phase-array coil. For assessment of LV dimensions and function steady-state free-precession breath-hold cine images were acquired in the following orientations: vertical and horizontal long-axis, short-axis and left ventricular (LV) outflow tract view. Standard parameters were: repetition time/echo time, 3.6/1.8 ms; sense factor, 2, flip angle, 60°; section thickness, 8 mm; matrix, 160 × 256; field of view, 300 mm; pixel size, 1.6 × 1.6 mm; number of phases, 30; phase percentage, 67%. The vertical long-axis was determined on the transverse images by positioning an image plane connecting the middle of the mitral valve with the LV apex. On the vertical long-axis, the same anatomical landmark points were used to define the horizontal long-axis plane. The cardiac short-axis was defined on the horizontal long-axis using an image plane perpendicular to the interventricular septum. Care was taken to position the most basal short-axis slice at end-diastole exactly through the mitral valve ring. The set of short-axis images encompassed the left ventricle entirely. Between slices a gap of 2 mm was used. Per breath-hold, two short-axis CMR slices were acquired. For the late gadolinium enhancement studies, a dose of 0.15 mL of gadobutrol (Gadovist; Bayer, Leverkusen, Germany) per kilogram of body weight, was administered, and we used breath-hold T1-weighted three-dimensional contrast-enhanced phase-sensitive inversion-recovery (PSIR) gradient-echo sequence in cardiac short-axis, vertical long-axis and horizontal long-axis view (TR, 4.8 ms; TE, 2.3 ms; flip angle, 15°; PSIR flip angle, 5°; acquisition matrix, 168 × 159 reconstructed to 256 × 256 × 20; parallel imaging acceleration factor, 2.5 and 2; fold-over suppression, 2 × 25 mm; BW, 365 Hz; FOV, 320 × 320 × 100 mm; pixel size; 1.9 × 2.0 × 5 mm; reconstructed pixel size 1.3 × 1.3 × 5.0 mm). PSIR-images were acquired 10-15 min post-contrast administration. To reduce total acquisition time, we performed cine imaging in SA and LV outflow tract after contrast administration using the available time before start of late gadolinium enhancement imaging.
Ventricular volumes and function
All images were sent to an off-line workstation and analysed using commercially available software package (ViewForum; Philips Medical Systems, Best, Netherlands) by a single observer with more than 2 years of experience in CMR (MBP). LV volumes, mass and function were measured using manual planimetry of the endocardial and epicardial borders from the short-axis set at end-diastole and end-systole (see Table 1 and ESM). The papillary muscles were considered part of the LV cavity. In the dilated cardiomyopathy group, presence of ventricular asynchrony and apical rocking were visually scored. A similar visual approach was used to score the presence of left valvar disease. Late gadolinium enhancement images were analysed for the presence of myocardial enhancement, and the number of segments was quantified using the 17-segment classification as defined by the American Heart Association [20]. In the infarct group the location of enhancement was determined as anteroseptal, inferior or lateral.
LV myocardial feature tracking analysis
For myocardial feature tracking analysis cine images were uploaded in the different CMR-FT software packages, i.e. (1) TomTec Arena (ver. 1.3.0.124), (2) QStrain Medis (ver. 2.0.12.0), (3) Circle cvi42 (ver. 5.5) and (4) Segment Medviso (ver. 2.0 R4988). In a first step, in cardiac short-axis, a basal, mid and apical LV slice was defined for each patient which was used to calculate global circumferential and radial myocardial strain (GCS and GRS, respectively). Global long-axis strain (GLS) calculation was performed in vertical and horizontal long-axis and in the LV outflow tract view. The analysis of cine images was performed as recommended by the different vendor user manuals. In cardiac short-axis all software packages required drawing of an endo- and epicardial contour. In long-axis direction, TomTec and Medis required only an endocardial contour while Circle and Medviso required drawing of an endo- and epicardial contour. According to the user manual, Medviso and Circle required drawing of LV contours on an end-diastolic image, Medis and TomTec drawing of contours on an end-systolic image with subsequent tracking of the contours over the remainder of the images. If tracking was suboptimal, Medviso allowed adaptation of the contours on the (initial) end-diastolic image whereas the other packages allowed end-diastolic and end-systolic contour correction before re-running the tracking analysis. Moreover, Circle allowed if the strain borders visible in the overlay were suboptimal to draw contours in a different cardiac phase, before re-running the analysis. All CMR studies were analysed by two skilled CMR readers (M.B.P. and D.C.), one reader (M.B.P.) repeated the analyses respecting an interval of one week between repeated analyses. Before starting the CMR-FT analyses in the study group, both readers performed together analyses with the different software packages in five subjects not belonging to the study group. To compare the practical performance of the different software packages, we measured the total time employed, the number of mouse clicks (Mousotron, Blacksun software) and the number of tracking corrections needed in short-axis and long-axis direction. These analyses were performed in ten randomly selected subjects.
Statistics
Summary statistics for continuous variables are presented as mean ± standard deviation (SD) or medians with interquartile ranges (IQR), as appropriate. Categorical variables are presented as numbers with percentages in parentheses. Differences in clinical characteristics and CMR findings between the three groups were tested with one-way analysis of variance (ANOVA) with post hoc unpaired t-tests with Bonferroni correction. Differences in post-processing time and in global myocardial strain values between the different vendors were tested with repeated measurements ANOVA with post hoc paired t-tests with Bonferroni correction. Because there is no gold-standard measure of global myocardial strain, the measurements of each vendor were compared with the average global myocardial strain of the four vendors using Bland-Altman analysis with 95% limits of agreement. Differences between vendors were analysed with one-way ANOVA with post hoc paired t-tests with Bonferroni correction. For this analysis, the results of the first reading of the first reader (M.B.P.) were used. Inter-observer (two readers) and intra-observer (two readings) reproducibility for the different vendors were assessed by using the absolute mean error, intraclass correlation coefficients (ICCs), and the coefficient of variation (CV). Reproducibility was defined as poor (ICC <0.4), good (0.4< ICC <0.75) or excellent (ICC >0.75) [21]. The CV was calculated as the SD of the differences between repeated analyses normalised to the mean. A p value <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed by using R Statistical Software v.3.4.0 (Foundation for Statistical Computing) and SPSS Statistics v.20.0 (IBM Corp).
Results
Mean age of the study population was 55 ± 14 years (range, 25-79 years; 58% males) with a large range of LV volumes and LV EF (Table 1). GLS, GCS, and GRS values were -13.9% ± 5.4% (range, -23.8% to -3.9%), -12.2% ± 5.8% (range, -25.1 to -1.0%), and 32.0% ± 14.7% (range, 3.6-67.8%), respectively. Amongst vendors, we found significant differences with regard to the time employed and number of mouse clicks needed to analyse LV strain in short- and long-axis direction (Table 2). TomTec and Medis were significantly faster and required a lower number of mouse clicks than the two other vendors (p < 0.01). Also, the number of tracking corrections differed amongst vendors. Overall, Circle needed most contour corrections for adequate tracking. Medviso needed initial tracking corrections in particular on long-axis cine images at the basal LV segments. Figure 1 shows the strain overlay at end-diastole and peak-systole in a control subject by the different vendors (see also online video 1-4). In Fig. 2 is shown an example of the strain-time curve obtained with the different CMR-FT algorithms in a control subject, an infarct, and DCM patient.
Strain versus time curves obtained with the four different software packages in a normal subject, an DCM and infarct patient. Medviso (green), Circle (blue), TomTec (purple) and Medis (red). All values in the y-axis represent percentages (%). DCM dilated cardiomyopathy, GCS global circumferential strain, GLS global longitudinal strain, GRS global radial strain
Inter-vendor reproducibility
The mean global strain values per vendor are summarised in Fig. 3. Bland-Altman analyses with 95% limits of agreement confirmed no significant bias between the different vendors and the mean of all vendors (Fig. 4). ANOVA showed significant differences between vendors for GRS (p < 0.001) and GLS (p = 0.018), not for GCS (p = 0.379). For GRS, post-hoc analysis showed significant differences between Medviso on the one hand and TomTec and Medis on the other hand, and between Circle and TomTec (Fig. 3). For GLS, post-hoc analysis showed significant differences between Medviso on the one hand and TomTec and Medis on the other hand (Fig. 3).
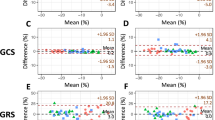
Intra-observer and inter-observer reproducibility
No significant bias was found for both intra- and inter-observer reproducibility for all four software packages (Figs. 5 and 6, Table 3). ICC for inter- and intra-observer reproducibility ranged from 0.828 to 0.991 and 0.902 to 0.997, respectively. Medviso showed the lowest CV ranging from 2.8 to 5.0% for intra-observer reproducibility and 4.0 to 5.2% for inter-observer reproducibility. The other vendors showed higher CV values. In particular GRS measured by TomTec and Medis showed high CV values (up to 28.8%).
Discussion
As CMR-FT software is increasingly used to assess myocardial strain as part of a comprehensive CMR exam, it is essential to appraise how accurate and reproducible the different CMR-FT software packages are to assess myocardial strain. As we had no “gold standard” (e.g. calibrated cardiac phantom) to assess accuracy, only the reproducibility issue could be addressed which, however, is crucial when it comes to repeated studies or when comparing strain values obtained by different vendors, readers or readings. Our study results show significant differences for global myocardial strain measurements between vendors, in particular for assessment of global longitudinal and radial strain. With regard to intra- and inter-observer reproducibility, overall all packages perform very well (ICC > 0.75) [21]. While Medviso is superior with the highest ICC and lowest CV values, some vendors suffer to reproducibly measure global radial strain.
Currently, several vendors offer CMR-FT dedicated software, but only a few studies have been reported on the inter-vendor agreement [9, 16]. For the current study, we compared four CMR-FT algorithms commercialised by TomTec, Medis, Circle and Medviso. The first three vendors use optical flow technology for CMR-FT. Following contouring of a first, reliable image, the traced contours are then followed in time by searching the same features in the following frames. The tracked features can be the myocardial-cavity boundary or anatomical elements that are different along the tissue [6]. Both TomTec and Medis use Advanced Medical Imaging Development (AMID) software for their CMR-FT program. The fourth vendor, Medviso, uses a non-rigid, elastic algorithm for segmentation and registration for myocardial strain quantification [17,18,19]. Although Bland-Altman analyses showed no bias between the different vendors and the mean of all vendors, ANOVA showed significant differences in GRS and GLS between vendors. For GRS, Medviso yielded significantly lower values than TomTec and Medis, while Circle yielded lower values than TomTec (Fig. 3). A similar finding was noted for GLS with lower strain values for Medviso compared to TomTec and Medis. With regard to the inter- and intra-observer agreement, all software packages perform excellent with regard to circumferential and longitudinal strain measurement, but some of them (Medis, TomTec) suffer to reproducibly measure radial strain. A clear-cut explanation for these observations is not available, but as Medis and TomTec use a similar algorithm, it is plausible that this algorithm performs less well for radial strain measurement. Previous studies on CMR-FT reproducibility—mainly using TomTec software—showed similar findings [9, 15, 22]. Most likely, small differences in the manual contouring of endo/epicardial borders between readers or readings has a major impact on radial strain values, whereas the impact on circumferential/longitudinal strain reproducibility is much smaller. Here, Medviso outperforms clearly the other packages in terms of inter- and intra-observer reproducibility, with excellent ICC values and CV values of 5% or lower. This suggests that a non-rigid algorithm is likely superior to the optical flow methods to reproducibly trace contours over time, independent of the reading/reader, as well as the level of experience of the reader as recently reported by Morais et al. [19] comparing the impact of observer experience on CMR-FT reproducibility using a non-rigid algorithm.
We found significant differences in total analysis time between vendors—the shortest for TomTec and Medis—which is likely related to the design of the contouring program (e.g. number of clicks needed), the number and need for contour correction, and to a large extent to the complexity of the used CMR-FT algorithm (e.g. blood-myocardial border tracing versus non-rigid, elastic registration algorithm). TomTec and Medis do not need epicardial contouring on long-axis and require contour tracing of short-axis images at end systole yielding good tracking propagation towards end diastole, with only minimal corrections. In contrast, Medviso and Circle necessitate both endocardial and epicardial contouring at end diastole rather than end systole, substantially increasing the number of mouse clicks. The Circle software often needed substantial contour adjustments, while Medviso needed regularly corrections at the basal LV borders in long-axis direction. Finally, the time needed to perform the strain analysis is significantly longer for Medviso (>50 s on long-axis and >100 s on short-axis) compared to the other software packages (all <10 s).
Limitations
To assess the reproducibility of different CMR-FT packages, and in contrast to most previous studies, we selected a cohort of patients with a wide range of cardiac performances, including patients with normal findings at CMR (‘control group’) as well as patients with focal pathology (‘acute myocardial infarction group’) and diffuse pathology (‘dilated cardiomyopathy group’). The control group does not necessarily imply that this group can be considered as a normal group as all subjects were referred for magnetic resonance imaging (MRI) with complaints (see ESM). Moreover, we cannot extrapolate our findings to other cardiac pathologies such as patients presenting thick-walled left ventricles (e.g. hypertrophic cardiomyopathy, cardiac amyloidosis). Kuetting et al. [12] reported, using TomTec software, lower myocardial strains and impeded reproducibility on post-contrast cine images as a result of the blood-myocardium contrast. In our routine clinical setting, we perform part of the cine studies post-contrast administration. The rationale is twofold, first to shorten total imaging time using the time between intravenous contrast administration and LGE imaging to perform cine imaging in short-axis and LV outflow tract, and second because of the improved tissue characterisation of contrast-enhanced cine images in the presence of focal myocardial pathology such as acute myocardial infarction [16, 23, 24]. As our study was not designed to address this issue, we cannot appropriately evaluate the impact of contrast administration on strain values and reproducibility by the different vendors. In the present study, we reported only on global myocardial strain. As assessment of segmental strain is important as well, certainly in the presence focal pathology, studies comparing this issue amongst vendors is crucial too. A recent study by Bourfiss et al. [16] studying RV myocardial strain in subjects with preclinical presentation of arrhythmogenic right ventricular cardiomyopathy/dysplasia with a similar set-up (comparing four vendors) showed that segmental strain values are not interchangeable between vendors. Similarly, recent work in the field of speckle tracking echocardiography in patients with a history of previous myocardial infarction has shown substantial bias in segmental strain calculation amongst vendors [25, 26]. Finally, to fully appreciate the reproducibility of strain measurements, not only the different CMR-FT tracking software algorithms should be compared but also the impact of the source images should be addressed comparing the different CMR vendors, such an initiative was recently realised in the field of speckle tracking strain imaging [27].
Conclusions
In conclusion, comparing four commercially available software package for CMR-FT analysis in a cohort of subjects with a wide range of cardiac performances, we found that global myocardial strain values, in particular longitudinal and radial strain values, are not interchangeable between vendors, and that inter-observer and intra-observer reproducibility for GRS remains challenging for some vendors.
Abbreviations
- CMR:
-
Cardiovascular magnetic resonance
- CV:
-
Coefficient of variation
- DCM:
-
Dilated cardiomyopathy
- EF:
-
Ejection fraction
- FT:
-
Feature-tracking
- GCS:
-
Global circumferential strain
- GLS:
-
Global longitudinal strain
- GRS:
-
Global radial strain
- LV:
-
Left ventricle
References
Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S (2016) Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 37:1196–1207
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100:1673–1680
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63:2751–2768
Luetkens JA, Schlesinger-Irsch U, Kuetting DL et al (2017) Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 27:4661–4671
Maret E, Todt T, Brudin L et al (2009) Functional measurements based on feature tracking of cine magnetic resonance images identify left ventricular segments with myocardial scar. Cardiovasc Ultrasound 7:53
Hor KN, Baumann R, Pedrizzetti G et al (2011) Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp 48:e2356
Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S (2016) Cardiovascular magnetic resonance myocardial feature tracking. Concepts and clinical applications. Circ Cardiovasc Imaging 9:e0004077
Zweerink A, Allaart CP, Kuijer JPA et al (2017) Strain analysis in CRT candidates using the novel segment length in cine (SLICE) post-processing technique on standard CMR cine images. Eur Radiol 27:5158–5168
Schuster A, Stahnke V-C, Unterberg-Buchwald C et al (2015) Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol 70:989–998
Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E (2012) Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson 14:43
Singh A, Steadman CD, Khan JN et al (2015) Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 41:1129–1137
Kuetting DL, Dabir D, Homsi R et al (2016) The effects of extracellular contrast agent (Gadobutrol) on the precision and reproducibility of cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 18:30
Kowallick JT, Morton G, Lamata P et al (2016) Inter-study variability of left ventricular torsion and torsion rate quantification using MR myocardial feature tracking. J Magn Reson Imaging 43:128–137
Aurich M, Keller M, Greiner S et al (2016) Left ventricular mechanics assessed by two-dimensional echocardiography and cardiac magnetic resonance imaging: comparison of high-resolution speckle tracking and feature tracking. Eur Heart J Cardiovasc Imaging 17:1370–1378
Taylor RJ, Moody WE, Umar F et al (2015) Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 16:871–881
Bourfiss M, Vigneault DM, Aliyari Ghasebeh MA et al (2017) Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy: a multisoftware feasibility and clinical implementation study. J Cardiovasc Magn Reson 19:66
Heyde B, Bouchez S, Thieren S et al (2013) Elastic image registration to quantify 3-D regional myocardial deformation from volumetric ultrasound: experimental validation in an animal model. Ultrasound Med Biol 39:1688–1697
Morais P, Heyde B, Barbosa D, Queirós S, Claus P, D’hooge J (2013) Cardiac motion and deformation estimation from tagged MRI sequences using a temporal coherent image registration framework. International Conference on Functional Imaging and Modeling of the Heart. Springer, Berlin Heidelberg
Morais P, Marchi A, Bogaert JA et al (2017) Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm. Assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson 19:24
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105:539–542
Fleiss JL, Levin B, Paik MC (2003) Statistical methods for rates and proportions, 3rd edn. Wiley, Hoboken
Schmidt B, Dick A, Treutlein M et al (2017) Intra- and inter-observer reproducibility of global and regional magnetic resonance feature tracking derived strain parameters of the left and right ventricle. Eur J Radiol 89:97–105
Masci PG, Dymarkowski S, Rademakers FE, Bogaert J (2009) Determination of regional ejection fraction in patients with myocardial infarction by using merged late gadolinium enhancement and cine MR: feasibility study. Radiology 250:50–60
Nordlund D, Heiberg E, Carlsson M et al (2016) Extent of myocardium at risk for left anterior descending artery, right coronary artery, and left circumflex artery occlusion depicted by contrast-enhanced steady-state free precession and T2-weighted short-tau inversion recovery magnetic resonance imaging. Circ Cardiovasc Imaging 9(7)
Mirea O, Pagourelias ED, Duchenne J et al (2018) Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 11:25–34
Mirea O, Pagourelias ED, Duchenne J et al (2018) Variability and reproducibility of segmental longitudinal strain: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 11(1):15–24
Farsalinos KE, Daraban AM, Ûnlü S, Thomas JD, Badano LP, Voigt JU (2015) Head-to-head comparison of global longitudinal strain measurements among nine different vendors. The EACVI/ASE Inter-vendor comparison study. J Am Soc Echocardiogr 28:1171–1181
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jan Bogaert
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• cross sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Barreiro-Pérez, M., Curione, D., Symons, R. et al. Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR-feature tracking algorithms. Eur Radiol 28, 5137–5147 (2018). https://doi.org/10.1007/s00330-018-5538-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5538-4