Abstract
Objectives
To characterize interhemispheric functional and anatomical connectivity and their relationships with impulsive behaviour in codeine-containing cough syrup (CCS)-dependent male adolescents and young adults.
Methods
We compared volumes of corpus callosum (CC) and its five subregion and voxel-mirrored homotopic functional connectivity (VMHC) in 33 CCS-dependent male adolescents and young adults and 38 healthy controls, group-matched for age, education and smoking status. Barratt impulsiveness scale (BIS.11) was used to assess participant impulsive behaviour. Abnormal CC subregions and VMHC revealed by group comparison were extracted and correlated with impulsive behaviour and duration of CCS use.
Results
We found selective increased mid-posterior CC volume in CCS-dependent male adolescents and young adults and detected decreased homotopic interhemispheric functional connectivity of medial orbitofrontal cortex (OFC). Moreover, impairment of VMHC was associated with the impulsive behaviour and correlated with the duration of CCS abuse in CCS-dependent male adolescents and young adults.
Conclusions
These findings reveal CC abnormalities and disruption of interhemispheric homotopic connectivity in CCS-dependent male adolescents and young adults, which provide a novel insight into the impact of interhemispheric disconnectivity on impulsive behaviour in substance addiction pathophysiology.
Key Points
• CCS-dependent individuals (patients) had selective increased volumes of mid-posterior corpus callosum
• Patients had attenuated interhemispheric homotopic FC (VMHC) of bilateral orbitofrontal cortex
• Impairment of VMHC correlated with impulsive behaviour in patients
• Impairment of VMHC correlated with the CCS duration in patients
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The corpus callosum (CC) is the largest interhemispheric commissure, which consists of 200 million fibres connecting the two brain hemispheres [1]. Abnormalities in CC morphometry and diffusivity have been consistently observed in patients with diverse substance dependencies, including alcohol [2], nicotine [3], opiates [4, 5] and cocaine [6]. These CC abnormalities have also been correlated with addictive behaviours [7]. However, previous studies mainly focused on adult addicts who are dominant in illicit drug abuse [4–6]. According to the Annual Report of National Drug Abuse Monitoring for 2012, young adult and adolescent addicts have become a crucial group. Codeine cough syrup (CCS) dependence has become one of the most popular forms of drug abuse in young people in the world [8]. The number of callosal fibres is already fixed around birth, but structural changes in the CC continue to occur during postnatal development as a result of fibre myelination, redirection and pruning [9]. Substance abuse during this period may affect CC development. Previous studies have revealed the effects of alcohol and marijuana on adolescent CC development [10, 11]. However, few studies have focused on the effects of prescription drug addiction on CC development during this period.
The CC, which primarily connects homologous regions of the cortex, is the major conduit for information transfer between the two cerebral hemispheres. The CC topographically exhibits functional specialization of callosal subregions connecting to various cortical lobes [12]. The genu of the CC connects the orbitofrontal and frontal cortices, whereas the body and the splenium connect the temporal, parietal and occipital regions [13]. Thus, CC abnormalities may contribute to disruption of interhemispheric homotopic functional connectivity, which may contribute to addictive behaviours, particularly impulsivity. However, to the best of our knowledge, the links between selective CC abnormalities, disruption of interhemispheric homotopic functional connectivity and addictive behaviours in CCS-dependent adolescents and young adults remain unclear.
To address these gaps, we combined resting-state functional magnetic resonance imaging (fMRI), high resolution 3D T1-weighted imaging and the Barratt impulsiveness scale (BIS.11) to investigate the CC, interhemispheric homotopic functional connectivity and impulsive behaviour in CCS-dependent individuals and healthy controls. We hypothesized that, compared with healthy controls, CCS-dependent adolescents and young adults would have impaired interhemispheric coordination (CC morphometric abnormalities and interhemispheric functional disconnection) and higher levels of impulsivity. Such alternations in connectivity, coupled with higher levels of impulsive behaviour, would imply that interhemispheric disconnection may underlie higher impulsivity in CCS-dependent adolescents and young adults.
Materials and methods
Participants
We recruited CCS-dependent Chinese adolescents and young adults from patients seeking treatment at the Addiction Medicine Division of Guangdong No. 2 Provincial People’s Hospital. Patients were screened on the basis of medical history and the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, DSM-IV) criteria, along with a urine test and an interview. Patients reported that they regularly used cigarettes, but denied any use of other psychotropic agents in the month prior to the resting-state fMRI scan. We recruited controls from the nearby community. Of 103 participants, we excluded 26 for not completing the MRI scan or impulsivity assessment, and three for incidental MRI findings (one had venous malformation, and two had hyper intense signals around the ventricle). We also excluded three participants because of excessive head motion during the resting-state fMRI (two subjects) or motion artefacts in 3D T1-weighted imaging data (one subject, see details in the Electronic Supplementary Material, ESM). The final sample included 38 healthy controls and 33 CCS-dependent male adolescents and young adults (Table 1). Inclusion criteria for the control subjects were lack of diagnosis of substance abuse or dependence. Exclusion criteria for all participants included neurological illness, schizophrenia or bipolar disorder, prior significant head trauma, positive HIV status, diabetes, hepatitis C, other major medical illness and left-handedness. This prospective study was approved by the Research Ethics Review Board of the Institute of Mental Health at the Guangdong No. 2 Provincial People’s Hospital, Guangzhou, China. Written informed consent was obtained from all subjects.
Impulsivity assessment
We assessed impulsivity using the BIS.11, one of the oldest and most widely used self-report measures of impulsive personality traits. The BIS.11 is a 30-item self-rated scale, having three oblique factors: (1) attentional/cognitive, which measures tolerance for cognitive complexity and persistence, (2) motor, which measures the tendency to act on the spur of the moment and (3) non-planning impulsivity, which measures the lack of sense of the future. Items are rated from 1 (rarely/never) to 4 (almost always/always). To determine an overall impulsiveness scores, ratings for all items were summed, with higher scores indicating greater impulsivity [14]. BIS.11 is a valid and reliable instrument for healthy and psychiatric Chinese populations [15].
MRI scanning
MR data were obtained by means of a Philips Achieva 1.5T Nova dual MR scanner using a 16-channel neurovascular (NV) coil. Each participant underwent a T1-weighted structural MRI and a resting-state fMRI in the same session. Imaging parameters are described in the ESM.
CC volume calculation
To calculate the volumes of the corpus callosum (CC) and its subregions, we used the FreeSurfer software package (Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard University, https://surfer.nmr.mgh.harvard.edu/, software package version 5.3) following a previous approach [16]. The automated procedures for subcortical volume measurements of different brain structures are described elsewhere [17] (see details in the ESM). This FreeSurfer-based automatic procedure has been shown to be statistically comparable with manual segmentation [17].
We calculated the volume of each brain structure by counting the number of voxels within it. The CC was automatically identified and divided into five segments using the FreeSurfer processing software, with each region encompassing 20 % of the anterior-posterior distance through the corpus callosum that broadly corresponded to functional subdivisions, including anterior (CC1), mid-anterior (CC2), central (CC3), mid-posterior (CC4) and posterior portions (CC5; Supplementary Fig. 1). We calculated the total CC volume as the sum of the five segments. We visually inspected images to ensure accuracy of registration, skull stripping, segmentation and cortical surface reconstruction.
Resting-state fMRI data preprocessing
Resting-state fMRI data preprocessing was performed using the Data Processing & Analysis of Brain Imaging (DPABI 1.2) based on statistical parametric mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm), following previous approaches [18] (see the ESM for details).
After quality control, all participants had minimum absolute translation and rotation (not exceeding 1.5 mm or 1.5°, respectively). Further, the mean framewise displacement (FD) was computed by averaging the FD from every time point for each subject. The FD was then included as a covariate in statistical analyses [19].
Voxel-mirrored homotopic functional connectivity derivation
We followed the methods for computing VMHC described in Zuo et al. [20, 21]. We computed the VMHC using DPABI software and examined global differences in VMHC in the whole brain. For each subject, we computed the homotopic functional connectivity as the Pearson correlation coefficient between each voxel’s residual time series and that of its symmetrical interhemispheric counterpart. Correlation values were then transformed using Fisher’s z to improve normality. The resulting subject-specific VMHC z-score maps were used for further statistical analyses.
Statistical analysis
Independent two-sample t test analyses on six volume indices of CC (total CC and five subregions), demographics, impulsive behaviour and motion parameters were performed to determine group differences. The results were reported at the significance level of p < 0.05 (two-tailed) and corrected for multiple comparisons [Bonferroni correction, for the CC (divided by 6) and for impulsivity (divided by 4)]. All statistical analyses were performed using SPSS software (v.23.0, IBM, USA).
Group differences in VMHC were determined by performing whole-brain voxelwise analysis based on subject-specific VMHC maps, with age, education, smoking and head motion as nuisance variables. The results were reported at p < 0.05, corrected with a single voxel height of p < 0.01 and a cluster volume greater than 1836 mm3 using software [AFNIAlphaSim; https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf]) with a grey matter mask produced by the symmetrical template outlined previously.
To test associations between brain interhemispheric coherence and impulsive behaviour, duration of CCS abuse and initial age of CCS use in CCS-dependent adolescents and young adults, we used SPSS to perform correlation analyses between brain measures (VMHC and CC volume) and impulsive behaviour, duration of CCS abuse and initial age of CCS. First, for each subject, the mean VMHC values of regions showing group differences in group comparisons were extracted using REST [22] (http://resting-fmri.sourceforge.net). Pearson’s correlations were then computed between the brain measures, six CC volume indices and mean VMHC values, and impulsive behaviour, duration of CCS abuse and initial age of CCS use. The results were reported at a significance level of p < 0.05 with Bonferroni correction for multiple comparisons (4 impulsivity scores × 6 CC volume indices; 4 impulsivity measures × 1 VMHC region).
Results
Demographics and clinical characteristics
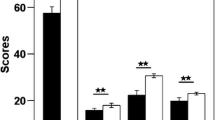
There were no group differences in age, education, smoking, head motion, mean grey matter VMHC or total intracranial volume (Table 1). There were significant group differences in the impulsivity test (Fig. 1).
Impulsivity differences between CCS-dependent adolescents and young adults and non-addict controls. CCS-dependent adolescents and young adults had higher BIS total scores, attentional impulsivity scores, motor impulsivity scores and non-plan impulsivity scores than control group. **P < 0.01 (multiple comparison correction with Bonferroni)
Selective increased volume of CC in CCS-dependent adolescents and young adults
The CCS-dependent male adolescents and young adults had a significantly larger volume for the total CC and mid-posterior (CC2, CC3, CC4 and CC5) subregions compared with the control group (Fig. 2). Furthermore, we confirmed the mid-posterior (CC2, CC3, CC4 and CC5) subregions’ abnormalities using midsagittal area measurements [23]. Details can be found in the ESM.
Corpus callosum (CC) subregions differences between CCS-dependent adolescents and young adults and non-addict control. After we controlled for age, education and total intracranial volume (TIV), the CCS-dependent adolescents and young adults had significantly smaller volumes of mid-posterior subregions of CC, including CC2, CC3, CC4 and CC5, than the control group. **P < 0.01 (multiple comparison correction with Bonferroni); *P < 0.05 (multiple comparison correction with Bonferroni). CCS codeine-containing cough syrups, Ctrl non-addict control, CC1 anterior CC, CC2 middle anterior CC, CC3 central CC, CC4 middle posterior CC, CC5 posterior CC
Disruption of interhemispheric homotopic functional connectivity in bilateral orbitofrontal cortices (OFC) in CCS-dependent male adolescents and young adults
After we controlled for age, education, smoking and head motion, CCS-dependent adolescents and young adults had lower interhemispheric homotopic functional connectivity between the bilateral OFC compared with the controls (Figs. 3, 4 and Supplementary Table 1). No significant increase in interhemispheric homotopic functional connectivity was found in CCS-dependent adolescents and young adults. Given the detection of signal differences between groups in the medial OFC, the region prone to fMRI signal loss [24], we have included a qualitative comparison of the mean T2* signal in this region. To do so, we created mean T2* maps for each group by adding one volume (the 20th) from each subject’s time series and dividing by the number of subjects. The mean T2* image for each of the two groups is shown in Supplementary Fig. 2 along with the medial OFC cluster to demonstrate that there was adequate signal in this region.
Interhemispheric homotopic functional connectivity differences between CCS-dependent adolescents and young adults and non-addict control, and their correlation with the impulsive behaviour and duration of CCS consumption. a After we controlled for age, education and head motion, CCS-dependent adolescents and young adults had significantly reduced VMHC in orbitofrontal cortex (OFC) than controls (p < 0.05, Alphasim corrected). Colour bars indicate the T value. b VMHC of OFC correlated with the years of CCS consumption. c VMHC of OFC correlated with the BIS total scores
Post hoc analyses of interhemispheric homotopic functional connectivity differences between CCS-dependent adolescents and young adults and non-addict control. On the basis of the regions showing group differences in VMHC (two sample t test, Fig. 3a), we performed post hoc analyses on the extracted cluster-mean VMHC values per subject. After we controlled for age, education, smoking and head motion, CCS-dependent adolescents and young adults had lower interhemispheric homotopic functional connectivity in the OFC compared to healthy controls
Interhemispheric homotopic functional connectivity of the OFC correlates with impulsivity and duration of CCS abuse in CCS-dependent adolescents and young adults
The mean VMHC of the OFC had significant negative correlations with the BIS total scores and with duration of CCS abuse (Fig. 3).
Discussion
Using high resolution structural MR imaging and resting-state fMRI, we investigated possible abnormalities of the CC and disruption of interhemispheric homotopic functional connectivity in patients with CCS abuse. In support of our hypothesis, our findings provide evidence that CCS-dependent male adolescents and young adults exhibit aberrant increased volumes of CC in the middle to posterior sections and attenuated interhemispheric homotopic functional connectivity of the OFC. Attenuated interhemispheric homotopic functional connectivity, but not CC abnormalities, correlated with duration of CCS abuse. More importantly, impairment of interhemispheric homotopic functional connectivity of the OFC was associated with impulsive behaviour in CCS-dependent male adolescents and young adults. These findings provide a novel insight into the impact of interhemispheric disconnectivity on impulsive behaviour in substance addiction pathophysiology of adolescent and young adults.
Selective increased volumes of CC in CCS-dependent adolescents and young adults
The number of callosal fibres is already fixed around birth, but structural changes of the CC continue to occur during postnatal development as a result of fibre myelination, redirection and pruning [9]. Callosal structural abnormalities, particularly increased subregion volume, suggest that chronic CCS abuse may affect axon pruning in adolescent and young adult CCS addicts. Research with monkeys [25], cats [26], and hamsters [27] has shown that approximately two-thirds of callosal axons are eliminated postnatally through adulthood [28]. Therefore, early arrest of this normal process of axonal pruning could contribute to the increased callosal white matter volume observed in CCS-dependent adolescents and young adults.
Increased callosal area or thickness has been found in several disorders that are thought to be neurodevelopmental or genetic, including psychotic disorders [33], schizophrenia [29], schizotypal personality disorder [30] and developmental language disorder [31]. The similar increased CC volumes observed in CCS-dependent male adolescents and young adults observed in the present study agree with previous reports, which showed that CCS misuse in adolescent or young adults frequently presented as mood and psychotic symptoms [32]. Thus, CCS abuse in adolescent and young adults during the period of CC development may have effects similar to these neurodevelopmental disorders. The selective increased volumes, which may indicate reduced pruning, of mid-posterior CC sections (CC2, CC3, CC4 and CC5 subregions) in CCS-dependent adolescent and young adults are consistent with prior studies in children and adolescents, which revealed trends for more pronounced development in posterior versus anterior callosal sections [33, 34].
However, our present findings are inconsistent with previous studies regarding the effects of alcohol and marijuana use on adolescent white matter development. Squeglia and colleagues compared grey and white matter volume trajectories between 75 youths who began drinking during adolescence and 59 continuously non-using controls over 4 years. They found that heavy-drinking youths showed abnormal neurodevelopmental trajectories compared to continuously non-using controls, including accelerated decreases in grey matter volume and attenuated increases in white matter volume over the follow-up, even after controlling for marijuana and other substance use [35]. Regarding white matter microstructure, adolescents with extensive marijuana and alcohol-use histories showed worsening white matter integrity over an 18-month [36] and 3-year follow-up in association, projection and interhemispheric white matter tracts when compared to non-using youths [37, 38]; however, volume changes may not be parallel with the diffusivity information [39]. These discrepancies may suggest a differential effect of CCS and other more common substance (alcohol and marijuana) use on adolescent brain development.
Aberrant interhemispheric homotopic functional connectivity in the OFC in CCS-dependent adolescents and young adults
In contrast to the mid-posterior CC abnormalities, we only found decreased interhemispheric homotopic functional connectivity in the OFC in CCS-dependent adolescents and young adults. The OFC is a prefrontal cortex region in the frontal lobe of the brain, which is involved in cognitive processing of decision-making. Aberrant OFC structure and function has been consistently found in substance addicts. Tanabe et al. [40] found decreased grey matter volume specifically in the bilateral medial OFC in substance-dependent individuals compared with control subjects. Several other previous functional neuroimaging studies have also found medial OFC abnormalities in substance-dependent individuals [41–45]. Our current finding provides evidence of disconnectivity between homotopic regions in the OFC, which complements previous studies.
Interhemispheric homotopic functional connectivity associated with impulsive behaviour and duration of CCS abuse
Interestingly, interhemispheric homotopic functional connectivity of the OFC correlated with duration of CCS abuse in CCS-dependent adolescents and young adults. This finding supports a previous hypothesis that substance addiction has cumulative effects; longer periods of substance abuse would lead to lower interhemispheric homotopic functional connectivity of the OFC [4, 44, 46–48]. If this hypothesis is true, early intervention may be particularly important for treatment of CCS addiction. However, as a cross-sectional study, the present results do not necessarily imply causality; a longitudinal study is needed in future.
It is noteworthy that we also found a significant correlation between interhemispheric homotopic functional connectivity of the OFC and impulsive behaviour in CCS-dependent adolescents and young adults. These findings support the idea that impulsivity is related to the bilateral medial OFC [49, 50]. Furthermore, they also suggest that interhemispheric homotopic functional connectivity might serve as a reliable biomarker when exploring brain function.
Uncoupled CC abnormalities and interhemispheric functional connectivity disruption in CCS-dependent adolescents and young adults
In the present study, we found selective increased volumes in the mid-posterior CC subregion but relatively reduced interhemispheric homotopic functional connectivity of the OFC. Although the mechanisms of these uncoupled findings are still not clear, the following hypotheses are consistent with these findings: (1) Functional and structural connectomes are not equivalent. They do not exhibit a one-to-one relationship, and functional connectivity cannot be considered merely a physiological index of anatomical connectivity [51]. (2) The aberrant larger CC may indicate reduced pruning of white matter, which can hinder the transmission of information and in turn contribute to the reduction of the interhemispheric functional connectivity. (3) The CC and its subregion volumes are a rough index of the morphometry. Diffusivity information can reflect microstructural integrity of white matter, but volume changes may not be parallel with the diffusivity information, which is a more powerful index for structural connectivity. However, we did not find diffusivity abnormalities of the CC in the subgroup of CCS-dependent adolescents and young adults in our previous study [48]. Diffusivity was maintained at a normal level even though fibres were unpruned. Future studies combined with animal experiments should be performed to verify the mechanism of these uncoupled changes.
Limitations
Several limitations should be considered. First, we used a standard symmetrical brain template for the calculation of voxel-based homotopic interhemispheric functional connectivity. We applied spatial smoothing to improve the spatial correspondence between homotopic areas and minimize the potential effect of intersubject variability. Second, because there are no obvious anatomical landmarks to define clearly how to subdivide the CC, we used FreeSurfer to equally subdivide the CC. The main disadvantage of this method was that either fibre compositions or fibre connections were not accurately reflected in the subregions. Third, as a cross-sectional study, we could only observe abnormal interhemispheric functional connectivity and aberrant larger CC subregion volumes in CCS-dependent adolescents and young adults. We cannot rule out the possibility that such detriments preceded initiation of CCS addiction and might constitute a predisposition to development of substance-related disorders. Fourth, we only enrolled male participants, so female participants must be included in future studies to fully understand CCS effects on brain development.
Conclusion
To the best of our knowledge, this is the first study to verify region-specific increased volumes of CC and homotopic interhemispheric functional disconnectivity in CCS-dependent male adolescents and young adults, as well as their relationships with impulsive behaviour. Selective increased volumes of mid-posterior CC was present in CCS-dependent adolescents and young adults, and decreased homotopic interhemispheric functional connectivity of the OFC was detected. Impairment of interhemispheric homotopic functional connectivity was associated with the impulsive behaviour and correlated with the duration of CCS abuse. These findings provide a novel insight into the impact of interhemispheric disconnectivity on impulsive behaviour in substance addiction pathophysiology.
References
Tomasch J (1954) Size, distribution, and number of fibres in the human corpus callosum. Anat Rec 119:119–135
Pfefferbaum A, Sullivan EV (2002) Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 15:708–718
van Ewijk H, Groenman AP, Zwiers MP et al (2015) Smoking and the developing brain: altered white matter microstructure in attention-deficit/hyperactivity disorder and healthy controls. Hum Brain Mapp 36:1180–1189
Qiu Y, Jiang G, Su H et al (2013) Progressive white matter microstructure damage in male chronic heroin dependent individuals: a DTI and TBSS study. PLoS One 8, e63212
Bora E, Yucel M, Fornito A et al (2012) White matter microstructure in opiate addiction. Addict Biol 17:141–148
Ma L, Hasan KM, Steinberg JL et al (2009) Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend 104:262–267
Lin F, Zhou Y, Du Y et al (2012) Abnormal white matter integrity in adolescents with internet addiction disorder: a tract-based spatial statistics study. PLoS One 7, e30253
Shek DT, Lam CM (2006) Adolescent cough medicine abuse in Hong Kong: implications for the design of positive youth development programs in Hong Kong. Int J Adolesc Med Health 18:493–503
Luders E, Thompson PM, Toga AW (2010) The development of the corpus callosum in the healthy human brain. J Neurosci 30:10985–10990
Squeglia LM, Gray KM (2016) Alcohol and drug use and the developing brain. Curr Psychiatry Rep 18:1–10
Squeglia LM, Jacobus J, Tapert SF (2009) The influence of substance use on adolescent brain development. Clin EEG Neurosci 40:31–38
De Lacoste MC, Kirkpatrick JB, Ross ED (1985) Topography of the human corpus callosum. J Neuropathol Exp Neurol 44:578–591
Abe O, Masutani Y, Aoki S et al (2004) Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr 28:533–539
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774
Yao S, Yang H, Zhu X et al (2007) An examination of the psychometric properties of the Chinese version of the Barratt Impulsiveness Scale, 11th version in a sample of Chinese adolescents. Percept Mot Skills 104:1169–1182
Fischl B (2012) FreeSurfer. Neuroimage 62:774–781
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Ji GJ, Zhang Z, Xu Q, Zang YF, Liao W, Lu G (2014) Generalized tonic-clonic seizures: aberrant interhemispheric functional and anatomical connectivity. Radiology 271:839–847
Yan CG, Cheung B, Kelly C et al (2013) A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76:183–201
Yan CG, Zang YF (2010) DPARSF: a MATLAB toolbox for "pipeline" data analysis of resting-state fMRI. Front Syst Neurosci 4:13
Zuo XN, Kelly C, Di Martino A et al (2010) Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci 30:15034–15043
Song XW, Dong ZY, Long XY et al (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6, e25031
Herron TJ, Kang X, Woods DL (2012) Automated measurement of the human corpus callosum using MRI. Front Neuroinform 6
Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE (1997) Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage 6:156–167
LaMantia A, Rakic P (1990) Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci 10:2156–2175
Bressoud R, Innocenti GM (1999) Typology, early differentiation, and exuberant growth of a set of cortical axons. J Comp Neurol 406:87–108
Halloran MC, Kalil K (1994) Dynamic behaviors of growth cones extending in the corpus callosum of living cortical brain slices observed with video microscopy. J Neurosci 14:2161–2177
Raine A, Lencz T, Taylor K et al (2003) Corpus callosum abnormalities in psychopathic antisocial individuals. Arch Gen Psychiatry 60:1134–1142
Narr KL, Thompson PM, Sharma T, Moussai J, Cannestra AF, Toga AW (2000) Mapping morphology of the corpus callosum in schizophrenia. Cereb Cortex 10:40–49
Downhill JE, Buchsbaum MS, Wei T et al (2000) Shape and size of the corpus callosum in schizophrenia and schizotypal personality disorder. Schizophr Res 42:193–208
Preis S, Steinmetz H, Knorr U, Jancke L (2000) Corpus callosum size in children with developmental language disorder. Brain Res Cogn Brain Res 10:37–44
Tang AK, Tang WK, Liang HJ, Chan F, Mak SC, Ungvari GS (2012) Clinical characteristics of cough mixture abusers referred to three substance abuse clinics in Hong Kong: a retrospective study. East Asian Arch Psychiatry 22:154–159
Giedd JN, Blumenthal J, Jeffries NO et al (1999) Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry 23:571–588
Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW (2000) Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature 404:190–193
Squeglia LM, Tapert SF, Sullivan EV et al (2015) Brain development in heavy-drinking adolescents. Am J Psychiatry 172:531–542
Bava S, Jacobus J, Thayer RE, Tapert SF (2013) Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res 37:E181–E189
Jacobus J, Squeglia LM, Bava S, Tapert SF (2013) White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res 214:374–381
Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF (2013) White matter integrity pre-and post marijuana and alcohol initiation in adolescence. Brain Sci 3:396–414
Lebel C, Caverhill-Godkewitsch S, Beaulieu C (2010) Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage 52:20–31
Tanabe J, Tregellas JR, Dalwani M et al (2009) Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry 65:160–164
Ersche KD, Fletcher PC, Lewis SJ et al (2005) Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 180:612–623
Botelho MF, Relvas JS, Abrantes M et al (2006) Brain blood flow SPET imaging in heroin abusers. Ann N Y Acad Sci 1074:466–477
Volkow ND, Wang GJ, Ma Y et al (2005) Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci 25:3932–3939
Qiu YW, Han LJ, Lv XF et al (2011) Regional homogeneity changes in heroin-dependent individuals: resting-state functional MR imaging study. Radiology 261:551–559
Yw Q, Jiang G, Ma Xf SHH, Xf L, Fz Z (2016) Aberrant interhemispheric functional and structural connectivity in heroin‐dependent individuals. Addict Biol. doi:10.1111/adb.12387
Qiu Y, Lv X, Su H et al (2013) Reduced regional homogeneity in bilateral frontostriatal system relates to higher impulsivity behavior in codeine-containing cough syrups dependent individuals. PLoS One 8, e78738
Qiu YW, Lv XF, Jiang GH et al (2014) Reduced ventral medial prefrontal cortex (vmPFC) volume and impaired vmPFC-default mode network integration in codeine-containing cough syrups users. Drug Alcohol Depend 134:314–321
Qiu YW, Su HH, Lv XF, Jiang GH (2015) Abnormal white matter integrity in chronic users of codeine-containing cough syrups: a tract-based spatial statistics study. AJNR Am J Neuroradiol 36:50–56
Bechara A, Damasio H, Tranel D, Damasio AR (1997) Deciding advantageously before knowing the advantageous strategy. Science 275:1293–1295
Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6:691–702
Kelly C, Castellanos FX (2014) Strengthening connections: functional connectivity and brain plasticity. Neuropsychol Rev 24:63–76
Acknowledgements
This work was supported by the grants from the Natural Scientific Foundation of China [Grant No. 81201084, 81560283], the Natural Scientific Foundation of Jiangxi Province, China [Grant No. 20151BAB205049], and Planned Science and Technology Project of Guangdong Province, China [Grant No. 2011B031800044]. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
The scientific guarantor of this publication is Professor Junzhang Tian. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. No study subjects or cohorts have been previously reported. Methodology: prospective, case–control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 197 kb)
Rights and permissions
About this article
Cite this article
Qiu, Yw., Lv, Xf., Jiang, Gh. et al. Larger corpus callosum and reduced orbitofrontal cortex homotopic connectivity in codeine cough syrup-dependent male adolescents and young adults. Eur Radiol 27, 1161–1168 (2017). https://doi.org/10.1007/s00330-016-4465-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4465-5