Abstract
Purpose
To determine if magnetic resonance imaging (MRI) histogram analyses can help predict response to chemotherapy in patients with colorectal hepatic metastases by using response evaluation criteria in solid tumours (RECIST1.1) as the reference standard.
Materials and methods
Standard MRI including diffusion-weighted imaging (b=0, 500 s/mm2) was performed before chemotherapy in 53 patients with colorectal hepatic metastases. Histograms were performed for apparent diffusion coefficient (ADC) maps, arterial, and portal venous phase images; thereafter, mean, percentiles (1st, 10th, 50th, 90th, 99th), skewness, kurtosis, and variance were generated. Quantitative histogram parameters were compared between responders (partial and complete response, n=15) and non-responders (progressive and stable disease, n=38). Receiver operator characteristics (ROC) analyses were further analyzed for the significant parameters.
Results
The mean, 1st percentile, 10th percentile, 50th percentile, 90th percentile, 99th percentile of the ADC maps were significantly lower in responding group than that in non-responding group (p=0.000–0.002) with area under the ROC curve (AUCs) of 0.76–0.82. The histogram parameters of arterial and portal venous phase showed no significant difference (p>0.05) between the two groups.
Conclusion
Histogram-derived parameters for ADC maps seem to be a promising tool for predicting response to chemotherapy in patients with colorectal hepatic metastases.
Key Points
• ADC histogram analyses can potentially predict chemotherapy response in colorectal liver metastases.
• Lower histogram-derived parameters (mean, percentiles) for ADC tend to have good response.
• MR enhancement histogram analyses are not reliable to predict response.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colorectal carcinoma is the third commonest form of cancers worldwide with approximately 14.5 % proportion of both synchronous liver metastases and 5-year cumulative metachronous liver metastases [1]. The prognosis of colorectal liver metastases mainly depend on the progression of liver metastases. In patients with resectable colorectal liver metastasis (CLM), preoperative MR imaging have the potential to improve the detection of lesions and to determine the correct therapeutic strategy [2, 3], which may lead to better prognosis and survival. However, the majority of patients with CLM are not suitable for resection due to the size, location, extent of disease, etc. Systemic chemotherapy contributes to reduce risk of recurrence after resection [4], facilitates the resection of lesions initially not suitable for resection [5, 6] and improves survival of patients with unresectable liver metastases [7]. However, chemotherapy results in a variety of side effects on the liver parenchyma such as steatosis, steatohepatitis, and sinusoidal obstruction syndrome (SOS) [8], which are associated with higher morbidity and mortality after liver resection [9–11]. Therefore, considering the high cost and hepatic toxicity of chemotherapies, prediction of treatment response before chemotherapy is not only a clinical necessity, but also an economic requirement.
Traditionally, the treatment effect of chemotherapy is determined by using response evaluation criteria in solid tumours (RECIST 1.1) criteria, which is based on measuring changes in the longest axial tumour diameter after chemotherapy on computed tomography (CT) or MRI; however, it lags behind. Recently, some novel imaging approaches, such as perfusion computed tomography (CT), positron emission tomography (PET)/CT, perfusion magnetic resonance imaging (MRI), diffusion weighted imaging (DWI) [12–16], are being developed to predict tumour response before chemotherapy. Previously published studies focusing on the value of perfusion CT [12, 13] or perfusion MRI [14, 15] for predicting the response of chemotherapy according to RECIST 1.1 show conflicting results. Furthermore, problems caused by the dual blood supply of liver, lack of standardization, and respiratory movement during the imaging acquisition of liver perfusion imaging need to be solved. Previous studies reported that pretreatment PET/CT had potential value for prediction of size-based response categories [13, 14]. Challenges for PET/CT include limited availability, cost, and ionizing radiation. DWI is a functional MR imaging technique that can detect the mobility of tissue water in vivo, thus can give information at a microscopic level. Apparent diffusion coefficient (ADC) describes the velocity and scope of molecular diffusion movement in various directions. Previous studies have indicated the potential usefulness of DW MR imaging in helping predict early response to therapy in liver metastases [16–18].
In addition to mean value measurements, histogram as an advanced technique which can examine the heterogeneity of voxel, has been indicated to have more advantages when applied to radiologic imaging [19–22]. Studies had demonstrated that pre-treatment ADC histogram analysis could be as useful predictor for chemotherapy/radiotherapy response in patients with other malignant tumours [23–25]. To our knowledge, there are no reports yet on the potential value of MR histogram analysis to predict the response to chemotherapy according to RECIST 1.1 criteria in patients with colorectal liver metastases.
The purpose of this study, therefore, was to investigate the value of pretreatment MR enhancement and ADC histogram analyses in prediction for chemotherapy response in patients with colorectal liver metastases by using the RECIST1.1 criteria as the reference standard.
Materials and methods
This single-institution study comprised a retrospective evaluation of image data from patients undergoing pre-treatment staging MR for liver metastases from colorectal cancer. This retrospective study was approved by institutional review board of our hospital and the requirement for informed consent was waived.
Patients
Fifty-three patients (34 male, 19 female) with synchronous or metachronous liver-limited metastases from colorectal cancer (stage IV), who underwent fluorouracil-based chemotherapy at our institution between March 2009 and April 2014, were included. The study group was selected from the institutional archive according to the following exclusion criteria: (1) pre-treatment MR not performed at our department; (2) images of MR or CT after chemotherapy not available; (3) patients who received liver local treatment as follows: transarterial chemoembolization, radiofrequency, radiotherapy or resection during follow-up; (4) patients who were discovered with other extra-hepatic metastases before chemotherapy.
MR examination
All MR images were acquired on a 1.5T MR system (Magnetom Avanto; Siemens Medical Solutions, Erlangen, Germany) with a body phased-array coil anterior and a spine array coil posterior. An axial breath-hold DW (b=0, 500 s/mm2) sequence with a single-shot, echo-planar sequence was performed by using generalized auto-calibrating partially parallel acquisition (GRAPPA,R=2) with the following parameters: repetition time(TR)/echo time(TE)= 2600 ms/66 ms, 128×112 matrix, 380–400×300–324-mm field of view, 7-cm section thickness with 2.1-mm gap, and 1500Hz/pixel bandwidth. Corresponding ADC maps were automatic calculated by the standard console software of the system. A 3D T1-weighted gradient echo sequence (volumetric interpolated breath-hold examination, VIBE)with GRAPPA (R=2) was performed before and after injection of the contrast media. The following parameters as followed: 5.04/2.31 (TR ms/TE ms), 12° flip angle, 256×192 matrix, 380–400×300–324-mm field of view, 24-cm slab thickness resulting in an interpolated 4-mm section thickness, and 300Hz/pixel/bandwidth. For dynamic contrast enhanced (DCE)-MRI, gadopentate dimeglumine (Magnevist, Bayer Schering Pharma AG, Berlin, Germany, 0.1 mmol/kg) was rapidly injected manually (at a rate of approximately 2 mL/s) by one investigator through a 20-gauge intravenous catheter placed in a cubital or cephalic vein. Immediately afterward, a 20-mL saline flush was injected at the same rate. Arterial phase (AP), portal venous phase (PVP) and equilibrium phase were obtained at 20–30 s, 70–80 s, and 180 s from the start of injection, respectively.
Histogram analyses
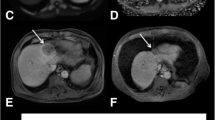
Histogram analyses were performed by using Mazda (MaZda for Windows, B11 ver. 4.6, www.eletel.p.lodz.pl/programy/mazda/) [26]. For each pre-treatment MR examination, images including ADC map, AP, and PVP were separately selected and transferred from the hospital’s PACS-system to a personal computer. For each patient, the histogram of the largest available metastatic lesion was assessed by a single radiologist with 2 years specific experience in abdominal imaging, who manually delineated free-hand region of interest (ROI) around the largest cross-sectional area of the tumour. Gray-level normalization of each ROI was performed, using the limitation of dynamics to μ ± 3σ (μ, gray-level mean; and σ, gray-level standard deviation), to minimize the influence of contrast and brightness variation. The histogram data was generated for the ROI (Figs. 1 and 2) and the following parameters were calculated: mean, variance, skewness, kurtosis, and 1st, 10th, 50th, 90th, and 99th percentile. These parameters are defined mathematically in Table 1.
Standard of reference
The evaluation of chemotherapy response was strictly in accord with RECIST guideline (version 1.1) [27]. For each patient, one (in cases of a solitary metastasis) or two individual metastases were selected as target lesions, and the largest diameter of these target lesions was measured on the portal venous phase of pre-treatment MR as well as post-chemotherapy MR or CT in hospital’s PACS-system. The response of target lesions were categorized as follows. CR (Complete Response): disappearance of target lesion; PR (Partial Response): at least a 30 % decrease in the diameters of target lesions; PD (Progressive Disease): at least a 20 % increase in the diameters of target lesions; SD (Stable Disease): neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD. Note: the appearance of new lesions or extra-hepatic metastases is also considered PD. Patients with SD and PD were classified as non-responding while CR and PR were classified as responding.
Statistical analyses
Baseline characteristics of the patients were expressed as mean and standard deviation or count and proportion. Continuous variables were compared with Student’s t test (or Mann-Whitney U test when not normally distributed), and categorical variables were compared by the chi-squared test or Fisher’s exact test as appropriate. Mean values of histogram parameters were compared between the groups of responders and non-responders using Student’s t test, or Mann-Whitney U test when not normally distributed. For the significant measures, receiver operating characteristics (ROC) analyses were constructed to determine their potential diagnostic performance for differentiating the good responders from the poor responding patients. Corresponding area under the ROC curve (AUCs) with 95 % confidence intervals (95 % CI) were calculated. Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, version 21.0, Inc., Chicago, IL, USA) and MedCalc (MedCalc for Windows, version11.5.0.0, www.medcalc.be). Differences with a P-value less than 0.05 were considered statistically significant.
Results
Response to chemotherapy treatment
Patient characteristics and the chemotherapeutic regimens are detailed in Table 2. According to the RECIST version 1.1 criteria, 38 out of 53 patients were classified as non-responders (13 progressions, 25 stable diseases), while the other 15 patients were classified as responders (14 patients showed a partial response after chemotherapy, and one patient showed a complete response). With regard to age, gender, target therapy (19 patients treated with cetuximab, three patients with bevacizumab), synchronous or metachronous metastases, tumour size in largest lesion diameter, the site of target lesion, time frame between pre- and post-treatment imaging, necrosis, chemotherapy (without target therapy), there were no statistically significant differences between the two groups.
The histogram measurements
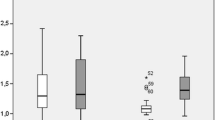
The mean values of histogram parameters (mean, variance, skewness, kurtosis, and 1st, 10th, 50th, 90th, and 99th percentile) for ADC map, AP, and PVP images are provided in Table 3. Mean, 1st percentile, 10th percentile, 50th percentile, 90th percentile, and 99th percentile of ADC maps were significantly lower in the responding group than that in non-responding group (p=0.000–0.002). Mean, 1st percentile, 10th percentile, 50th percentile, 90th percentile, and 99th percentile of AP and PVP images were slightly higher in responding group compared with non-responding group, but the difference was not statistically significant (p=0.36–0.67). For variance, skewness and kurtosis of ADC maps, AP, and PVP images, there were no significant differences between the responder and non-responder (p=0.14–0.85).
Diagnostic performance for assessment of responder
For the abovementioned parameters that showed a significant difference in the histogram analyses between responder and non-responder (see Table 3), ROC curve analyses were performed to evaluate the performance for assessment of responder to chemotherapy. Corresponding AUCs, sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) were showed in Table 4 and Fig. 3. The AUCs of the above-mentioned significant parameters of ADC histogram analyses in prediction of responder to chemotherapy were 0.79, 0.76, 0.76, 0.79, 0.80, 0.82 for Mean, 1st percentile, 10th percentile, 50th percentile, 90th percentile, 99th percentile, respectively.
Discussion
Our results indicated that histogram-derived parameters for ADC maps were useful for discrimination between responders (PR and CR) and non-responders (SD and PD) to chemotherapy in patients with colorectal hepatic metastases with AUCs for predicting responders ranging between 0.76–0.82. In contrast, histogram-derived parameters for DCE-MR images including AP and PVP images were not reliable to discriminate between responders and non-responders.
Previously published data focusing on ADC measurement showed conflicting results for predicting response to chemotherapy in patients with liver metastases [12, 16, 17]. Koh et al [17] reported that mean pretreatment ADC (b values of 150 and 500 s/mm2) of 1.69 ×10–3 mm2/s had 60 % sensitivity and 100 % specificity for identification of colorectal hepatic metastases not responding to chemotherapy. Cui et al [16] also demonstrated that pretreatment ADC values in responding lesions were significantly lower than those of non-responding lesions in the patients with colorectal and gastric hepatic metastases. However, the results of study by Anzidei did not support the use of ADC as a potential response predictor despite the limitation of the statistical sample in their study [12]. The findings of our study showed that mean, 1st percentile, 10th percentile, 50th percentile, 90th percentile, and 99th percentile of the ADC histogram parameters were significantly lower in responding group with AUCs up to 0.82 for identifying responder to chemotherapy.
In heterogeneous tumours, regions with densely cellular concentration have lower ADC, whereas in tissues with less densely packed obstacles (i.e. necrosis, non-neoplastic tissue) the ADC values are higher [28]. Both animal models and clinical studies had also shown that necrotic tissues had higher ADCs and that lower ADCs in tumours indicated high viability [29]. Herneth et al [30] demonstrated that the areas with an increased ADC within tumour correlated well with areas of necrosis (reduced cell density) in the animal model with squamous cell tumour. In our study, lesions with lower pretreatment ADC tended to have good response to chemotherapy, The possible explanations might be that regions of high cell density within a tumour are usually well perfused, resulting in high delivery and retention of chemotherapeutic drugs in these areas. Furthermore, tumour cells in hypoperfusion areas are exposed to a more hypoxic and acidic environment, which diminish the effectiveness of chemotherapy.
Apart from the mean values, histograms provide data on other parameters and give us insight on the distribution of imaging biomarkers over all voxels for ADC maps. Central necrosis which is typical in colorectal liver metastases might increase the ADC value. We chose to analyze an ADC histogram of the tumour that would enable depiction of the heterogeneity of a lesion (viable portions would show lower ADC values than those of the necrotic portions). In our study, the 99th percentile of the ADC results in the highest diagnostic performance for predicting responding to chemotherapy (AUC, 0.82). The 99th percentile is a measure indicating the value below which 99 % of the ADC observations within the study group fall. In other words, the 1 % maximum values of the histograms are excluded. These 1 % maximum values might represent small areas of free fluid (necrosis) within tumour. Exclusion of these 1 % maximum values may thus lead to ADC data that are better representative for the actual diffusion characteristics of the tumour itself.
In the present study, we failed to demonstrate a benefit for MR enhancement histogram analyses to predict the response to chemotherapy. The effect of chemotherapy relies on the delivery of chemotherapeutic agents which is associated with vascular supply of liver metastases. In theory, the mean and heterogeneity of contrast enhancement, implying blood supply of tumour, might potentially provide predictive information for treatment response. This apparent discrepancy might reflect the fact that enhancement of tumour is a non-specific marker that is influenced by several physiological correlates including blood flow, capillary permeability, blood volume, and extravascular leakage space. Studies reported that the pretreatment parameters of blood flow and blood volume derived from perfusion CT were not useful for prediction of response [12, 13], while capillary permeability was demonstrated as a potential response predictor [12]. Of interest, however, was our finding that a subtle trend towards increased mean, 1st percentile, 10th percentile, 50th percentile, 90th percentile, and 99th percentile of AP and PVP images in responders compared with non-responders, which implies increased vascular supply delivering more chemotherapeutic agents in responders. In our study, histogram analyses of signal intensity at a single time point (AP and PVP) may not sufficiently reflect perfusion status of hepatic metastases, which could not discriminate vascular supply of responders from non-responders. One remedy to this problem might be by the application of the analysis of dynamic enhanced enhancement, which is a relatively new approach to determine perfusion parameters and quantify the microcirculatory status of hepatic tumour [15, 31]. Therefore the heterogeneity of blood perfusion as assessed using histogram analyses might become more evident on MR perfusion parameter maps.
There are several limitations in our study. Firstly, the selection bias is inevitable because it is a retrospective study. Secondly, we performed DWI using only two b-values of 0 and 500s/mm2, further studies on the diffusion-weighted imaging with multiple b-values for response evaluation would be helpful [18]. Thirdly, the systemic chemotherapy was variable. In theory, tumours treated with target drugs and standard chemotherapy increase the response rates compared with standard chemotherapy alone. However, our study showed no significant difference for target drugs between responders and non-responders groups. Fluorouracil was included as a basic chemotherapeutic drug in the treatment regimen in most patients in our study. Hence, we believe that the effect of chemotherapy regimens on our study results was limited. Despite small sample, we found that ADC histogram could be a useful predictor. We plan to pursue future research in this field with the inclusion of more patients and more variables such as interval changes in ADC histogram. Fourthly, we did not use the pathologic response as reference standard. In our study, only some patients underwent liver metastases resection. However, treatment response is mainly assessed by RECIST1.1 in daily clinical practice. Finally, the cases included synchronous and metachronous liver metastases and the pre-metastatic chemotherapy in metachronous liver metastases might have potential influence on the treatment in this study.
In conclusion, histogram analyses of ADCs showed high accuracy for predicting response of chemotherapy in patients with colorectal liver metastases while MR enhancement histogram analyses were not reliable. Furthermore, 99th percentile of ADC map might be the potential treatment predictor.
Abbreviations
- MRI:
-
magnetic resonance imaging
- RECIST:
-
response evaluation criteria in solid tumours
- ADC:
-
apparent diffusion coefficient
- ROC:
-
receiver operator characteristics
- AUCs:
-
area under the ROC curve
- CLM:
-
colorectal liver metastasis
- SOS:
-
sinusoidal obstruction syndrome
- CT:
-
computed tomography
- PET:
-
positron emission tomography
- DWI:
-
diffusion weighted imaging
- TR:
-
repetition time
- TE:
-
echo time
- VIBE:
-
volumetric interpolated breath-hold examination
- DCE:
-
dynamic contrast enhanced
- AP:
-
arterial phase
- PVP:
-
portal venous phase
- ROI:
-
region of interest
- CR:
-
complete response
- PR:
-
partial response
- PD:
-
progressive disease
- SD:
-
stable disease
- PPV:
-
positive predictive values
- NPV:
-
negative predictive values
References
Manfredi S, Lepage C, Hatem C et al (2006) Epidemiology and management of liver metastases from colorectal cancer. Ann Surg 244(2):254–259
Sofue K, Tsurusaki M, Murakami T et al (2014) Does Gadoxetic acid-enhanced 3.0T MRI in addition to 64-detector-row contrast-enhanced CT provide better diagnostic performance and change the therapeutic strategy for the preoperative evaluation of colorectal liver metastases? Eur Radiol 24(10):2532–2539
Scharitzer M, Ba-Ssalamah A, Ringl H et al (2013) Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol 23(8):2187–2196
Nordlinger B, Van Cutsem E, Gruenberger T et al (2009) Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol 20:985–992
Bruera G, Cannita K, Giuliante F et al (2012) Effectiveness of Liver Metastasectomies in Patients With Metastatic Colorectal Cancer Treated With FIr-B/FOx Triplet Chemotherapy Plus Bevacizumab. Clin Colorectal Cancer 11(2):119–126
Nordlinger B, Sorbye H, Glimelius B et al (2008) Perioperative chemotherapy with FOLFOX4 and surgery vs. surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet 371:1007–1016
Lehmann K, Rickenbacher A, Weber A, Pestalozzi BC, Clavien PA (2012) Chemotherapy before liver resection of colorectal metastases: friend or foe? Ann Surg 255(2):237–247
Robinson SM, Wilson CH, Burt AD et al (2012) Chemotherapy-Associated Liver Injury in Patients with Colorectal Liver Metastases: A Systematic Review and Meta-analysis. Ann Surg Oncol 19:4287–4299
Kneuertz PJ, Maithel SK, Staley CA, Kooby DA (2011) Chemotherapy associated liver injury: impact on surgical management of colorectal liver metastases. Ann Surg Oncol 18:181–190
Marsman HA, Van Der Pool AE, Verheij J et al (2011) Hepatic steatosis assessment with CT or MRI in patients with colorectal liver metastases after neoadjuvant chemotherapy. J Surg Oncol 104:10–16
Vauthey JN, Pawlik TM, Ribero D et al (2006) Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 24:2065–2072
Anzidei M, Napoli A, Zaccagna F et al (2011) Liver Metastases From Colorectal Cancer Treated With Conventional and Antiangiogenetic Chemotherapy: Evaluation With Liver Computed Tomography Perfusion and Magnetic Resonance Diffusion-Weighted Imaging. J Comput Assist Tomogr 35:690–696
Kim DH, Kim SH, Im SA et al (2012) Intermodality comparison between 3D perfusion CT and 18F-FDG PET/CT imaging for predicting early tumor response in patients with liver metastasis after chemotherapy: Preliminary results of a prospective study. Eur J Radiol 81:3542–3550
De Bruyne S, Van Damme N, Smeets P et al (2012) Value of DCE-MRI and FDG-PET/CT in the prediction of response to preoperative chemotherapy with bevacizumab for colorectal liver metastases. Br J Cancer 106(12):1926–1933
Coenegrachts K, Bols A, Haspeslagh M et al (2012) Prediction and monitoring of treatment effect using T1-weighted dynamic contrast-enhanced magnetic resonance imaging in colorectal liver metastases: Potential of whole tumour ROI and selective ROI analysis. Eur J Radiol 81:3870–3876
Cui Y, Zhang XP, Sun YS et al (2008) Apparent Diffusion Coefficient: Potential Imaging Biomarker for Prediction and Early Detection of Response to Chemotherapy in Hepatic Metastases. Radiology 248:894–900
Koh DM, Scurr E, Collins D et al (2007) Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol 188:1001–1008
Kukuk GM, Murtz P, Traber F et al (2014) Diffusion-weighted imaging with acquisition of three b-values for response evaluation of neuroendocrine liver metastases undergoing selective internal radiotherapy. Eur Radiol 24(2):267–276
Filss CP, Stoffels G, Galldiks N et al (2014) Histogram analysis reveals a better delineation of tumor volume from background in 18F-FET PET compared to CBV maps in a hybrid PET–MR studie in gliomas. Nucl Inst Methods Phys Res A 734:175–178
Kim JY, Kim JK, Kim N et al (2008) CT Histogram Analysis: Differentiation of Angiomyolipoma without Visible Fat from Renal Cell Carcinoma at CT Imaging. Radiology 246:472–479
Kyriazi S, Collins DJ, Messiou C et al (2011) Metastatic Ovarian and Primary Peritoneal Cancer: Assessing Chemotherapy Response with Diffusion-weighted MR Imaging —Value of Histogram Analysis of Apparent Diffusion Coefficients. Radiology 261:182–192
Nowosielski M, Recheis W, Goebel G et al (2011) ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53:291–302
Pope WB, Lai A, Mehta R et al (2011) Apparent Diffusion Coefficient Histogram Analysis Stratifies Progression-Free Survival in Newly Diagnosed Bevacizumab-Treated Glioblastoma. AJNR Am J Neuroradiol 32:882–889
Pope WB, Kim HJ, Huo J et al (2009) Recurrent Glioblastoma Multiforme: ADC Histogram Analysis Predicts Response to Bevacizumab Treatment. Radiology 252:182–189
Pope WB, Qiao XJ, Kim HJ et al (2012) Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol 108:491–498
Szczypiński PM, Strzelecki M, Materka A et al (2009) MaZda--a software package for image texture analysis. Comput Methods Prog Biomed 94(1):66–76
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Lyng H, Haraldseth O, Rofstad EK (2000) Measurement of cell density and necrotic fraction in human melanoma xenografts by diffusion weighted magnetic resonance imaging. Magn Reson Med 43:828–836
Deng J, Rhee TK, Sato KT et al (2006) In vivo diffusion-weighted imaging of liver tumor necrosis in the VX2 rabbit model at 1.5 Tesla. Invest Radiol 41:410–414
Herneth AM, Guccione S, Bednarski M (2003) Apparent diffusion coefficient: a quantitative parameter for in vivo tumor characterization. Eur J Radiol 45(3):208–213
Rao SX, Chen CZ, Liu H et al (2013) Three-dimensional whole-liver perfusion magnetic resonance imaging in patients with hepatocellular carcinomas and colorectal hepatic metastases. BMC Gastroenterol 13:53
Acknowledgments
The scientific guarantor of this publication is Sheng-Xiang Rao. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. This study has received funding by the National Science Foundation of China (Grant No. 81371543. No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, case-control study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, HY., Huang, YQ., Yang, ZX. et al. Potential of MR histogram analyses for prediction of response to chemotherapy in patients with colorectal hepatic metastases. Eur Radiol 26, 2009–2018 (2016). https://doi.org/10.1007/s00330-015-4043-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4043-2