Abstract
Objectives
To investigate the diagnostic value of cardiac magnetic resonance (CMR) feature-tracking (FT) myocardial strain analysis in patients with suspected acute myocarditis and its association with myocardial oedema.
Methods
Forty-eight patients with suspected acute myocarditis and 35 control subjects underwent CMR. FT CMR analysis of systolic longitudinal (LS), circumferential (CS) and radial strain (RS) was performed. Additionally, the protocol allowed for the assessment of T1 and T2 relaxation times.
Results
When compared with healthy controls, myocarditis patients demonstrated reduced LS, CS and RS values (LS: -19.5 ± 4.4% vs. -23.6 ± 3.1%, CS: -23.0 ± 5.8% vs. -27.4 ± 3.4%, RS: 28.9 ± 8.5% vs. 32.4 ± 7.4%; P < 0.05, respectively). LS (T1: r = 0.462, P < 0.001; T2: r = 0.436, P < 0.001) and CS (T1: r = 0.429, P < 0.001; T2: r = 0.467, P < 0.001) showed the strongest correlations with T1 and T2 relaxations times. Area under the curve of LS (0.79) was higher compared with those of CS (0.75; P = 0.478) and RS (0.62; P = 0.008).
Conclusions
FT CMR myocardial strain analysis might serve as a new tool for assessment of myocardial dysfunction in the diagnostic work-up of patients suspected of having acute myocarditis. Especially, LS and CS show a sufficient diagnostic performance and were most closely correlated with CMR parameters of myocardial oedema.
Key Points
• Myocardial strain measures are considerably reduced in patients with suspected myocarditis.
• Myocardial strain measures can sufficiently discriminate between diseased and healthy patients.
• Myocardial strain measures show basic associations with the extent of myocardial oedema/inflammation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Myocarditis is an important cause of cardiac morbidity and mortality, accounting for up to 20% of sudden unexpected deaths in young adults [1]. In addition, dilated cardiomyopathy may result from chronic inflammatory disease in patients with inadequate immune response [2]. Correct diagnosis is often challenging, as patients with acute myocarditis present in many different ways, often accompanied by unspecific symptoms [3]. Although endomyocardial biopsy is still regarded the gold standard for diagnosis [3], non-invasive cardiac magnetic resonance (CMR) is increasingly used for the diagnostic evaluation of patients suspected of having acute myocarditis [4, 5]. In these patients, CMR can reliably characterize acute inflammatory myocardial alterations by the use of a combination of imaging sequences that detect oedema, hyperaemia and necrosis. This combined imaging approach is the essential part of the so called “Lake Louise” criteria (LLC), with a diagnosis of myocarditis being made if two of three criteria are met [6]. Newer, quantitative imaging techniques like myocardial T1 and T2 mapping can also assess diffuse myocardial inflammation and further enhance the diagnostic performance of CMR [4, 5, 7]. Another novel CMR approach, which may help in the diagnostic evaluation of patients with suspected acute myocarditis, might be the assessment of regional or global myocardial dysfunction using the recently introduced feature-tracking (FT) technique [4, 8]. This technique allows for quantitative segmental and global myocardial strain analysis based on routinely acquired cine CMR images. Such functional markers such as myocardial strain analyses might add additional diagnostic information and further broaden the diagnostic targets of CMR. In this prospective study, a comprehensive CMR examination was performed in patients suspected of having acute myocarditis, which included an additional myocardial strain analysis besides the assessment of the LLC, T1 relaxation times and T2 relaxation times. The purpose of our study was to investigate: (1) whether FT derived strain parameters can differentiate between patients with acute myocarditis and control subjects, (2) to which degree myocardial strain parameters correlate with other markers of myocardial inflammation (e.g. myocardial oedema), and (3) which myocardial strain parameters offer the best diagnostic performance in patients suspected of having myocarditis.
Methods
The institutional review board approved this prospective study and all subjects gave written informed consent prior to CMR. The study population consisted of patients with clinically defined acute myocarditis and control subjects. The diagnosis of acute myocarditis was made solely on the basis of clinical observations. This clinical evidence was the reference standard against which the diagnostic performance of myocardial strain parameters was tested. Patients with clinically suspected myocarditis had the following: acute chest pain, evidence of acute myocardial injury (ECG changes and/or elevated troponin), and a history of viral infection during the last 4 weeks with elevated serum markers indicating infectious disease (e.g. C-reactive protein). Coronary artery disease was ruled out by invasive cardiac catheterization prior to CMR in all patients. Exclusion criteria for the study were contraindications for CMR, previous acute myocardial conditions (myocarditis or myocardial infarction), or other medical history of cardiac disease. The control group consisted of healthy volunteers and outpatients referred for nonspecific thoracic pain in which a detailed diagnostic workup and clinical follow-up were unremarkable and without signs of cardiac disease.
Cardiac magnetic resonance
CMR scans were performed on a 1.5 Tesla CMR system (Ingenia 1.5 T, Philips Healthcare, Best, The Netherlands). For functional analysis ECG-gated steady-state free precession cine images were obtained in short axis, four-chamber and two-chamber views. Oedema-sensitive black blood T2-weighted short-tau inversion-recovery (STIR) sequences were acquired in the short axis orientation. To correct for torso coil related signal inhomogeneities, a signal intensity correction algorithm based on a calibration measurement using the body coil was performed. Early gadolinium enhancement was assessed using transverse free-breathing fast spin echo T1-weighted images, which were acquired in three identical slices both before and after intravenous injection of a bolus of 0.2 mmol/kg of body weight of gadobutrol (Gadovist, Bayer Healthcare, Leverkusen, Germany) using the body coil for signal reception. For late gadolinium enhancement (LGE) imaging segmented inversion-recovery gradient-echo sequences were acquired in short axis, four-chamber and two-chamber views. Optimal inversion time was determined by using the Look-Locker technique [9]. T1 and T2 mapping were performed in end-diastole in short-axis orientation (basal, mid-ventricular and apical sections). For myocardial T1 mapping a 3(3)3(3)5 modified Look-Locker inversion recovery (MOLLI) acquisition scheme was applied [10]. T1 maps were acquired before and 10 minutes after contrast administration. For myocardial T2 mapping an optimized 6-echo gradient spin echo (GraSE) sequence was used [11]. Detailed sequence parameters are given in Table S1 (online supplement).
Image analysis
Two physicians experienced in CMR analysed the data and performed the measurements. Readers were blinded to the patient information. Cardiac functional analysis was performed offline using dedicated software (IntelliSpace Portal 6, Philips Healthcare). Papillary muscles were included in the left ventricular cavity volume. The presence of focal myocardial oedema on T2 STIR and/or non-ischemic lesions on LGE images was visually assessed by consensus agreement of the two readers. T2 signal intensity ratio for the presence of global myocardial oedema as well as the early gadolinium enhancement ratio (EGEr) for the presence of inflammation-induced hyperaemia were calculated as recommended for the assessment of the LLC [6]. Myocardial T1 and T2 relaxation times were extracted from the relaxation maps using freely available software (Segment, version 1.9, R2783; http://segment.heiberg.se) [12]. Myocardial T1 and T2 maps were analysed by a segmental approach [13], and global T1 and T2 relaxation times were calculated from the segmental data. Haematocrit-corrected extracellular volume fraction (ECV) values were calculated separately from pre- and post-contrast T1 values as previously described [5].
Myocardial strain analysis
Strain measurements were performed using a dedicated CMR FT software (Image-Arena 4.6; TomTec Imaging Systems, Unterschleissheim, Germany). For strain analysis of the left ventricle a 16-segment model according to the model of the American Heart Association was applied [13]. Circumferential and radial strain and strain rate measurements were derived from the basal, mid-ventricular, and apical sections of short axis cine images. Longitudinal strain and strain rate values were derived from four-chamber and two-chamber view cine images. For strain analysis, an initial endocardial contour is drawn in an end-diastolic phase with optimal contrast between blood and myocardium. The FT software then propagates the contour throughout the cardiac cycle. In case of faulty contour propagation the software allows editing throughout the cardiac cycle. Global systolic radial, circumferential and longitudinal strain and strain rate values were calculated from peak segmental data.
Statistical analysis
Statistical analysis was performed using SPSS Statistics 22.0 (IBM, Armonk, NY, USA) and MedCalc 11.0 (MedCalc Software bvba, Ostend, Belgium). Patient characteristics are presented as mean ± standard deviation or as absolute frequency. Continuous variables were checked for normal distribution. The independent two-sample Student’s t test was used for comparison of continuous variables between two different groups. Dichotomous variables were compared using the χ2 test (with a cell count >5) or Fisher’s exact test (with a cell count ≤5). Correlation analysis was performed using Spearman’s rank correlation coefficient. To explore basic relationships between functional and inflammatory variables, correlation coefficients were tested for being different from zero. Diagnostic performance of strain parameters was analysed by plotting receiver operating characteristic curves (ROCs) and comparing the area under the ROCs. Areas under the ROCs were compared as previously described [14]. Cut-off values were chosen by maximizing the reclassification accuracy for the predictive variables, and (reclassification) sensitivity, specificity, and accuracy were calculated. For the combination of single predictive variables, scores were derived based on logistic regression analysis. The level of statistical significance was set to P < 0.05.
Results
Population characteristics
A total of 83 subjects were included in this study (48 patients with acute myocarditis and 35 control subjects). Mean time from admission to CMR was 2.7 ± 1.9 days. Mean age of myocarditis patients was 43.6 ± 18.8 (range: 18–80) years. Mean age of healthy controls was 41.1 ± 17.2 (range: 18–76) years. Age (P = 0.525), sex (P = 0.384), and body mass index (P = 0.241) did not differ significantly between both groups. Clinical characteristics for myocarditis and control subjects are given in Table 1.
CMR parameters and strain values
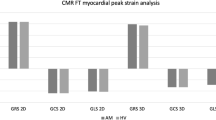
All CMR parameters indicating inflammatory alterations of the myocardium were elevated in the myocarditis group compared to the control group: T2 ratio (1.8 ± 0.4 vs.1.6 ± 0.3, P = 0.001), EGEr (3.9 ± 2.8 vs. 1.9 ± 1.4, P < 0.001), native T1 relaxation time (1050.8 ± 49.1 ms vs. 966.7 ± 30.6 ms, P < 0.001), T2 relaxation time (62.2 ± 8.8 ms vs. 52.3 ± 2.5 ms, P < 0.001), and ECV (32.9 ± 8.0% vs. 26.7 ± 4.8%, P < 0.001). Non-ischemic LGE was found in 37/48 (77.1%) and visible myocardial oedema was seen in 36/48 (75.0%) of all myocarditis patients. Positive LGE was mostly visible in the basal and midventricular inferolateral segments. Global peak systolic longitudinal (-19.5 ± 4.4% vs. -23.6 ± 3.1%, P < 0.001), circumferential (-23.0 ± 5.8% vs.-27.4 ± 3.4%, P < 0.001), and radial (29.0 ± 8.5% vs. 32.4 ± 7.4%; P = 0.049) strain values were considerably reduced in the myocarditis group compared to the control group (see Fig. 1). All CMR parameters evaluated are given in Table 2.
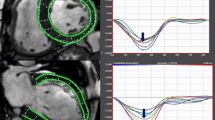
Longitudinal strain curves and LGE images (4-chamber view) of a healthy control (28-year-old male) and a patient with acute myocarditis (22-year-old male). The color-coded strain graph shows the longitudinal strain of each segment vs. time. Average longitudinal strain is clearly reduced in the patient with acute myocarditis. On LGE imaging typical patchy epicardial and midmyocardial inflammatory/necrotic lesions are visible
Correlation of strain indices with CMR parameters of myocardial inflammation
Global peak systolic longitudinal strain was the only strain parameter, which showed a significant correlation between all other continuous CMR parameters of myocardial inflammation/oedema (T2 ratio: r = 0.238, P = 0.030; EGEr: r = 0.296, P = 0.008, native T1 relaxation time: r = 0.462, P < 0.001; ECV: r = 0.258, P = 0.019, T2 relaxation time: r = 0.436, P < 0.001). Global peak circumferential strain showed moderate correlations between T1 (r = 0.429, P < 0.001) and T2 relaxation times (r = 0.467, P < 0.001), whereas the correlations between global peak radial strain and T1 (r = -0.226, P = 0.041) and T2 (r = -0.229, P = 0.038) relaxation times were only weak (see Fig. 2 and Table 3).
On a segmental analysis, there were significant correlations between mean segmental circumferential and radial strain values and T1 (circumferential: r = 0.205, P < 0.001, radial: r = -0.118, P < 0.001) and T2 relaxation times (circumferential: r = 0.190, P < 0.001, radial: r = -0.150, P < 0.001) (see Fig. 3). Segments with positive LGE or visible oedema had a reduced mean segmental circumferential strain (LGE+: -21.4 ± 19.9% vs. 25.3 ± 12.0%, P < 0.001; oedema+: -21.1 ± 10.7% vs. 25.3 ± 11.5%, P < 0.001). Mean segmental radial stain did not show an association with positive LGE or visible oedema (LGE+: 28.1 ± 18.5% vs. 30.6 ± 19.7%, P = 0.127; oedema+: 28.6 ± 20.9% vs. 30.6 ± 19.4%, P = 0.283).
Diagnostic performance of strain indices and strain parameters in patients with preserved ejection fraction
Global peak systolic longitudinal and circumferential strain showed a good diagnostic performance with area under curve (AUC) values of 0.79 (longitudinal strain) and 0.75 (circumferential strain). Both parameters had significantly higher AUC values compared to global peak systolic longitudinal strain rate (0.67), global peak systolic circumferential strain rate (0.65), global peak systolic radial strain (0.62) and global peak systolic radial strain rate (0.52) (P < 0.05, respectively) (see Fig. 4). In our study collective, the LCC yielded an excellent diagnostic performance with an AUC of 089. However, the diagnostic performance of a combination score of global peak longitudinal strain with T1 and T2 mapping (AUC: 0.98) was significantly higher when compared to LLC (P = 0.003) (see Fig. 5). Sensitivities, specificities, accuracies, positive predictive values and negative predictive values for all parameters are given in Table 4.
Graph showing receiver operating characteristic curves (ROCs) for global peak systolic longitudinal strain (AUC: 0.79), global peak systolic circumferential strain (AUC: 0.75), global peak systolic longitudinal strain rate (AUC: 0.67), global peak systolic circumferential strain rate (AUC: 0.65), global peak systolic radial strain (AUC: 0.62) and global peak systolic radial strain rate (AUC: 0.52) (P < 0.05, respectively)
Thirty of 48 (62.5%) patients with acute myocarditis had a preserved ejection fraction (ejection fraction ≥55%). In these patients, only global peak longitudinal strain was reduced compared to healthy controls (-21.3 ± 3.1% vs. -23.6 ± 3.1%; P < 0.002), whereas global peak circumferential strain (-25.9 ± 4.3% vs. -27.4 ± 3.4%; P = 0.112) and radial strain (31.9 ± 8.0% vs. 32.4 ± 7.4%; P = 0.821) showed no significant group differences. Global peak longitudinal strain yielded a fair AUC value of 0.73 for the diagnosis of myocarditis patients with preserved ejection fraction (sensitivity: 70.0%, specificity: 71.4%; cutoff value: ≥-22.3%).
Discussion
In this prospective study, we evaluated the diagnostic value of CMR FT derived myocardial strain parameters in patients suspected of having acute myocarditis. The main findings of our study are that (1) myocardial strain parameters were considerably reduced in patients with acute myocarditis compared to control subjects, (2) global peak longitudinal and circumferential strain were the only strain parameters to be closely correlated with other CMR parameters of myocardial inflammation (e.g. myocardial T1 and T2 relaxation times), and (3) global peak longitudinal and circumferential strain provided the best diagnostic performance of all strain parameters evaluated.
Differences in myocardial strain parameters
In accordance with previous CMR and echocardiographic studies we found considerably reduced strain values in patients with suspected acute myocarditis [8, 15,16,17,18,19]. Especially global longitudinal and circumferential strain values showed high group differences, which indicates that both parameters are useful to detect alterations of myocardial function in acute myocarditis. Furthermore, we could show that longitudinal strain was also reduced in myocarditis patients with preserved ejection when compared to healthy controls. This observation is in accordance with the results of a previous CMR FT study [19], which could also detect significant group differences between patients with preserved ejection fraction and control subjects. Overall these results suggest that especially longitudinal strain parameters are capable to detect even subtle alterations of myocardial function. Group differences in global radial strain values, however, were least pronounced. This might be due to two reasons: First, previous FT CMR studies reported global radial strain to offer the highest intra- and inter-observer variability compared with global longitudinal and circumferential strain and thus to be less reliable [20, 21]. Furthermore, FT global radial strain is also associated with the lowest inter-study reproducibility [22]. Second, the myocardium of the left ventricle typically consists of three myocardial layers: the inner oblique, the middle circular, and the outer oblique myocardial layers. During systole the inner oblique layer undergoes the greatest dimensional changes [23, 24], and functional alterations predominantly occur when the endocardial layer is affected [25]. Oedematous and necrotic inflammatory changes in acute myocarditis, however, most commonly affect the epicardial layer of the myocardium [6]. Therefore, radial function might be less affected in a disease, which mostly does not affect the endocardium. Severe wall motion abnormalities occur infrequently in patients with acute myocarditis and ejection fraction is mostly in preserved [26].
Correlations of strain parameters with myocardial inflammation and oedema
In our study collective, we not only assessed the inflammatory CMR parameters EGEr (marker for hyperaemia) and T2 ratio (marker for myocardial oedema), which are part of the established Lake Louis criteria [6], but also two newer parameters of myocardial inflammation namely T1 and T2 relaxation times. Especially, T1 and T2 relaxation times have recently been reported to represent an excellent measure of myocardial oedema [4, 5, 27, 28]. Interestingly, global longitudinal strain was the only strain parameter to be significantly correlated with all parameters of myocardial inflammation. The closest correlations, however, were found between myocardial T1 and T2 relaxation times and longitudinal and circumferential strain measurements. In accordance with our results, a previous echocardiographic study reported echocardiographic longitudinal strain to be correlated with myocardial oedema assessed by CMR [16]. Overall these results implicate that besides being a sensitive tool for detecting myocardial dysfunction, global longitudinal and circumferential strain also allow for an indirect quantification of myocardial oedema in acute myocarditis. In cases of limited accessibility to CMR, echocardiography myocardial deformation analysis might therefore not only be useful in the initial diagnostic evaluation of patients with suspected myocarditis, but also in the follow-up of patients with acute myocarditis, when a decrease of myocardial oedema might be expected. On a segmental basis mean segmental circumferential strain was also significantly reduced in segments with positive LGE or visible oedema. Focal/segmental contrast enhancement is a frequent finding in the clinical setting of suspected acute myocarditis and areas of enhancement are known to be especially associated with active inflammation on histopathology [29]. Our findings indicate that focal inflammation also leads to a focal impairment of myocardial function. Thus, myocardial strain analysis may be regarded as a non-invasive marker for the detection of focal myocardial damage/necrosis in acute myocarditis.
Diagnostic performance of strain indices
According to our datasets global longitudinal and circumferential strain displayed a good accuracy in detecting acute myocarditis (75% and 70%, respectively), suggesting that myocardial deformation analysis can provide incremental diagnostic information in suspected acute myocarditis. In a retrospective echocardiographic collective, a sensitivity and specificity for longitudinal strain of 78% and 93% had been reported for global longitudinal strain [15]. In our study, the sensitivity and specificity for global longitudinal strain was 81% and 71%. In combination with the newer quantitative parameters T1 and T2 relaxation times, global longitudinal strain could even outperform standard LLC in our study collective.
The following clinical implications may be inferred from our study results: First, overall these data propose that feature tracking derived myocardial strain analysis from conventional cine CMR images might serve as a novel parameter for the detection of inflammatory alterations of the myocardium in acute myocarditis. Second, myocardial strain might be an especially valuable additional parameter in patients with contraindications for gadolinium-based contrast media, as no addition of contrast media is necessary for analysis. Third, strain parameters represent a physiological standardized unit of measurement and can be determined using various imaging techniques (e.g. CMR or echocardiography). This might be of especially importance for the clinical follow-up of myocarditis patients.
Limitations
In this study a systematic endomyocardial biopsy as a reference standard was not performed to diagnose acute myocarditis. Instead, the presence of acute myocarditis was defined by combining typical clinical features, exclusion of coronary artery disease, and elevated biomarkers as reported previously in multiple cardiac MR validation studies [4, 5]. Most of the patients included in our study were of younger age and were admitted to our hospital because of persistent typical anginal chest pain suggestive of an acute myocardial infarction. Therefore, the results of this study are only valid for the described subgroup of patients with suspected myocarditis and caution must be exercised when transferring the results to patients with other clinical presentations. Furthermore, it is important to know that alterations of myocardial strain parameters are non-specific and can be found in many cardiac diseases (e.g. in patients with significant coronary artery disease [25]), leaving a possible diagnostic uncertainty in the differentiation of myocarditis and potentially other pre-existing cardiac diseases in a real-world setting. Therefore, quantitative myocardial strain analyses can only be interpreted within the appropriate clinical context. Although the reported correlations indicate a basic relationship between myocardial strain parameters and the extent of myocardial oedema/inflammation, their utility for an individual prediction might be reduced. Because of the small sample size, validations of the chosen cutoff values could not be performed in this study. Therefore, further studies are necessary to substantiate the results of this study.
Conclusions
In patients suspected of having acute myocarditis, FT derived myocardial strain measurements allowed for a sufficient discrimination between diseased and healthy patients. Especially, global peak systolic and longitudinal strain showed good diagnostic accuracies in confirming the presence of acute myocarditis. Both parameters were most closely correlated with CMR measures of myocardial oedema. Global peak systolic and longitudinal strain may serve as novel parameters in detecting global and regional myocardial dysfunction in patients suspected of having acute myocarditis and therefore further broaden the diagnostic targets of CMR.
Abbreviations
- AUC:
-
Area under the curve
- CMR:
-
Cardiac magnetic resonance
- EGEr:
-
Early gadolinium enhancement ratio
- ECV:
-
Extracellular volume fraction
- FT:
-
Feature tracking
- GraSE:
-
gradient spin echo
- LGE:
-
Late gadolinium enhancement
- LLC:
-
“Lake Louise” criteria
- MOLLI:
-
Modified Look-Locker inversion recovery
- ROC:
-
Receiver operating characteristic
- STIR:
-
Short-tau inversion-recovery
References
Drory Y, Turetz Y, Hiss Y et al (1991) Sudden unexpected death in persons less than 40 years of age. Am J Cardiol 68:1388–1392
Liu PP, Mason JW (2001) Advances in the understanding of myocarditis. Circulation 104:1076–1082
Caforio AL, Pankuweit S, Arbustini E et al (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648, 2648a-2648d
Luetkens JA, Homsi R, Sprinkart AM et al (2016) Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17:154–161
Luetkens JA, Doerner J, Thomas DK et al (2014) Acute myocarditis: multiparametric cardiac MR imaging. Radiology 273:383–392
Friedrich MG, Sechtem U, Schulz-Menger J et al (2009) Cardiovascular magnetic resonance in myocarditis: a JACC white paper. J Am Coll Cardiol 53:1475–1487
Lurz P, Luecke C, Eitel I et al (2016) Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis: the MyoRacer-Trial. J Am Coll Cardiol 67:1800–1811
Weigand J, Nielsen JC, Sengupta PP, Sanz J, Srivastava S, Uppu S (2015) Feature tracking-derived peak systolic strain compared to late gadolinium enhancement in troponin-positive myocarditis: a case-control study. Pediatr Cardiol. doi:10.1007/s00246-015-1333-z
Look DC, Locker DR (1970) Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum 41:250–251
Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP (2004) Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med 52:141–146
Sprinkart AM, Luetkens JA, Traber F et al (2015) Gradient Spin Echo (GraSE) imaging for fast myocardial T2 mapping. J Cardiovasc Magn Reson 17:12
Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H (2010) Design and validation of segment--freely available software for cardiovascular image analysis. BMC Med Imaging 10:1
Cerqueira MD, Weissman NJ, Dilsizian V et al (2002) Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Int J Cardiovasc Imaging 18:539–542
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Hsiao JF, Koshino Y, Bonnichsen CR et al (2013) Speckle tracking echocardiography in acute myocarditis. Int J Cardiovasc Imaging 29:275–284
Logstrup BB, Nielsen JM, Kim WY, Poulsen SH (2015) Myocardial oedema in acute myocarditis detected by echocardiographic 2D myocardial deformation analysis. Eur Heart J Cardiovasc Imaging. doi:10.1093/ehjci/jev302
Uppu SC, Shah A, Weigand J et al (2015) Two-dimensional speckle-tracking-derived segmental peak systolic longitudinal strain identifies regional myocardial involvement in patients with myocarditis and normal global left ventricular systolic function. Pediatr Cardiol 36:950–959
Baessler B, Schaarschmidt F, Dick A, Michels G, Maintz D, Bunck AC (2016) Diagnostic implications of magnetic resonance feature tracking derived myocardial strain parameters in acute myocarditis. Eur J Radiol 85:218–227
Andre F, Stock FT, Riffel J et al (2016) Incremental value of cardiac deformation analysis in acute myocarditis: a cardiovascular magnetic resonance imaging study. Int J Cardiovasc Imaging 32:1093–1101
Andre F, Steen H, Matheis P et al (2015) Age- and gender-related normal left ventricular deformation assessed by cardiovascular magnetic resonance feature tracking. J Cardiovasc Magn Reson 17:25
Taylor RJ, Moody WE, Umar F et al (2015) Myocardial strain measurement with feature-tracking cardiovascular magnetic resonance: normal values. Eur Heart J Cardiovasc Imaging 16:871–881
Morton G, Schuster A, Jogiya R, Kutty S, Beerbaum P, Nagel E (2012) Inter-study reproducibility of cardiovascular magnetic resonance myocardial feature tracking. J Cardiovasc Magn Reson 14:43
Adamu U, Schmitz F, Becker M, Kelm M, Hoffmann R (2009) Advanced speckle tracking echocardiography allowing a three-myocardial layer-specific analysis of deformation parameters. Eur J Echocardiogr 10:303–308
Sabbah HN, Marzilli M, Stein PD (1981) The relative role of subendocardium and subepicardium in left ventricular mechanics. Am J Physiol 240:H920–926
Sarvari SI, Haugaa KH, Zahid W et al (2013) Layer-specific quantification of myocardial deformation by strain echocardiography may reveal significant CAD in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol Img 6:535–544
Pinamonti B, Alberti E, Cigalotto A et al (1988) Echocardiographic findings in myocarditis. Am J Cardiol 62:285–291
Ferreira VM, Piechnik SK, Dall'Armellina E et al (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson 14:42
Luetkens JA, Doerner J, Schwarze-Zander C et al (2016) Cardiac magnetic resonance reveals signs of subclinical myocardial inflammation in asymptomatic HIV-infected patients. Circ Cardiovasc Imaging 9, e004091
Mahrholdt H, Goedecke C, Wagner A et al (2004) Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation 109:1250–1258
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Dr. Daniel Thomas.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
The authors state that this work has not received any funding.
Statistics and biometry:
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• prospective
• case-control study
• performed at one institution
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(DOCX 36 kb)
Rights and permissions
About this article
Cite this article
Luetkens, J.A., Schlesinger-Irsch, U., Kuetting, D.L. et al. Feature-tracking myocardial strain analysis in acute myocarditis: diagnostic value and association with myocardial oedema. Eur Radiol 27, 4661–4671 (2017). https://doi.org/10.1007/s00330-017-4854-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4854-4