Abstract
We have tested the predictive value of apparent diffusion coefficient (ADC) histogram analysis in stratifying progression-free survival (PFS) and overall survival (OS) in bevacizumab-treated patients with recurrent glioblastoma multiforme (GBM) from the multi-center BRAIN study. Available MRI’s from patients enrolled in the BRAIN study (n = 97) were examined by generating ADC histograms from areas of enhancing tumor on T1 weighted post-contrast images fitted to a two normal distribution mixture curve. ADC classifiers including the mean ADC from the lower curve (ADC-L) and the mean lower curve proportion (LCP) were tested for their ability to stratify PFS and OS by using Cox proportional hazard ratios and the Kaplan–Meier method with log-rank test. Mean ADC-L was 1,209 × 10−6mm2/s ± 224 (SD), and mean LCP was 0.71 ± 0.23 (SD). Low ADC-L was associated with worse outcome. The hazard ratios for 6-month PFS, overall PFS, and OS in patients with less versus greater than mean ADC-L were 3.1 (95 % confidence interval: 1.6, 6.1; P = 0.001), 2.3 (95 % CI: 1.3, 4.0; P = 0.002), and 2.4 (95 % CI: 1.4, 4.2; P = 0.002), respectively. In patients with ADC-L <1,209 and LCP >0.71 versus ADC-L >1,209 and LCP <0.71, there was a 2.28-fold reduction in the median time to progression, and a 1.42-fold decrease in the median OS. The predictive value of ADC histogram analysis, in which low ADC-L was associated with poor outcome, was confirmed in bevacizumab-treated patients with recurrent GBM in a post hoc analysis from the multi-center (BRAIN) study.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glioblastoma (GBM), the most aggressive and lethal primary brain tumor, respond unpredictably to standard therapy, resulting in highly variable patient survival [1]. Because of this variable response, a biomarker to predict treatment susceptibility could help guide patient care and avoid side effects from ineffective therapies. In clinical practice, the reference standards of patient response to therapy are 6-month progression-free and overall survival (PFS and OS) [2]. The MacDonald criteria, based on measurable changes in contrast-enhancing lesions[3], and the recently proposed response assessment in neuro-oncology (RANO) criteria that also takes into account non-enhancing tumor, have been the primary paradigms for assessing response in recent years [4]. However, tumor burden may be difficult to accurately quantify, especially in patients undergoing anti-angiogenic therapy [5]. Recently, the FDA approved the anti-angiogenic drug bevacizumab (a humanized monoclonal antibody to vascular endothelial growth factor, VEGF) for use in patients with recurrent GBM. Currently, there are no prospectively validated predictive or prognostic biomarkers for bevacizumab response. Biomarkers that either predict clinical outcome following a specific treatment such as bevacizumab, or those that are early markers of tumor response after treatment initiation, are of major interest, as well as a challenge, in clinical oncology research [6].
The apparent diffusion coefficient (ADC), derived from diffusion-weighted imaging (DWI), is a physiologic parameter calculated based on characteristics of water diffusion within the tissue of interest [7]. In neoplasms, lower ADC values have been shown to correlate with higher cell density [8]. Conversely, higher ADC values have been observed in regions of necrosis and edema [9]. ADC has been investigated as a biomarker for glioma response in the setting of anti-angiogenic therapy [10–12]. In our previous study [10], we developed a strategy in which whole ADC histograms extracted from enhancing tumor volumes on pre-bevacizumab treatment MR images were fitted with two normal distribution mixture curves. The subsequently generated ADC classifiers, mean ADC from the lower curve (ADC-L), and the mean lower curve proportion (LCP) were shown to accurately stratify 6-month PFS in bevacizumab-treated patients with recurrent GBM. However, the study was conducted in a relatively small patient cohort (n = 41) in a single medical center (UCLA). In the current study, we analyzed patient data from the BRAIN trial [13], one of the largest multicenter studies of recurrent GBM patients treated with bevacizumab, to verify the observed predictive feature of ADC histogram analysis in stratifying the outcomes of bevacizumab-treated patients with recurrent GBM.
Methods
Patients
All patients in the current study were part of the BRAIN trial, which was performed to assess the effectiveness of bevacizumab or bevacizumab and CPT-11 (Irinotecan) in patients with recurrent GBM [13]. For this trial, 167 patients from multiple participating centers who had histologically confirmed GBM at first or second relapse were enrolled. Disease progression that led to enrollment in the study was identified on magnetic resonance imaging (MRI) ≤14 days before the baseline treatment. These patients had failed the initial standard care plan including concurrent radiotherapy (RT) and temozolomide (TMZ), and were required to be at least 8 weeks from the completion of radiation therapy. The baseline was defined as the first day when bevacizumab or bevacizumab + CPT-11 treatment was given. Bevacizumab was given at a dose of 10 mg/kg. The dose of CPT-11 varied based on different clinical scenarios. All patients were treated for 104 weeks or until disease progression or discontinuation. Other inclusion criteria included Karnofsky performance status (KPS) ≥70 %; life expectancy ≥12 weeks; and adequate hematologic, hepatic, and renal function. Patients receiving corticosteroids were required to be on a stable or decreasing dose for at least 5 days before the baseline MRI scan.
Of the 167 patients from the BRAIN study, 97 patients from 9 centers were used in the current analysis, based on the availability of both clinical data and pre-treatment T1 post-contrast images and ADC maps of sufficient quality (Table 1). Clinical data were acquired from the study investigators independent of the sponsor. Data acquisition was performed in compliance with all applicable Health Insurance Portability and Accountability Act regulations (HIPAA).
To account for the possible confounding effects from radiation therapy (RT), 6-month PFS, a strong indicator of overall-survival for GBM patients [2], was compared in two patient subgroups. Patients in subgroup 1 received RT more than 3 months before the baseline treatment (n = 80) and patients in subgroup 2 received RT more than 12 months before the baseline study (n = 16), minimizing the likelihood of pseudo-progression in this cohort.
Assessments
Disease progression and survival data were provided by the study investigators according to modified Macdonald criteria [3]. Only contrast-enhancing lesions were measured by MRI. Non-contrast-enhancing lesions were considered non-target lesions in tumor assessment. Progression was determined by contrast-enhancing and non–contrast-enhancing lesions. Any new areas of non-enhancing T2 or fluid-attenuated inversion-recovery (FLAIR) signal consistent with tumor were considered as progressive disease. In the absence of radiographic documentation, clinical progression was used to determine progression. At the time of the last assessment (ranging from May 2009 to August 2010 for participating centers), 89 of 97 patients had progressed. All patients were observed until discontinuation from the study, loss to follow-up, study termination, or death.
Tumor volume acquisition and ADC histograms
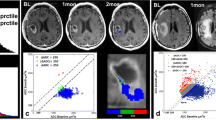
Tumor volumes were segmented on pre-bevacizumab treatment contrast-enhancing T1-weighted images at baseline (pretreatment) by using a semi-automated adaptive thresholding technique as previously described [10]. Non-enhancing regions of macroscopic necrosis as well as cystic areas were excluded by this method. The resulting regions of interest (ROI) encompassing the entire enhancing tumor volume were verified by a board-certified neuro-radiologist, blinded to clinical outcome, and mapped to the ADC images (Fig. 1). ADC values were then calculated on a pixel-by-pixel basis and fitted to a two normal distribution histogram. The ADC classifiers, lower curve mean (ADC-L) and lower curve proportion (LCP), were generated.
Generation of ADC histograms: total enhancing tumor volume was segmented on axial post-contrast T1-weighted images in a 36-year-old man with recurrent GBM (a) and co-registered to the corresponding ADC maps (b) for generation of ADC histograms (c, d). A single distribution curve provided a poor fit for the asymmetrical, broad and dual-peaked histogram (c). A two normal distribution fitting curve improved data analysis by separating ADC histogram into two components, with lower mean (ADC-L) and higher mean (ADC-H), respectively. In this case, ADC-L = 1,428, LCP = 0.67
Statistical analysis
Based on the mean values of the ADC classifiers generated from the ADC histograms, ADC-L and LCP were dichotomized. Sensitivity and specificity of the dichotomized ADC classifiers for predicting 6-month PFS were calculated. The Kaplan–Meier method with log-rank test was used to estimate the duration of overall PFS and OS and to compare the proportions defined by the dichotomized ADC classifier. Multivariate Cox models were used to test ADC-L and LCP covariates, adjusting for patient age and enhancing tumor volume (at recurrence) on 6-month PFS, overall PFS, and OS. A test of the proportional hazards assumption was used after fitting a multivariate Cox model, and 95 % confidence intervals of all the covariates were generated. For the primary analysis, using two ADC classifiers from three multivariate Cox regression models, P < 0.008 was accepted as indicating statistical significance to control for the family-wise error rate of 0.05. For the rest of the exploratory univariate analysis, P value of 0.05 was accepted as statistically significant. Analysis was performed with statistical software (Stata 10, 2008; Stata, College Station, TX, USA).
Results
Patient characteristics
Table 1 shows the baseline characteristics of patients with recurrent GBM from the multiple participating centers. Steroid dose was variable among individual patients in this group.
Univariate Cox model
In a univariate Cox model, ADC-L alone and combined ADC-L and LCP values were predictive of 6-month PFS (ADC-L hazard ratio of 2.1, P = 0.01; combined ADC-L and LCP HR of 2.3, P = 0.003). This predictive value was also significant using the log-rank test (P = 0.0078 for ADC-L; P = 0.0019 for combined ADC-L and LCP). Sensitivity, specificity, and accuracy of ADC-L alone and combined ADC-L and LCP were calculated (Table 2).
Multivariate Cox model
Consistent with the previous study, patient age at the time of tumor recurrence was not a significant predictor for 6-month PFS, overall PFS, or OS (P = 0.33, 0.88, 0.49, respectively). The predictive power of ADC-L was confirmed when analyzing 6-month PFS (HR of 3.1, P = 0.001), overall PFS (HR of 2.3, P = 0.002), and OS (HR of 2.4, P = 0.002). LCP alone was only predictive of 6-month PFS (HR of 2.3, P = 0.021), but not overall PFS (P = 0.07) and OS (P = 0.15). In a multivariate Cox model, baseline enhancing tumor volume was shown to be predictive of 6-month PFS, overall PFS, and OS (HR 1.5, P = 0.00433; HR 1.4, P = 0.00488; HR 1.6, P <=0.00149, respectively; Table 3).
Kaplan–Meier method with log-rank test
ADC-L alone and combined ADC-L and LCP (Fig. 2) were significant predictors of overall PFS (higher versus lower ADC-L: 1.71-fold increase in median PFS, P = 0.015; ADC-L >1,209 and LCP <0.71 versus the rest: 2.02-fold increase in PFS, P < 0.001), and OS (higher versus lower ADC-L: 1.18-fold increase in median time to survival, P = 0.027; ADC-L >1,209 and LCP <0.71 versus the rest: 1.42-fold increase in median time to survival, P = 0.0023).
Kaplan–Meier curves for overall progression-free survival (PFS) and overall survival (OS). ADC classifiers (ADC-L, ADC-L and LCP) were examined in stratifying overall PFS (a, b) and OS (c, d). The x-axis shows days after baseline treatment with the vertical lines noting 6- and 12-month PFS (a, b), 6- and 12-month OS (c, d), respectively. The y-axis represents the percentage of event-free patients
Controls for radiation treatment effect/pseudoprogression
The significant difference between 6-month PFS was maintained for patients with ADC-L >1,209 versus ADC-L <1,209 (P = 0.016), and for patients with ADC-L >1,209 and LCP <0.71 versus the rest (P = 0.022) when the subgroup who had radiation therapy within 3 months of baseline treatment (n = 14) were excluded from the analysis. The examination of the subgroup who had radiation therapy more than 12 months prior to the baseline (n = 16) also showed a significant difference between patients with ADC-L >1,209 and LCP <0.71 versus the rest (P < 0.001; median time to progression, 291 vs. 83 days), but only yielded a trend between patients with ADC-L >1,209 versus ADC-L <1,209 (P = 0.08; median time to progression, 238 vs. 91 days), potentially due to the small sample size available for this subgroup.
Discussion
Highly variable response of GBM to currently used therapies coupled with short survival times underlie the need for biomarkers that can accurately predict treatment outcome or are early markers for treatment response. Our current study aimed to verify that ADC histogram analysis could serve as a noninvasive imaging biomarker to predict progression-free survival (PFS) and overall survival (OS) in patients with GBM treated with bevacizumab at recurrence. To do this, we performed a post hoc analysis of data obtained from a well-run, large, multi-center clinical trial with a relatively homogenous patient population and well-defined inclusion criteria (the BRAIN trial) [13]. We confirmed a relationship between ADC values and survival.
Recently, advanced MRI using sequences dependent on physiologic processes have been advocated as a possible way to further the development of biomarkers in cancer therapy. Diffusion sensitive imaging techniques are one such method as they are dependent on the microscopic structure of tissue, and are sensitive to cell density, necrosis as well as vasogenic and cytotoxic edema. Thus, ADC histogram analysis may provide information on tumors that is additive to standard MRI sequences. ADC histogram analysis has the advantage of being able to extract quantitative physiologic data on all voxels from an MRI scan, sort extracted voxels according to their values, and group similar values together. For GBM, the resultant dataset can then be closely fitted with two normal distribution curves in almost all patients. Our hypothesis is that the lower histogram curve may be more dependent on the viable cellular component of the tumor in areas of limited necrosis, whereas the higher histogram (ADC-H) curve may more closely reflect the edematous/necrotic tissue component associated with areas of increased water diffusivity. This analysis may provide a method to separate these components, and better characterize their potentially independent response, even though the two are mixed together without discernable boundaries in actual tumor tissue.
It has been demonstrated for this patient cohort from the BRAIN trial that objective response is a predictor of survival [14]. However, response is, of course, determined subsequent to therapy initiation. The benefit of the current method of analysis is that response prediction is acquired prior to treatment. Previously we demonstrated a relationship between 6-month progression free survival and ADC values in a smaller cohort of patients (n = 41 compared to 97 for the current study). In our initial report, the relationship between ADC values and survival held for bevacizumab-treated patients, but not control patients. This supports the contention that the ADC histogram analysis has a predictive, rather than just prognostic, component. The advance of the present study is that, in addition to using more patients from multiple sites, we used overall survival as an endpoint. Even though imaging analysis was entirely post hoc and no standardization of imaging protocols with respect to diffusion imaging acquisitions was performed among the centers, our analysis confirmed that the ADC classifiers retained significance as a biomarker of bevacizumab response. It is notable that the longest survivors (those living more than 600 days), were all identified by this method.
The accuracy of the predictive model for 6-month PFS was 63 % based on ADC-L alone in the current investigation, less than the accuracy of the prior pilot study (73 %). Maximum specificity of 87 % for progression-free survival was achieved by combining ADC-L and LCP. Further improvements in predictive value may be achievable by combining imaging with other clinical or molecular data [15], and by improving standardization of the diffusion imaging protocols. The ability to predict response prior to treatment initiation distinguishes the ADC histogram analysis from several other potential markers of anti-angiogenic therapy [15–17].
The issue of radiation treatment effect is important to consider in the context of this study. Radiation treatment effect can be mistaken for tumor progression, a phenomenon known as “pseudoprogression” [18, 19]. This is more likely to occur within 3–6 months of the end of radiation therapy [20]. Since necrosis/edema, which can be associated with pseudoprogression, is thought to increase ADC, this could bias the results in favor of tumors with high ADC. Therefore, we split the analysis into patients that recurred less versus more than 3 months from the end of radiation treatment, and were still able to show predictive significance for the ADC analysis.
Advanced MRI techniques, such as diffusion imaging, are now commonly acquired both in academic and private practice settings. DWI has the advantage of being quickly obtained, does not require contrast injection, and is more reproducible than other advanced physiologic imaging techniques (such as perfusion imaging) [21]. In future clinical trials, optimization of diffusion protocols will likely result in higher image quality and repeatability, potentially improving the accuracy, sensitivity, and specificity of the ADC histogram analysis. For a biomarker to be applied broadly in clinical trials, it needs to be both easy to implement and reproducible. Some studies achieve greater reproducibility in MRI interpretation by using a central review, but this can add cost, particularly if extensive post-processing of images is required. For the ADC histogram analysis, we have developed computer software that automatically selects enhancing voxels that are above a cut-off value, which should increase reproducibility compared to operator-defined regions of interest (ROIs). However, the ROIs do sometimes require hand-editing, especially for lesions with very small or highly irregular and varying enhancing components. This may decrease reproducibility and may generate errors during fusion to the ADC images. Further validation is required to demonstrate reproducibility of the ROIs and ease of use of the image processing among radiologists at different institutions. Furthermore, although the two normal histograms are automatically generated according to a well-established mathematical formula, it will be important to determine how potentially subtle changes in ROI editing can affect ADC classifiers, and whether this can lead to a change in the prediction of patient response.
Multicenter trials present several challenges for standardization of imaging [22]. These include numerous vendors and platforms for MRI, as well as variations in acquisition parameters. However, our results show that, even in the face of these challenges, ADC histogram analysis may provide a biomarker predicting response to bevacizumab therapy in the recurrent setting. Currently, there are no validated biomarkers for anti-VEGF therapy, although some promising candidates are being investigated [23]. The development of such markers could be an important stratification for future studies, potentially allowing investigators to change patient subgroups analysis, and also allow for reduced subject numbers by focusing on patients who are less likely to respond to bevacizumab-containing regimens.
In conclusion, our study confirmed the potential value of ADC histogram analysis in stratifying response to salvage chemotherapy with bevacizumab-based regimens in patients with recurrent GBM. Further prospective evaluation in clinical trials with the implementation of standardized imaging methodology may optimize the benefit of this promising marker.
References
Herbert C, Williams M, Sawyer H et al (2011) Treatment of Glioblastoma Multiforme with radiotherapy and concomitant and adjuvant temozolomide: translation of randomised controlled trial evidence into routine clinical practice. Clin Oncol 27:372–373
Lamborn KR, Yung WK, Chang SM et al (2008) Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol 10:162–170
Macdonald DR, Cascino TL, Schold SC Jr et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Weller M, Wick W, Hegi ME et al (2010) Should biomarkers be used to design personalized medicine for the treatment of glioblastoma? Future Oncol 6:1407–1414
Bode MK, Ruohonen J, Nieminen MT et al (2006) Potential of diffusion imaging in brain tumors: a review. Acta Radiol 47:585–594
Ellingson BM, Malkin MG, Rand SD et al (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31:538–548
Chenevert TL, Sundgren PC, Ross BD (2006) Diffusion imaging: insight to cell status and cytoarchitecture. Neuroimaging Clin N Am 16:619–632, viii–ix
Pope WB, Kim HJ, Huo J et al (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252:182–189
Jain R, Scarpace LM, Ellika S et al (2010) Imaging response criteria for recurrent gliomas treated with bevacizumab: role of diffusion weighted imaging as an imaging biomarker. J Neuro Oncol 96:423–431
Nowosielski M, Recheis W, Goebel G et al (2011) ADC histograms predict response to anti-angiogenic therapy in patients with recurrent high-grade glioma. Neuroradiology 53:291–302
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27:4733–4740
Prados M, Cloughesy T, Samant M et al (2011) Response as a predictor of survival in patients with recurrent glioblastoma treated with bevacizumab. Neuro Oncol 13:143–151
Batchelor TT, Sorensen AG, di Tomaso E et al (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Ellingson BM, Cloughesy TF, Lai A et al (2011) Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol 13:401–409
Pope WB, Young JR, Ellingson BM (2011) Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep 11:336–344
Chamberlain MC, Glantz MJ, Chalmers L et al (2007) Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neuro Oncol 82:81–83
Sanghera P, Perry J, Sahgal A et al (2010) Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neuro Sci 37:36–42
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22:633–638
Bedekar D, Jensen T, Schmainda KM (2010) Standardization of relative cerebral blood volume (rCBV) image maps for ease of both inter- and intrapatient comparisons. Magn Reson Med 64:907–913
Ashton E (2010) Quantitative MR in multi-center clinical trials. J Magn Reson Imaging 31:279–288
Sorensen AG, Batchelor TT, Zhang WT et al (2009) A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res 69:5296–5300
Conflict of interest
Whitney B. Pope, Albert Lai, David Schiff, Lauren Abrey, Tom Mikkelsen, Nina A. Paleologos, and Timothy Cloughesy served on the Genentech/Roche advisory board; Albert Lai, Vinay K. Puduvalli, Tom Mikkelsen, and Patrick Y. Wen were supported by research funds from Genentech/Roche; Nina A. Paleologos, Lauren Abrey, and Tom Mikkelsen received honoraria from Genentech/Roche; All other authors declare no conflict of interest.
Ethical Standard
This is to declare that the studies performed in this manuscript complied with current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pope, W.B., Qiao, X.J., Kim, H.J. et al. Apparent diffusion coefficient histogram analysis stratifies progression-free and overall survival in patients with recurrent GBM treated with bevacizumab: a multi-center study. J Neurooncol 108, 491–498 (2012). https://doi.org/10.1007/s11060-012-0847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-012-0847-y