Abstract
Objectives
The aim of this prospective study was to compare the diagnostic performance of 64-row MDCT and gadoxetic-acid-enhanced MRI at 3.0 T in patients with colorectal liver metastases in correlation with histopathological findings.
Methods
Lesions detected at MDCT and MRI were interpreted by three blinded readers and compared with histopathological workup as the term of reference. Two subgroups of lesions were additionally evaluated: (1) metastases smaller than 10 mm and (2) lesions in patients with and without steatosis of the liver, assessed histopathologically.
Results
Surgery and histopathological workup revealed 81 colorectal liver metastases in 35 patients and diffuse metastatic involvement in 3 patients. In a lesion-by-lesion analysis, significant sensitivity differences could only be found for reader 1 (P = 0.035) and reader 3 (P = 0.003). For segment-based evaluation, MRI was more sensitive only for reader 3 (P = 0.012). The number of false-positive results ranged from 3 to 12 for MDCT and 8 to 11 for MRI evaluation. In the group of small lesions, the sensitivity differed significantly between both methods (P = 0.003). In patients with hepatic steatosis, MRI showed a trend toward better performance than MDCT, but without statistical performance.
Conclusions
The 3.0-T MRI with liver-specific contrast agents is the preferred investigation in the preoperative setting, especially for the assessment of small colorectal liver metastases.
Key Points
• Potential surgical treatment requires accurate radiological assessment of colorectal liver metastases
• Magnetic resonance imaging with gadoxetic acid is the preferred imaging investigation.
• MRI is better than multidetector CT for detecting small liver metastases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical resection of colorectal liver metastasis has evolved as a potentially curative treatment in patients with metastatic disease confined to the liver. Recent advances in multimodality treatment, with the implementation of atypical parenchyma-sparing resections, have reduced postoperative mortality, and the success of neoadjuvant chemotherapy has increased the number of patients who are candidates for liver surgery. Thus, in the last several decades, the 5-year overall survival after hepatic resection of colorectal liver metastases has increased to 35–58 % [1–3]. Even multiple metastases in both liver lobes are no longer a contraindication for surgical resection if sufficient amounts of liver tissue remain [4, 5]. However, the complete elimination of intrahepatic metastases is mandatory for curative purposes. This requires an accurate localisation of all intrahepatic lesions prior to surgical resection and underlines the importance of diagnostic imaging [6].
The detection of focal liver lesions with multidetector-row CT (MDCT) or gadolinium-enhanced MRI is based on morphological criteria and specific enhancement pattern following intravenous (IV) contrast agents. In order to improve the performance of liver lesion detection with MR imaging, several liver-specific MRI contrast media have been developed. Gadoxetic acid (Primovist®, Bayer Healthcare, Berlin, Germany) is a liver-specific agent that can be used for both perfusion and hepatocyte-specific imaging. After IV injection, a considerable and prolonged [7] enhancement of the liver parenchyma due to hepatocellular uptake can be observed, whereas non-hepatocellular lesions show only minimal or no enhancement. Several clinical studies have proven an increase in the detection of liver lesions with these hepatocyte-specific contrast media, with a diagnostic performance comparable or even superior to standard non-specific gadolinium chelates or spiral CT [8–11].
Only a few studies have compared gadoxetic-acid-enhanced 1.5-T MRI and contrast-enhanced MDCT for the detection of liver metastases [12–15]. MRI at a higher field strength as well as 64-row MDCT with more slices results in an improved image resolution and increased detection rate of liver lesions [16, 17]. To our knowledge, studies comparing 3.0-T and 64-slice CT in patients with colorectal liver metastases, with histopathological verification, are still lacking. The purpose of this study was to compare gadoxetic acid-enhanced MRI at 3.0 T with contrast-enhanced 64-row MDCT for the detection of colorectal liver metastases in patients who were candidates for surgical liver resection in correlation with histopathological findings.
Materials and methods
Patient population
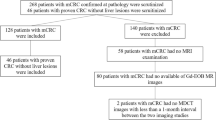
The study was designed as a prospective trial with the aim to compare the sensitivity of gadoxetic acid-enhanced MRI and 64-row MDCT in the detection of hepatic colorectal metastases. The protocol for this study was reviewed and approved by the Institutional Review Board of our institution. All patients gave their written informed consent to both investigations. The patients enrolled in this study were highly suspected of having hepatic colorectal metastases on the basis of their history of colon carcinoma and previous ultrasound or CT studies performed at an outside institution. We included 43 patients, who underwent preoperative gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced 64-row MDCT (time interval, 0–8 days) between November 2006 and February 2008 for highly suspected hepatic metastases from colon cancer. Six of them were excluded from the study because of diffuse metastatic involvement, and, in two patients, the MR examination had to be terminated before contrast administration because of claustrophobia (n = 1) and severe agitation (n = 1). Thus, 35 patients completed imaging and subsequently underwent resection of their liver metastases (Fig. 1). In these patients, the mean time interval between surgical resection and the second imaging examination (either MDCT or MRI) was 3 days (range, 0–19 days). In this final study group (35 men, 10 women), the mean age was 65 years (range, 41–87 years).
Magnetic resonance imaging
MRI examinations were performed on a superconducting magnet operating at 3.0 T (Magnetom Trio Tim; Siemens Medical Solutions, Erlangen, Germany) and equipped with a phased-array coil, which was placed on the upper abdomen, and a spine coil that was placed dorsally. Routine liver MR images were acquired in transverse planes using the following sequences: a 2D dual-echo breath-hold T1-weighted GRE (FLASH) sequence, field-of-view (FOV) 350 mm, at in-phase [repetition time (TR)/effective echo time (TE), 130/2.46]; an opposed-phase (130/3.69), slice thickness 5 mm; a navigator-triggered T2-weighted turbo spin-echo (TSE) sequence 2,000/95 (TR/TE), FOV 370 mm, slice thickness 5.5 mm; and a breath-hold T2-weighted HASTE with fat supression, 1,800/151 (TR/TE), FOV 400 mm, slice thickness 5.5 mm. In 23 patients, diffusion-weighted MR imaging using a breath-hold, axial, single-shot 2D echo-planar imaging (EPI) sequence (3,049/76), FOV 380 mm, slice thickness 6 mm, was performed. Diffusion-weighted MR imaging was obtained with b-values of 50 s/mm2, 400 s/mm2, and 800 s/mm2. ADC maps were calculated on a voxel-by-voxel basis with an implemented algorithm.
After intravenous administration of a bolus of gadoxetate disodium (Primovist®, Bayer Healthcare; 0.025 mmol/kg body weight) through an intravenous cubital line, followed by a saline flush of 20 ml, dynamic T1-weighted MRI was performed using a breath-hold T1-weighted fat-suppressed volumetric interpolated sequence (VIBE) at 30, 70, and 130 s after contrast material administration during the hepatic arterial, portal, and equilibrium phases, respectively (TR/TE 3.13/1.24, slice thickness 1.7 mm, FOV 350 mm), followed by the same sequence in the coronal plane (TR/TE 3.13/2.46, 2 mm slice thickness, 400-mm FOV), and then by a T1-weighted 2D breath-hold fat-saturated FLASH GRE sequence, 5 mm slice thickness. This sequence was repeated 20 min after the injection of gadoxetic acid. In addition, there was a T1-weighted fat-suppressed VIBE sequence in the axial (slice thickness 1.7 mm) and coronal planes (slice thickness 2 mm).
Multidetector CT imaging
All CT examinations were performed using 64-slice multidetector CT (Sensation 64; Siemens) with a detector configuration of 64 × 0.6 mm. The following parameters were used: tube voltage, 120 kV; tube current, 220 mAs with automatic dose modulation; gantry rotation time, 0.5 s; detector collimation, 64 × 0.6 mm, pitch 1. Precontrast CT images of the upper abdomen were obtained, then nonionic contast medium (iomeprol 300 mg/ml) was administered intravenously as a bolus of 2 ml/kg body weight, up to a maximum of 150 ml, using a power injector with a flow rate of 5 ml/s and followed by a 40 ml saline flush. Image acquisition was obtained in the arterial and portal venous phases after contrast-medium injection using a bolus tracking programme provided by the manufacturer of the CT unit. All images were reconstructed with a section thickness of 3 mm and an increment of 2 mm for axial slices and coronal multiplanar reformations.
Image evaluation
Three radiologists (W.S., H.R., and A.B., all with more than 10 years' experience in CT and MRI of the abdomen) independently reviewed the CT and MRI images to identify all of the focal intrahepatic lesions. They were informed that this study included only patients with colorectal metastases, but were not provided with any other information about the patients. For the detection and characterisation of liver lesions, all images from each CT examination were analysed together. Analysis of MR images was performed using all available MR pulse sequences. To minimise any decision threshold bias due to reading-order effects, the CT and MRI reading sessions were presented in a randomised sequence and separated by at least 8 weeks. Readers were aware that all patients had known colorectal hepatic metastases. All images were evaluated on a 2K monitor (Barco, Kortrijk, Belgium), and the optimal window setting was adjusted individually in each patient, as required by the reviewer.
The whole liver was divided into eight hepatic segments according to Couinaud’s classification [18]. For each assessment, liver maps were completed by drawing each liver lesion on a respective template according to the Couinaud system of liver anatomy. This was done for MR and CT images separately. Then, the reader assessed the lesion for size (defined as a lesion’s maximum diameter on the most representative transverse image) and the likelihood for metastatic disease. The criteria for the radiological diagnoses of hepatic metastasis were irregular or ill-defined borders, low T1 signal intensity and variably high T2 signal intensity, and lack of or only minimal enhancement on hepatobiliary phase images (Fig. 2). The three readers graded the likelihood that a lesion represented a metastasis using a five-point scale: 1, definitely benign lesion; 2, probably benign lesion; 3, equivocal; 4, probably metastasis; 5, definitely metastasis.
Coexisting benign and malignant lesion in the same patient. a Contrast-enhanced MDCT in the portal venous phase shows a minimally hyperattenuating lesion near the gallbladder (arrow) and a large hypoattenuating lesion in the right lobe (arrowhead). b Unenhanced MRI (T1-weighted 2D GRE) slightly delineates the lesion in segment IV (arrow) and a hypointense large lesion in the right lobe (arrowhead). c After gadoxetic acid administration (T1-weighted VIBE arterial phase), homogeneous enhancement of the lesion in segment IV is shown (arrow) without enhancement of the other lesion (arrowhead). d Hepatobiliary phase MRI (T1-weighted 2D GRE) confirms the hepatocellular origin of the lesion (FNH) in segment IV, with uptake of liver-specific contrast material (arrow) and metastatic origin of the lesion in the right lobe (arrowhead)
The readers classified each liver segment (1–8, according to the classification of Couinaud) as free of metastases or as containing at least one metastasis using a five-point scale: 1, definitely no metastasis; 2, probably no metastasis; 3, equivocal; 4, probably metastasis within a segment; 5, definitely metastasis within liver segment. When a lesion had invaded two or more segments, the readers were asked to evaluate all involved segments. In total, 280 segments were assessed.
A fourth radiologist (M.S.) correlated the scored lesions with the histopathological reference standard on the basis of the description regarding the size and location of the lesion as a coordinate. To calculate the sensitivity, specificity, and accuracy of each investigation, scores of 5, 4, and 3 were considered positive, and scores of 1 or 2 were considered negative.
Standard of reference
For all patients, diagnoses of metastases were based on histopathologic findings. The resected pathological specimens were routinely sectioned with a slice thickness of 5 mm. Assignment of detected liver lesions was enabled by matching the marked lesions on the evaluation sheets on the liver graphs to the histopathological report and the surgical report, which included a description of intraoperative sonography. In three patients, an exact correlation of histopathological findings and the reader’s evaluation was impossible due to diffuse metastatic hepatic involvement. In these three patients, histopathological workup ruled out other hepatic lesions than colorectal metastases. For lesion-based analysis, therefore, a maximum of five lesions was classified as correctly assessed if they were assigned to the correct liver segment by the different readers.
Statistical analysis
Data were analysed using a statistical software programme (IBM SPSS 19.0 for Windows, Chicago, IL). Metric data, such as numbers of detected lesions, are described using mean ± standard deviation. In order to compare the mean number of detected lesions, Greenhouse–Geisser-corrected, two-way repeated measures ANOVA was used. In addition, lesion- and segment-based sensitivities and specificities were calculated. In order to compare the two investigations and different readers, a logistic regression for repeated measures (GEE) was used, taking multiple measures per patient into account. Furthermore, McNemar tests were used for pairwise post hoc comparisons. In addition, two more subgroups of lesions were created: (1) a comparison between CT and MR imaging for the detection of lesions smaller than 10 mm; (2) a comparison of detection of all lesions on MDCT and gadoxetic-acid enhanced MR imaging in patients with and without steatosis of the liver. The amount of fatty infiltration of the liver was determined histopathologically by semiquantitative percent-wise estimation, and a minimum of 25 % of histopathologically proven fatty changes in the liver parenchyma was considered a markedly steatotic change in the liver parenchyma [9]. A P-value ≤0.05 was considered to indicate a statistically significant difference. An a priori power analysis was based on a paired t-test to compare the two methods with respect to the number of correctly identified lesions per patient. This test revealed that 34 patients were needed to obtain a power of 80 % (alpha 5 %, two-sided) to detect a medium effect (epsilon = 0.5).
Results
In 32 patients, a total of 81 metastases were diagnosed according to the reference standard. In the other three patients with diffuse hepatic spread of colorectal metasases, a maximum of five lesions was added to the list of correctly classified lesions if they were correctly assigned to the affected segment, resulting in a total of 96 hepatic metastases. The diameter of the metastases determined by histopathologic workup of the resected specimen ranged from 0.2 to 7.5 cm (mean: 2.3 cm). Thirteen patients had a solitary metastasis, seven had two metastases, three had three metastases, four had four metastases, three had five metastases, one had six metastases, one had eight metastases, and three had diffuse metastatic infiltration (Fig. 3). In total, 280 segments were assessed, and in 98 segments histopathology revealed metastatic spread of colorectal carcinoma.
Patient with multiple liver metastases. a Contrast-enhanced MDCT in the portal venous phase shows only two lesions (arrows). b Gadoxetic acid-enhanced MRI (T1-weighted VIBE portal venous phase) shows another two subcapsular lesions (arrows). c On delayed imaging (T1-weighted 2D GRE, 20-min post administration of gadoxetic acid), the smallest subcapsular lesion of non-hepatocellular origin (arrow) was clearly seen and assigned with high confidence
All sensitivity values of MDCT and MRI are reported in Table 1. No significant differences between both methods for all readers could be found (P = 0.063). The tendency for interaction (P = 0.061) showed that obviously not all readers achieved superior results with MRI. Accordingly, McNemar tests showed verifiable differences only for reader 1 (P = 0.035) and reader 3 (P = 0.003) for the lesion-based evaluation. A different sensitivity for the assessment of segmental involvement was found only for reader 3 (P = 0.012). The number of false-positive diagnoses is shown in Fig. 4. Specificity for CT evaluation was 100 % for reader 1, 94.5 % for reader 2, and 95.1 % for reader 3. For MRI evaluation, specificity values ranged from 97.3 % for reader 1, 96.7 % for reader 2, and 97.3 % for reader 3. Logistic regression measurements showed significant differences in specificity for segment-based evaluation between MDCT and MRI for all readers (P < 0.001) and for the two methods (P < 0.001). However, the McNemar test for each reader showed no significant results (P-value of 0.06 for reader 1, P-value of 0.34 for reader 2, and P-value of 0.29 for reader 3). Receiver-operating characteristic analysis resulted in AUCs curves for MDCT ranging from 0.916 to 0.949 and for MRI ranging from 0.940 to 0.962, respectively.
Considering the group of lesions ≤10 mm (n = 31), we found significant different sensitivity values between the two methods (P = 0.003) with an overall sensitivity of 63.4 % for MDCT and 80.6 % for MRI (Fig. 5). Furthermore, this difference depended on the rater (P = 0.03).
a Contrast-enhanced MDCT in the portal venous phase fails to show any lesion on this level. b Gadoxetic acid-enhanced MRI in the portal venous phase (T1-weighted VIBE) shows a small hypointense subcapsular lesion (arrow). c On the delayed postconstrast MR image (20 min, T1-weighted 2D GRE), the lesion without enhancement (arrow) is clearly seen. Specimen confirmed the diagnosis of colorectal metastasis
Eleven patients in our study group had a histopathologically proven fatty infiltration of more than 25 % of the liver parenchyma (i.e., at least moderate diffuse steatosis). In the analysis of these patients, MRI showed a trend toward better performance than the other technique (Table 2), but without statistical significance (Fig. 6). Differences in sensitivity values in patients with and without fatty infiltration were not statistical significant between raters (P = 0.847) or between methods (P = 0.594). Specificity values were 100 % for all readers for the interpretation of CT in patients with steatosis and in one reader in patients without steatosis compared to 96.3–100 % for MRI in patients with steatosis and 96–96.8 % in patients without fatty infiltration.
Patient with fatty liver with histopathological steatosis of 80 %. a Contrast-enhanced MDCT in the portal venous phase delineates no distinct suspicious lesion at this level. b T2-weighted MRI (T2-TSE) shows a hyperintense lesion near the portal vein division (arrow). c On gadoxetic acid-enhanced MRI (T1-weighted VIBE, portal venous phase) no lesion is distinguishable. d Hepatobiliary phase imaging (T1-weighted 3D GRE) shows a hypointense lesion (arrow), confirmed as a colorectal metastasis
Discussion
Several clinical trials have confirmed an improved long-term survival rate for patients who undergo liver resection of colorectal metastases, including survival rates of more than 5 years, even in patients with multiple metastases [2, 4]. For an increasing number of patients with potentially curative liver resection, preoperative diagnostic imaging has gained significantly more importance in achieving an accurate selection of potential surgical candidates. Nevertheless, the most appropriate imaging technique remains controversial. Multiple studies comparing the detection rates of MDCT and contrast-enhanced MRI in patients with hepatic metastases have shown inconsistent results [9, 13, 19–27]. A meta-analysis comparing the diagnostic performance values of CT, MR, FDG, PET, and FDG PET/CT in studies published between 1990 and 2010 confirmed that MRI should be the first-line investigation for evaluating colorectal liver metastases [28]. Nevertheless, the use of liver-specific contrast agents and nonspecific gadolinium-chelates did not provide improved results [28]. Other studies evaluating MR imaging with liver-specific contrast agents have demonstrated a marked improvement in the detection of colorectal metastases compared to spiral CT [29–31]. However, a direct comparison between both methods, with histopathological proof, has not yet been performed.
Gadoxetic acid, sometimes referred to as a “combined contrast agent”, has imaging properties of perfusion and hepatocyte-selective properties [32] and can be used to improve lesion detection, to characterise lesions as hepatocellular or nonhepatocellular, and to specifically characterise some hepatocellular lesions (Fig. 2). Hypovascular metastases, such as metastases deriving from a colorectal origin, appear hypointense in the arterial phase compared to surrounding liver parenchyma, which represents contrast uptake to a lesser degree than the surrounding liver parenchyma. In the hepatocyte phase, due to a lack of hepatocytes and the lack of an intracellular transport mechanism, almost all the contrast agent is washed out and no uptake is observed within the metastases. In our study, significant differences in sensitivity values between MDCT and MRI could not be found for all three readers together. However, further statistical evaluation revealed that only reader 1 and reader 3 showed significantly superior performance with MRI. Reader 2 as an expert in MDCT imaging did not equally profit from gadoxetic-enhanced MRI. We did not pay for the slightly higher sensitivity of MRI with a higher number of false-positive lesions and a lower specificity.
Nevertheless, the sensitivity of MRI on a per-lesion basis in the present study (90.5–91.8 %) was very similar to recently published results (81.1–88.2 %) [9, 14, 22, 30]. However, MDCT performance in the present study was better (80.0–87.4 %) than anticipated from the literature (70.4–78 %) [31, 33, 34]. Studies with an intraoperative standard of reference report a lesion-by-lesion sensitivity above 80 % for different liver lesions with MDCT and MRI imaging [26, 35–38]. A meta-analysis of studies, which compared different imaging investigations in patients with colorectal metastases between 1990 and 2010, could identify only one study that fulfilled the criteria of only including lesions with intraoperative and histopathologic verification [28]. A strength of this article is the histological workup that served as the standard of reference for each patient.
The detection of small lesions in particular remains a challenge. For lesions up to 10 mm, the sensitivity estimates for MR imaging were slightly but not significantly higher than those for CT (Fig. 3), whereas an increasing sensitivity for MR imaging was reported in articles published after January 2004 [28]. Differences in sensitivity values were found for both methods, with higher sensitivity rates for MRI evaluation (Table 3). Published data reported a higher accuracy for the detection of small hepatic lesions with liver-specific enhanced MRI at 1.5 T [11, 22], compared to DWI [39]. These results are in line with the present study at 3.0 T.
Fatty infiltration of the liver is a common occurrence in patients with colorectal cancer metastases undergoing neoadjuvant chemotherapy. Recently, it has been shown that fatty infiltration may impair detection of metastases with MDCT by diminishing the contrast between a hypodense hepatic lesion and surrounding liver tissue. However, statistical evaluation of our patients with a histologically proven fatty liver showed no significant differences between MDCT and MRI evaluation. Our results showed a higher specificity and lower sensitivity for both MRI and MDCT (Fig. 6) and agree with recently published results, which also showed no statistically significant differences between the two investigations for lesions >1 cm [40, 41].
A limitation of this study is the selected patient sample that included only patients scheduled for surgery, and no patients without liver metastases, which resulted in a higher level of suspicion on the readers’ part and a lower threshold for diagnosing a lesion. However, the readers were not aware of the distribution and/or the number of metastases, which showed a high variability in our patient group. On the other hand, inclusion of surgical patients only provided the best standard of reference (i.e., histopathology). A second limitation is the fact that the DWI pulse sequence was not included in the analysis. This was due to the fact that at the start of the study DWI was not routinely performed at our institution.
In conclusion, the question of the most appropriate preoperative diagnostic workup of liver metastases remains controversial. MRI evaluation, with its higher sensitivity, is the preferred investigation in the preoperative setting. However, MDCT still yields comparable detection rates, especially if the reporting radiologist is highly familiar with this method. Identification of small hepatic colorectal metastases remains a challenge, although high-field strength MRI with liver-specific contrast agents is superior to 64-row MDCT.
References
Abdalla EK, Vauthey JN, Ellis LM et al (2004) Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 239:818–825, discussion 825–817
Choti MA, Sitzmann JV, Tiburi MF et al (2002) Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235:759–766
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–318, discussion 318–321
Malik HZ, Hamady ZZ, Adair R et al (2007) Prognostic influence of multiple hepatic metastases from colorectal cancer. Eur J Surg Oncol 33:468–473
Clavien PA, Petrowsky H, DeOliveira ML, Graf R (2007) Strategies for safer liver surgery and partial liver transplantation. N Engl J Med 356:1545–1559
Tamandl D, Herberger B, Gruenberger B et al (2008) Adequate preoperative staging rarely leads to a change of intraoperative strategy in patients undergoing surgery for colorectal cancer liver metastases. Surgery 143:648–657
Hamm B, Staks T, Muhler A et al (1995) Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 195:785–792
Halavaara J, Breuer J, Ayuso C et al (2006) Liver tumor characterization: comparison between liver-specific gadoxetic acid disodium-enhanced MRI and biphasic CT—a multicenter trial. J Comput Assist Tomogr 30:345–354
Huppertz A, Balzer T, Blakeborough A et al (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230:266–275
Vogl TJ, Kummel S, Hammerstingl R et al (1996) Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200:59–67
Zech CJ, Herrmann KA, Reiser MF, Schoenberg SO (2007) MR imaging in patients with suspected liver metastases: value of liver-specific contrast agent Gd-EOB-DTPA. Magn Reson Med Sci 6:43–52
Kim YK, Park G, Kim CS, Yu HC, Han YM (2012) Diagnostic efficacy of gadoxetic acid-enhanced MRI for the detection and characterisation of liver metastases: comparison with multidetector-row CT. Br J Radiol 85:539–547
Muhi A, Ichikawa T, Motosugi U et al (2011) Diagnosis of colorectal hepatic metastases: comparison of contrast-enhanced CT, contrast-enhanced US, superparamagnetic iron oxide-enhanced MRI, and gadoxetic acid-enhanced MRI. J Magn Reson Imaging 34:326–335
Holzapfel K, Eiber MJ, Fingerle AA, Bruegel M, Rummeny EJ, Gaa J (2012) Detection, classification, and characterization of focal liver lesions: value of diffusion-weighted MR imaging, gadoxetic acid-enhanced MR imaging and the combination of both methods. Abdom Imaging 37:74–82
Hekimoglu K, Ustundag Y, Dusak A et al (2011) Small colorectal liver metastases: detection with SPIO-enhanced MRI in comparison with gadobenate dimeglumine-enhanced MRI and CT imaging. Eur J Radiol 77:468–472
Chang KJ, Kamel IR, Macura KJ, Bluemke DA (2008) 3.0-T MR imaging of the abdomen: comparison with 1.5T. RadioGraphics 28:1983–1998
Laghi A (2007) Multidetector CT (64 Slices) of the liver: examination techniques. Eur Radiol 17:675–683
Couinaud CC (1957) Le foie: e’tudes anatomiques et chirurgicales. Masson, Paris
Blakeborough A, Ward J, Wilson D et al (1997) Hepatic lesion detection at MR imaging: a comparative study with four sequences. Radiology 203:759–765
Blyth S, Blakeborough A, Peterson M, Cameron IC, Majeed AW (2008) Sensitivity of magnetic resonance imaging in the detection of colorectal liver metastases. Ann R Coll Surg Engl 90:25–28
Hagspiel KD, Neidl KF, Eichenberger AC, Weder W, Marincek B (1995) Detection of liver metastases: comparison of superparamagnetic iron oxide-enhanced and unenhanced MR imaging at 1.5T with dynamic CT, intraoperative US, and percutaneous US. Radiology 196:471–478
Hammerstingl R, Huppertz A, Breuer J et al (2008) Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol 18:457–467
Kim MJ, Kim JH, Chung JJ, Park MS, Lim JS, Oh YT (2003) Focal hepatic lesions: detection and characterization with combination gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology 228:719–726
Muller RD, Vogel K, Neumann K et al (1999) SPIO-MR imaging versus double-phase spiral CT in detecting malignant lesions of the liver. Acta Radiol 40:628–635
Reimer P, Jahnke N, Fiebich M et al (2000) Hepatic lesion detection and characterization: value of nonenhanced MR imaging, superparamagnetic iron oxide-enhanced MR imaging, and spiral CT-ROC analysis. Radiology 217:152–158
Semelka RC, Cance WG, Marcos HB, Mauro MA (1999) Liver metastases: comparison of current MR techniques and spiral CT during arterial portography for detection in 20 surgically staged cases. Radiology 213:86–91
Seneterre E, Taourel P, Bouvier Y et al (1996) Detection of hepatic metastases: ferumoxides-enhanced MR imaging versus unenhanced MR imaging and CT during arterial portography. Radiology 200:785–792
Niekel MC, Bipat S, Stoker J (2010) Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 257:674–684
Bartolozzi C, Donati F, Cioni D et al (2004) Detection of colorectal liver metastases: a prospective multicenter trial comparing unenhanced MRI, MnDPDP-enhanced MRI, and spiral CT. Eur Radiol 14:14–20
Floriani I, Torri V, Rulli E et al (2010) Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging 31:19–31
Rappeport ED, Loft A, Berthelsen AK et al (2007) Contrast-enhanced FDG-PET/CT vs. SPIO-enhanced MRI vs. FDG-PET vs. CT in patients with liver metastases from colorectal cancer: a prospective study with intraoperative confirmation. Acta Radiol 48:369–378
Dahlqvist Leinhard O, Dahlstrom N, Kihlberg J et al (2012) Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol 22:642–653
Eiber M, Fingerle AA, Brugel M, Gaa J, Rummeny EJ, Holzapfel K (2012) Detection and classification of focal liver lesions in patients with colorectal cancer: retrospective comparison of diffusion-weighted MR imaging and multi-slice CT. Eur J Radiol 81:683–691
Ward J, Robinson PJ, Guthrie JA et al (2005) Liver metastases in candidates for hepatic resection: comparison of helical CT and gadolinium- and SPIO-enhanced MR imaging. Radiology 237:170–180
Kuszyk BS, Bluemke DA, Urban BA et al (1996) Portal-phase contrast-enhanced helical CT for the detection of malignant hepatic tumors: sensitivity based on comparison with intraoperative and pathologic findings. AJR Am J Roentgenol 166:91–95
Numminen K, Isoniemi H, Halavaara J et al (2005) Preoperative assessment of focal liver lesions: multidetector computed tomography challenges magnetic resonance imaging. Acta Radiol 46:9–15
Valls C, Andia E, Sanchez A et al (2001) Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology 218:55–60
Vogl TJ, Schwarz W, Blume S et al (2003) Preoperative evaluation of malignant liver tumors: comparison of unenhanced and SPIO (Resovist)-enhanced MR imaging with biphasic CTAP and intraoperative US. Eur Radiol 13:262–272
Shimada K, Isoda H, Hirokawa Y, Arizono S, Shibata T, Togashi K (2010) Comparison of gadolinium-EOB-DTPA-enhanced and diffusion-weighted liver MRI for detection of small hepatic metastases. Eur Radiol 20:2690–2698
Berger-Kulemann V, Schima W, Baroud S et al (2012) Gadoxetic acid-enhanced 3.0T MR imaging versus multidetector-row CT in the detection of colorectal metastases in fatty liver using intraoperative ultrasound and histopathology as a standard of reference. Eur J Surg Oncol 38:670–676
Kulemann V, Schima W, Tamandl D et al (2011) Preoperative detection of colorectal liver metastases in fatty liver: MDCT or MRI? Eur J Radiol 79:e1–e6
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scharitzer, M., Ba-Ssalamah, A., Ringl, H. et al. Preoperative evaluation of colorectal liver metastases: comparison between gadoxetic acid-enhanced 3.0-T MRI and contrast-enhanced MDCT with histopathological correlation. Eur Radiol 23, 2187–2196 (2013). https://doi.org/10.1007/s00330-013-2824-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-013-2824-z