Abstract
Objectives
Our aim was to evaluate the inter-vendor reproducibility of cardiovascular MR feature tracking (CMR-FT) for the measurement of segmental strain (SS) of the left ventricle (LV) as well as to test the accuracy of CMR-FT to detect regional myocardial pathology.
Methods
We selected 45 patients: 15 with normal CMR findings, 15 with dilated cardiomyopathy, and 15 with acute myocardial infarction. Segmental longitudinal, circumferential, and radial strains were assessed with 4 different software. The inter-vendor difference as well as intra- and inter-observer variability was investigated. Furthermore, the accuracy of CMR-FT for the detection of structural (infarcted segments) as well as functional pathology (septal vs. lateral wall strain in left bundle branch block) was tested.
Results
Between vendors, there were significant differences in values for all strains (p < 0.001). The software using a non-rigid algorithm for image registration and segmentation demonstrated the best intra- as well as inter-observer variability with interclass correlation coefficient (ICC) > 0.962 and coefficient of variation (CV) < 24%. For infarct location, the same software yielded the highest area under the curve values for radial and circumferential SS (0.872 and 0.859, respectively). One of the other three software using optical flow technology performed best for longitudinal SS (0.799) and showed the largest differences in SS between septum and lateral wall in the dilated cardiomyopathy group.
Conclusion
SS values obtained by CMR-FT are not interchangeable between vendors, and intra- and inter-observer reproducibility shows substantial variability among vendors. Overall, the different packages score relatively well to depict focal structural or functional LV pathology.
Key Points
• Segmental myocardial strain values obtained by CMR feature tracking are not interchangeable between different vendors.
• Intra- and inter-observer reproducibility shows substantial variability among vendors.
• Segmental myocardial strains measured by CMR feature tracking score relatively well to depict focal structural or functional LV pathology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The assessment of left ventricular (LV) function has moved from simple measurement of volume changes to more advanced techniques, which tries to assess the intrinsic myocardial contractility—i.e., myocardial strain—in order to be able to accurately characterize cardiac mechanics. The measurement of strain in a myocardial segment is defined as the percentage change from the initial dimension, in a given direction (longitudinal, circumferential, radial) throughout the cardiac cycle, and is widely used with 2D speckle tracking, in echocardiography [1, 2].
Global longitudinal strain measurement has proven to yield prognostic value [3,4,5] as well as the capacity to detect myocardial dysfunction earlier than standard parameters of cardiac function like ejection fraction [6, 7].
Although strain measurements have been proven to be robust, studies still show large inter-vendor variability [8,9,10] hampering the clinical applicability and stressing the importance of standardization.
Cardiac magnetic resonance (CMR) is considered the gold standard for evaluation of global ventricular function, in addition to its excellent capability to detect focal myocardial pathologies such as necrosis and/or fibrosis. In analogy to cardiac ultrasound, feature tracking (FT)–based software packages using routinely acquired steady-state free-precession cine images have become available, allowing to quantify longitudinal, circumferential, and radial myocardial strain [11, 12]. Whereas most vendors (e.g., TomTec, Medis, and Circle) use optical flow technology to track “features,” i.e., ventricular boundaries; others such as Medviso use non-rigid algorithm for image registration and segmentation [13, 14]. In brief, optical flow uses the difference in signal intensity between myocardium and surrounding structures to compute myocardial strain, whereas an elastic, non-rigid algorithm estimates myocardial strain curves by computing inter-frame deformation fields between images using a B-spline tensor product transform [14]. Although the number of papers investigating the reproducibility of CMR-FT to assess global myocardial strain is growing, only a few have assessed how well the CMR-FT packages score to measure segmental myocardial strain [10, 13, 15]. In a recent study by Bourfiss et al in patients with arrhythmogenic cardiomyopathy, large differences and poor inter-vendor correlation were shown with regard to segment RV strain [15]. The first aim of the present study was to compare segmental LV values obtained by four different commercially available CMR-FT packages studying a representative study group, including patients with normal CMR findings, patients with focal LV pathology (i.e., acute myocardial infarction), and patients with diffuse LV pathology (i.e., dilated cardiomyopathy). The second aim was to test the accuracy of the different CMR-FT software packages to detect regional myocardial pathology.
Materials and methods
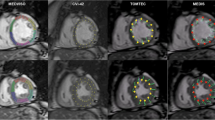
This study is a continuation of the paper recently published by our group on the inter-vendor CMR-FT differences in global strain measurements [10]. For the current study, we compared four commercially available software packages, i.e., (a) TomTec Arena (v. 1.3.0.124); (b) QStrain Medis (v. 2.0.12.0); (c) Circle cvi42 (v. 5.5), and (d) Segment Medviso (v. 2.0 R4988), which we will refer to as “TomTec,” “Medis,” “Circle,” and “Medviso,” respectively.
Study population
The same data-set was used as we reported before on the inter-vendor reproducibility of global LV myocardial strain [10]. In summary, a CMR database search was conducted on studies performed between 2016 and 2017, selecting those with a good to excellent image quality and sinus rhythm. Furthermore, 3 population groups were chosen, each composed of 15 subjects, generating a total study population of 45 patients. The first group included patients with normal CMR findings, i.e., LV ejection fraction ≥ 50%, end-diastolic LV wall thickness ≤ 12 mm, and no myocardial enhancement at late gadolinium enhancement imaging (control group). The other two groups were comprised of patients with myocardial pathology: a group of patients with dilated cardiomyopathy (DCM group) and finally patients with reperfused acute ST- elevation infarction (infarct group) (see Table 1). The CMR studies in the infarct group were performed 3 ± 2 days (range, 2–7 days) after the acute event. Dilated cardiomyopathy was defined as LV dilatation and dysfunction in the absence of coronary artery disease or severe valvular disease. Nine patients from the DCM group had a left bundle branch block (LBBB), with a mean QRS duration of 153 ± 20 ms. The study was conducted in accordance with the Declaration of Helsinki and was approved by the local research ethics committee.
Image acquisition and analysis
All CMR studies were performed on a 1.5-T unit (Ingenia; Philips Healthcare) by using commercially available CMR imaging software, electrocardiographic triggering, and a cardiac-dedicated phase-array coil.
For a detailed description of the image acquisition parameters, we refer to our paper on inter-vendor global strain measurements [10]. Balanced steady-state free-precession breath-hold cine images were acquired in the 3 long-axis views: vertical and horizontal long-axes and left ventricular (LV) outflow tract view. Furthermore, after contrast injection, a set of short-axis images with a between-slice gap of 2 mm, encompassing the left ventricle entirely, was performed.
For the late gadolinium enhancement studies, a dose of 0.15 mL of gadobutrol (Gadovist; Bayer) per kilogram of body weight was administered. At 10–15 min post-contrast administration, T1-weighted three-dimensional contrast-enhanced phase-sensitive inversion-recovery (PSIR) gradient-echo sequences were performed in cardiac short-axis, vertical long-axis, and horizontal long-axis view.
Standard image analysis for ventricular function, dimensions, and mass was performed by an experienced observer (MBP) utilizing commercially available software package (ViewForum; Philips Medical Systems). The papillary muscles were considered part of the LV cavity.
In the DCM group, the presence of apical rocking and septal flash, which suggest asynchrony, were visually assessed. Late gadolinium enhancement images were analyzed for the presence of myocardial enhancement, and the number of segments was quantified using the 17-segment classification as defined by the American Heart Association [16].
LV myocardial feature tracking analysis
For myocardial feature tracking analysis, cine images were uploaded in the different CMR-FT software packages. The analysis of cine images was performed as recommended by the different vendor user manuals. In a first step, in cardiac short-axis, a basal, mid, and apical LV slice was defined for each patient which was used to measure segmental circumferential and radial myocardial strain (SCS and SRS, respectively). Segmental longitudinal strain (SLS) tracing was performed in vertical and horizontal long-axes and in the LV outflow tract view. A 17-segment model of the LV was used. The software reported the strain values for each segment after the tracings of all 3 long-axis views were performed. Supplementary Table 1 shows the characteristics of tracings for the 4 vendors. All CMR studies were analyzed by two skilled CMR readers (M.B.P. and D.C.); one reader (M.B.P.) repeated the analyses after an interval of 1 week. For segmental strain, the end-systolic strains are reported for all vendors.
Statistical analysis
Statistical analysis was performed with SPSS Statistics software version 25 (IBM SPSS, Inc.) and MedCalc version 1.8 (MedCalc Software bvba). Continuous variables are presented as mean ± standard deviation (SD). Categorical variables are presented as numbers with percentages in parentheses.
The differences in clinical characteristics and CMR findings among the three groups were tested with one-way analysis of variance and post hoc unpaired t test with the Bonferroni correction.
The inter-vendor difference we assessed using repeated-measures analysis of variance with the Bonferroni correction for the post hoc pairwise comparison on the first readings of the first reader (M.B.P.). In order to have a visual depiction of the range of values obtained with all vendors, a Bland-Altman analysis was used, comparing the measurements obtained for each vendor to the mean of all vendors.
Intra-observer (2 readings) and inter-observer (two readers) variability were assessed using interclass correlation coefficient (ICC) and coefficient of variation (CV). Reproducibility was defined as poor (ICC < 0.4), good (0.4 < ICC < 0.75), or excellent (ICC > 0.75). The CV was calculated as the SD of the differences between repeated analyses normalized to the mean of the two values. The Bland-Altman analysis with 95% confidence intervals was constructed in order to visualize inter- and intra-observer variability.
Subgroup analysis was performed in the DCM group with LBBB (i.e., 9/15 patients), to compare inter-vendor differences in septal (antero- + inferoseptal wall) versus lateral wall (antero- + inferolateral wall) strains and in the infarct group, to compare strain values in segments with transmural enhancement (late gadolinium enhancement ≥ 75% of the wall width), versus normal, non-infarcted segments. Our patient population displayed mostly transmural infarcted segments, with only 1 segment showing isolated subendocardial scar, thus not permitting further testing of the ability of FT to distinguish degrees of transmurality. For this analysis, a repeated-measures analysis of variance with the Bonferroni correction for the post hoc pairwise comparison was used. The values obtained for each vendor for normal versus pathologic segments were compared with paired t tests. Receiver operating characteristic (ROC) curves were constructed for patients of the infarct group and the ability to discriminate transmural scar from normal segments was tested among vendors using the DeLong test.
A p value < 0.05 was considered to indicate statistical significance.
Results
Inter-vendor variability
Longitudinal, circumferential, and radial segmental strain measurements showed significant inter-vendor differences (p < 0.001, p = 0.005, and p < 0.001, respectively) (Fig. 1). Medviso consistently exhibited the lowest mean values in all strain directions, while TomTec and Medis displayed similar results for all measurements.
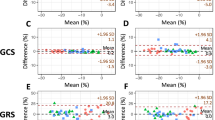
The Bland-Altman plots comparing the different vendors to the mean of all vendors are depicted in Fig. 2. Medis and TomTec demonstrated the narrowest confidence interval (CI) for SLS, while Medviso performed best with SRS, all these 3 vendors having similar CI when compared with the mean of all vendors for SCS. Circle displayed a wide CI for all three strain types measured.
Bland-Altman plots constructed for each vendor compared with the mean of all vendors, for all the three strain types. The horizontal green line depicts the mean; the 2 red lines depict the upper and lower limits of agreement (+ 1.96 SD and − 1.96, respectively). SLS, segmental longitudinal strain; SCS, segmental circumferential strain; SRS, segmental radial strain; DCM, dilated cardiomyopathy
Intra-observer and inter-observer reproducibility
Medviso demonstrated excellent intra- and inter-observer reproducibility for longitudinal circumferential as well as radial strain (ICC > 0.955) (Fig. 3a, b). TomTec and Medis also presented very good results, especially for longitudinal and circumferential strain (ICC values > 0.910 and > 0.930, respectively). Circle exhibited lower, but still good intra- and inter-observer reproducibility (ICC > 0.715) but a high coefficient of variability (up to 109%) (see Table 2). To better appreciate the reproducibility for segmental strain analysis throughout the left ventricle, we looked at the results per level, i.e., base (segments 1–6), mid (segments 7–12), and apical (segments 13–16) (Table 3). These results per level are highly consistent with the results for the entire left ventricle as mentioned above.
a Bland-Altman plot of intra-observer reproducibility, for all three strain types. The horizontal green line depicts the mean; the 2 red lines depict the upper and lower limits of agreement (+ 1.96 SD and − 1.96, respectively). b Bland-Altman plot of inter-observer reproducibility, for all three strain types. The horizontal green line depicts the mean; the 2 red lines depict the upper and lower limits of agreement (+ 1.96 SD and − 1.96, respectively). SLS, segmental longitudinal strain; SCS, segmental circumferential strain; SRS, segmental radial strain; DCM, dilated cardiomyopathy
Detection of focal myocardial pathology
All vendors displayed significant differences in segmental strain values between pathologic and normal segments (i.e., infarct group) and between septal and lateral strains in LBBB patients. The only exception was for SLS obtained by Medviso in the LBBB group (see Supplementary Tables 2 and 3). Circle showed the highest difference between normal and pathologic segments. Between vendors, there were significant differences in SLS, SCS, and SRS when measuring strain in infarcted versus normal segments (Fig. 4). The same was observed for strain measurement of the septal and lateral wall in patients with left bundle branch block (Fig. 5).
Infarct detection ability as shown by the ROC analysis showed moderate specificity and sensibility for longitudinal strain, but acceptable performance for circumferential and radial strain (see Fig. 6). Circle demonstrated significant better area under the curve (AUC) values than TomTec and Medis for longitudinal strain (0.799 versus 0.614 and 0.636, respectively). Medviso performed better than Circle for circumferential strain (AUC 0.859 versus 0.793). For radial strain, there was no significant inter-vendor difference in scar detection (values ranging from 0.810 to 0.872).
ROC curves, AUC, and 95% CI indicating the ability for transmural scar detection of the 4 vendors for SLS, SCS, and SRS ROC receiver operating characteristic. AUC, area under the curve; CI, confidence interval; SLS, segmental longitudinal strain; SCS, segmental circumferential strain; SRS, segmental radial strain
Discussion
The scope of the study was to assess how well the current commercially available software packages for CMR-FT perform to assess segmental LV strain in a selected group of patients with normal CMR findings or known with focal or with diffuse LV pathology. For this study, the same data-set was used as we reported before on the inter-vendor reproducibility of global LV myocardial strain [10]. The present study results show substantially higher CV and lower ICC values for all vendors when it comes to segmental strain analysis. Importantly, as mentioned for global strain analysis as well, segmental strain values are not interchangeable between vendors, and reproducibility is strongly influenced by the software package used for segmental strain analysis, regardless whether the results are expressed for the left ventricle or per level. With regard to the depiction of focal structural or functional LV pathology, however, most software packages perform reasonably well.
Although there are a fair number of publications on the CMR-FT reproducibility of global myocardial strain, studies on segmental strain are sparse. Kempny et al reported poor intra-observer agreement of segmental LV and right ventricular strain in a group of tetralogy of Fallot patients and normal volunteers using TomTec software with a CV of 29.6% for SLS, 23.7% for SCS, and 36.7% for SRS [17]. On the other hand, a study by Schmidt et al assessing patients with acute myocarditis and normal volunteers—also using similar software—showed good intra- and inter-observer reproducibility (ICCs range, 0.81–1.00 and 0.66–0.93, respectively) for longitudinal and circumferential strain but poor agreement for radial strain [18]. Only one study so far has assessed inter-vendor agreement for segmental myocardial strains, reporting low ICC values (0.373) for RV segmental strains in patients with preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy [15].
In a direct comparison with global strain reproducibility [10], segmental strain reproducibility is rather poor, i.e., CV 15–90% versus 2.8–27.7% and CV 16–109% versus 4.0–28.8% for intra- and inter-observer reproducibility, respectively. Moreover, these findings are consistent when the strain is assessed per LV level, i.e., base/mid/apex. Software packages that perform well for global strain analysis yield the best results for segmental strain analysis as well. Overall, Medviso performed the best, with an acceptable CV, especially for radial strain, i.e., CV of 15% and 16% for intra- and inter-observer reproducibility, respectively. TomTec and Medis showed reasonable CVs in line with previous reports, except for radial strain with CV values around 50%. Circle performed less well for segmental strain analysis with high CV values for all segmental strains. As discussed before, part of the observed differences is likely related to the software algorithm used for strain analysis, i.e., optical flow technology versus non-rigid, elastic algorithm. The lack of clear reference points—such as provided with myocardial tagging—hampers reliable tracking of regional deformation, and apparently the Medviso software is currently the most reliable with regard to this issue. As TomTec and Medis both use Advanced Medical Imaging Development software for CMR-FT, this likely explains the similarity in performance. Circle shows modest reliability and a high coefficient of variation, which were not noted for global strain. The widest confidence interval when compared with the mean of all vendors also suggests higher dispersions of values. This may be due to the fact that one can correct the tracings in all time frames, resulting in a more genuine tracking but inducing high variability between measures. We reported before the most contour corrections for Circle software [10].
The second aim of this study was to look at the discriminative power of segmental strain analysis, by selecting a group of patients with focal myocardial pathology, as well as patients with ventricular dyssynchrony due to LBBB. Our findings are in line with previous work. For instance, in patients with acute myocardial infarction, Khan et al reported an AUC of 0.772 and 0.572 for SCS and SLS, respectively [19]. Moreover, Schneeweis et al demonstrated a good performance of SCS for the detection of significant coronary artery disease during dobutamine stress testing [20]. Also, in patients with preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy, segmental strain measured by CMR-FT demonstrated a good discriminative ability to distinguish regional myocardial pathology [15]. Remarkably, in the present study, SRS and SCS performed better than SLS to detect infarcted segments. These values are in line with a recent inter-vendor study on the accuracy of longitudinal strain using echocardiographic speckle tracking to detect myocardial scar (i.e., AUC 0.74–0.83 compared to AUC 0.61 to 0.80 in our study) [21]. To assess how well the software packages perform to detect segmental strain differences in patients with inhomogeneous myocardial workload, we compared the strain differences in the septal and lateral LV wall in the subgroup of DCM patients presenting LBBB [22]. Indeed, in this small subgroup of 9 patients, we found significant differences in myocardial strain between the septal and lateral LV wall, suggesting that CMR-FT may be used to depict ventricular dyssynchrony. Circle consistently yielded the largest difference in strain values between the septal and lateral wall. The slight superiority shown in pathology detection for Circle may be explained by the large range of values but comes at the cost of a large amount of tracing correction and lack of reproducibility. This apparent superiority of Circle to the other software packages to discriminate focal LV pathology, however, needs some considerations. Remarkably, in the infarcted myocardium, Circle yields overall positive circumferential strains—i.e., systolic infarct bulging—which may be found in severe myocardial infarctions but in most infarcts, some circumferential strain is still preserved as shown by the three other vendors. A similar finding in the DCM group where Circle shows a mean circumferential septal stretch of 10.2% which does not seem realistic as most DCM patients had moderate to severe LV dilatation and dysfunction but not extreme forms. Again, the values obtained by the three other vendors seem more realistic.
The lack in discriminative power with regard to longitudinal strain in the DCM group for Medviso may be related to the overall lower strain values obtained by the non-rigid elastic algorithms, compared with the algorithms based on optical flow technology.
In addition to the limitations we mentioned in the paper on global strain reproducibility [10], with regard to segmental strain analysis, we would like to mention that part of the observed differences between vendors, readers or readings may be related to the differences how the 17 segments were defined. Small differences in segment definition may impact significantly reproducibility. Of all strains, apparently the radial strain is the most difficult to reproducibly measure for most software. The most likely explanation is that systolic strain in radial direction (i.e., systolic wall thickening) is in the order of millimeters. For instance, a myocardial wall with an end-diastolic wall thickness of 8 mm, thickening 50% during systole, means 4 mm in absolute values. With the current spatial resolution of CMR cine sequences, this makes strain assessment likely more challenging than in longitudinal or circumferential direction where strains are measured over a longer distance. Finally, although the subgroup of DCM patients with LBBB contained only nine patients, in each patient, we compared SS of the 6 septal segments with the 6 lateral segments, making a total of 54 septal and 54 lateral segments.
In conclusion, taking into account the moderate to high variability in segmental strain values with current CMR-FT packages, assessment of segmental myocardial LV strain remains challenging. Nevertheless, these packages have the reasonable discriminative ability for depicting regional pathology.
Abbreviations
- CMR-FT:
-
Cardiovascular magnetic resonance feature tracking
- CV:
-
Coefficient of variation
- ICC:
-
Interclass correlation coefficient
- LV:
-
Left ventricle
- SCS:
-
Segmental circumferential strain
- SLS:
-
Segmental longitudinal strain
- SRS:
-
Segmental radial strain
References
Voigt JU, Pedrizzetti G, Lysyansky P et al (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr 28(2):183–193
Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S (2016) Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J 37(15):1196–1207
Mignot A, Donal E, Zaroui A et al (2010) Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr 23:1019–1024
Stanton T, Leano R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2:356–364
Kalam K, Otahal P, Marwick TH (2014) Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart 100(21):1673–1680
Zamorano JL, Lancellotti P, Rodriguez Muñoz D et al (2016) 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J 37(36):2768–2801
Luetkens JA, Schlesinger-Irsch U, Kuetting DL et al (2017) Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 27(11):4661–4671
Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt JU (2015) Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr 28(10):1171–1181
Nagata Y, Takeuchi M, Mizukoshi K et al (2015) Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr 28(6):630–641
Barreiro-Pérez M, Curione D, Symons R, Claus P, Voigt JU, Bogaert J (2018) Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR-feature tracking algorithms. Eur Radiol 28(12):5137–5147
Hor KN, Baumann R, Pedrizzetti G et al (2011) Magnetic resonance derived myocardial strain assessment using feature tracking. J Vis Exp 12(48)
Schuster A, Hor KN, Kowallick JT, Beerbaum P, Kutty S (2016) Cardiovascular magnetic resonance myocardial feature tracking. Concepts and clinical applications. Circ Cardiovasc Imaging 9(4):e004077
Schuster A, Stahnke VC, Unterberg-Buchwald C et al (2015) Cardiovascular magnetic resonance feature-tracking assessment of myocardial mechanics: intervendor agreement and considerations regarding reproducibility. Clin Radiol 70(9):989–998
Morais P, Marchi A, Bogaert JA et al (2017) Cardiovascular magnetic resonance myocardial feature tracking using a non-rigid, elastic image registration algorithm. Assessment of variability in a real-life clinical setting. J Cardiovasc Magn Reson 19(1):24
Bourfiss M, Vigneault DM, Aliyari Ghasebeh MA et al (2017) Feature tracking CMR reveals abnormal strain in preclinical arrhythmogenic right ventricular dysplasia/cardiomyopathy: a multi-software feasibility and clinical implementation study. J Cardiovasc Magn Reson 19(1):66
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105(4):539–542
Kempny A, Fernández-Jiménez R, Orwat S et al (2012) Quantification of biventricular myocardial function using cardiac magnetic resonance feature tracking, endocardial border delineation and echocardiographic speckle tracking in patients with repaired tetralogy of Fallot and healthy controls. J Cardiovasc Magn Reson 31(14):32
Schmidt B, Dick A, Treutlein M et al (2017) Intra- and inter-observer reproducibility of global and regional magnetic resonance feature tracking derived strain parameters of the left and right ventricle. Eur J Radiol 89:97–105
Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP (2015) Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol 84(5):840–848
Schneeweis C, Qiu J, Schnackenburg B et al (2014) Value of additional strain analysis with feature tracking in dobutamine stress cardiovascular magnetic resonance for detecting coronary artery disease. J Cardiovasc Magn Reson 16:72
Mirea O, Pagourelias ED, Duchenne J et al (2018) EACVI-ASE-industry standardization task force. Intervendor differences in the accuracy of detecting regional functional abnormalities: a report from the EACVI-ASE Strain Standardization Task Force. JACC Cardiovasc Imaging 11(1):25–34
Cvijic M, Duchenne J, Ünlü S et al (2018) Timing of myocardial shortening determines left ventricular regional myocardial work and regional remodelling in hearts with conduction delays. Eur Heart J Cardiovasc Imaging 19(8):941–949
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Professor Jan Bogaert.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Several authors have significant statistical expertise.
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because of its retrospective nature.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The study subjects and cohorts have been previously reported in the paper published by our group: Barreiro-Pérez M, Curione D, Symons R, Claus P, Voigt JU, Bogaert J. Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR-feature tracking algorithms. Eur Radiol. 2018 Jun 5. [Epub ahead of print] doi:https://doi.org/10.1007/s00330-018-5538-4
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Dobrovie, M., Barreiro-Pérez, M., Curione, D. et al. Inter-vendor reproducibility and accuracy of segmental left ventricular strain measurements using CMR feature tracking. Eur Radiol 29, 6846–6857 (2019). https://doi.org/10.1007/s00330-019-06315-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06315-4