Abstract
Objective
To determine whether CT pulmonary angiography (CTPA) using low mA setting reconstructed with model-based iterative reconstruction (MBIR) is equivalent to routine CTPA reconstructed with filtered back projection (FBP).
Methods
This prospective study was approved by the institutional review board and patients provided written informed consent. Eighty-two patients were examined with a low mA MBIR-CTPA (100 kV, 20 mA) and 82 patients with a standard FBP-CTPA (100 kV, 250 mA). Region of interests were drawn in nine pulmonary vessels; signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were calculated. A five-point scale was used to subjectively evaluate the image quality of FBP-CTPA and low mA MBIR-CTPA.
Results
Compared to routine FBP-CTPA, low mA MBIR-CTPA showed no differences in the attenuation measured in nine pulmonary vessels, higher SNR (56 ± 19 vs 43 ± 20, p < 0.0001) and higher CNR (50 ± 17 vs 38 ± 18, p < 0.0001) despite a dose reduction of 93 % (p < 0.0001). The subjective image quality of low mA MBIR-CTPA was quoted as diagnostic in 98 % of the cases for patient with body mass index less than 30 kg/m2.
Conclusion
Low mA MBIR-CTPA is equivalent to routine FBP-CTPA and allows a significant dose reduction while improving SNR and CNR in the pulmonary vessels, as compared with routine FBP-CTPA.
Key Points
• Low mA MBIR-CTPA is equivalent to routine FBP-CTPA.
• MBIR-CTPA may be achieved with drastic (93 %) dose reduction.
• Low mA MBIR-CTPA should be studied in the setting of suspected PE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulmonary embolism is a serious medical condition associated with high mortality, ranging from 3 to 6 % [1, 2]. Computed tomography pulmonary angiography (CTPA) is now widely accepted as the standard test for diagnosing pulmonary embolism (PE) [3], despite its relatively high radiation dose, which varied from 13 to 40 mSv in 2008 [4], but slowly decreased to 3 to 5 mSv nowadays [3].
In order to reduce the radiation dose delivered by CTPA, several strategies have been introduced, including lowering the tube potential [5, 6] and lowering the tube current–time product [7, 8]. However, low dose examinations are associated with increased image noise that can now be reduced by newer reconstructions technologies such as iterative reconstructions [9–11].
Model-based iterative reconstruction (MBIR, Veo®, GE Healthcare, Milwaukee, Wis) is a novel fully iterative [12] reconstruction algorithm allowing reduction of the dose without loss of image quality [13–15]. It has already been demonstrated that chest CT with an x-ray dose equivalent to a posteroanterior and lateral chest x-ray is possible [16].
The primary aim of this study was to demonstrate equivalency (in terms of signal-to-noise ratio (SNR) in the pulmonary arteries) between standard FBP-CTPA and low mA (20 mA) MBIR-CTPA. Subjective image quality was also evaluated.
Materials and methods
This prospective equivalency trial was approved by the institutional review board of the Geneva University Hospital and each patient gave a written informed consent.
Sample size [17]
Necessary sample size was calculated for SNR as the primary outcome. If there is truly no difference between standard FBP-CTPA and low mA (20 mA) MBIR-CTPA, then 138 patients are required in order to be 80 % sure that the limits of a two-sided 90 % confidence interval will exclude a difference in SNR means in the main pulmonary artery of more than 10, assuming a standard deviation of 20.
Patients
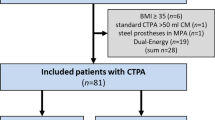
From January to May 2013, 167 consecutive patients, scheduled for an enhanced chest CT, were examined on a multidetector CT system (Discovery 750 HD, GE Healthcare, Milwaukee, Wis). Of these 167 consecutive patients, two patients refused to participate and one changed his mind and asked afterwards to be excluded from the study; thus 164 patients were included in the study. In half of the patients (n = 82), a clinical suspicion of PE was the reason for performing CT. These patients were examined with a routine CTPA protocol consisting of intravenous injection of contrast material (60 ml of Iohexol 350, containing 350 mg I/ml, GE Healthcare) at a rate of 3.5 ml/s, followed by a 30-ml saline flush at the same flow rate. The patients were asked to hold their breath after mid-inspiration. A region of interest (ROI) was drawn in the main pulmonary artery and imaging started 5 s after a threshold of 150 HU was reached. The acquisition parameters were as follows: 0.6-s gantry rotation time, 100 kVp, 0.984:1 beam pitch, 40-mm table feed per gantry rotation, a z-axis tube current modulation was used, with a noise index (NI) of 28 (min/max mA, 100/500) and a 64 × 0.625-mm detector configuration. All routine CTPA were reconstructed with the FBP algorithm (FBP-CTPA).
In the other half of the patients (n = 82), the reasons for chest CT were (1) follow-up of a tumour (n = 51), (2) search for complication of pneumonia (n = 16), (3) follow-up of interstitial pneumonia (n = 11), (4) miscellaneous (n = 4). In these patients, an enhanced chest CT was performed 60 s after contrast media administration (60 ml of Iohexol 350, GE Healthcare at a rate of 3.5 ml/s, followed by a 30-ml saline flush at the same flow rate) to answer the clinical questions. A low mA (20 mA) dose chest CT angiography was added for each patient just prior to the enhanced chest CT (an ROI was placed in the main pulmonary artery, imaging started 5 s after a threshold of 150 HU was reached). The patients were also asked to hold their breath after mid-inspiration. In order not to increase the dose received by the patient, the dose of the enhanced chest CT was decreased by 10 % by increasing the NI. Modification of the NI changes the mA (and only the mA) used to acquire the image. As previously published, increasing the NI by one unit will result in approximately 10 % decreased dose to the patient [18]. The x-ray dose saved was hence used to acquire the low mA MBIR-CTPA. The low mA MBIR-CTPA protocol was as follow: 0.6-s gantry rotation time, 100 kVp, 0.984:1 beam pitch, 40-mm table feed per gantry rotation, 20 mA without tube current modulation and a 64 × 0.625-mm detector configuration. All low mA MBIR-CTPA were reconstructed with the model-based iterative reconstruction algorithm.
A known allergy to iodinated contrast media, glomerular filtration rates less than 45 ml min−1 m2, pregnancy and being younger than 18 years old were considered as exclusion criteria.
Parameters of both protocols, as well as patient demographics, are summarized in Table 1.
Quantitative image analysis
Axial images were reconstructed at 0.625 mm slice thickness using a soft tissue kernel. All images were evaluated at standard CTPA window settings (window width 350, window level 40).
ROIs were drawn by XM in the (1) main pulmonary artery (MPA), (2) right pulmonary artery (RPA), (3) left pulmonary artery (LPA), (4) right upper lobe artery (RUA), (5) right middle lobe artery (RMA), (6) right lower lobe artery (RLA), (7) left upper lobe artery (LUA), (8) lingular artery (LIA) and (9) left lower lobe artery (LLA). The ROIs were sized to cover almost all the vessel diameters, taking care to avoid artefacts. The mean values (mean attenuation) of these nine ROIs served to calculate the SNR and contrast-to-noise ratio (CNR). Three ROIs were also drawn in each side of the patient (outside the patient) as well as on the anterior part of the patient to assess background noise. The ROIs were sized around 3 cm2 to cover as much air as possible, without including external objects surrounding the patient. The mean values of the three measurements served as background noise (mean background noise). ROIs (2 cm2) were also drawn in the paraspinal and subscapular muscle. The mean values of the two muscles served for contrast-to-noise calculation (mean muscle attenuation):
-
SNR = mean attenuation/mean background noise
-
CNR = (mean attenuation − mean muscle attenuation)/mean background noise
CT dose index volume (CTDIvol) and dose length product (DLP) were recorded for each examination.
The calculation of the effective dose (E) was based on the “European Guidelines on quality criteria on computed tomography” using the following formula: E = E DPL × DLP, where E is the effective dose in mSv and E DPL the dose length coefficient for the chest, which is equal to 0.017 [19].
The size-specific dose estimate (SSDE) was also calculated following the recommendation of American Association of Physicists in Medicine (AAPM) report n°204. Briefly, the anteroposterior (AP) and the lateral diameter of each patient were measured at the level of the tracheal bifurcation and summed. The sum of the lateral and AP dimension allows one to find a conversion factor (in the AAPM report n°204, based on a 32-cm-diameter PMMA phantom), which is used to multiply the CTDIvol reported by the scanner to obtain the SSDE [20, 21].
Subjective image quality
All the images were evaluated independently by two radiologists (XM with 10 years and ALH with 7 years of post-fellowship experience). Images were anonymised, aggregated in folders in a random way and evaluated in a standard CTPA windows setting (window width 350, window level 40). A five-point scale [5] was used to assess the overall image quality, defined as follows: 1, good image quality at the level of the main pulmonary artery (MPA); 2, good image quality at the level of the pulmonary arteries (RPA and LPA); 3, good image quality at the level of the lobar arteries; 4, good image quality at the level of the segmental arteries; 5, good image quality at the level of the subsegmental arteries.
Good image quality was defined as good visual homogeneous enhancement, without respiratory artefacts, allowing one to confidently exclude PE. In case readers did not reach a score of 5, they were asked to specify why image quality was insufficient to confidently exclude PE at a given level. The cause may be breathing artefacts, insufficient attenuation in the pulmonary vessels due to either bad timing of injection or a too high noise level.
Scores 1–3 were considered insufficient for diagnostic purposes, whereas scores of 4–5 were considered sufficient.
Such a score was used because there is still controversy in the literature as to whether subsegmental pulmonary emboli should be treated or not [3].
Ten cases of routine CTPA, not included in the study, were used to train the readers on image quality.
Statistical analysis
Statistical analysis was performed with Prism (Prism, version 6b, 2012; GraphPad Software, San Diego, CA, USA). For continuous values, the results are presented as mean ± standard error.
Non-normally distributed datasets (established from Kolmogorov–Smirnov tests) were compared using Friedman test with Dunn post hoc test. Normally distributed data sets were compared using ANOVA test with Bonferroni post hoc test. Two-sided testing was used. Differences were considered significant at p < 0.05.
Results
Patient demographics
The FBP-CTPA group comprised 46 men and 36 women with a mean age of 64 ± 15 years. The low mA MBIR-CTPA group comprised 49 men and 33 women with a mean age of 60 ± 14 years (Table 1).
Dose estimates
The low mA MBIR-CTPA had a significantly lower CTDIvol (0.59 ± 0.003 vs 8 ± 1.8) and DLP (21 ± 2 vs 282 ± 64) than the routine FBP-CTPA protocols (Table 2). Estimated effective doses were 0.3 ± 0.03 vs 4.1 ± 1.1 mSv, p < 0.0001 (Table 2), whereas SSDE were 0.74 ± 0.14 vs 13.3 ± 3.7 mGy (p < 0.0001) for low mA MBIR-CTPA and routine FBP-CTPA, respectively.
Quantitative image analysis
The low mA MBIR-CTPA yielded the same attenuation in each of the nine arteries of the lung, a lower noise and a better SNR and CNR, despite a significant x-ray dose reduction, when compared to classical FBP-CTPA (Table 2).
Qualitative image analysis
Table 3 summarizes the image quality of the low mA MBIR-CTPA and of the routine FBP-CTPA protocol for each reader. The inter-reader agreements were excellent for both routine FBP-CTPA and low mA MBIR-CTPA, with kappa values of 0.921 and 0.865, respectively. In the routine FBP-CTPA group, there was only one examination (for reader 1) which was considered as non-diagnostic. This was attributed to a suboptimal injection time. The non-diagnostic examinations in the low mA MBIR-CTPA groups (n = 7 for both readers) were mostly due to patients with a BMI greater than 30 kg/m2 (n = 6) and due to suboptimal injection time in one case.
Figure 1 illustrates a typical case of routine FBP-CTPA and low mA MBIR-CTPA for patients with BMI less than 20 kg/m2, BMI between 20 and 30 kg/m2, and BMI greater than 30 kg/m2.
Coronal reformatted thin slice CT (2 mm slice thickness) of routine FBP-CTPA (first row) and of low mA MBIR-CTPA (second row) of patients with a BMI less than 20 kg/m2 (a, d), with a BMI between 20 and 30 kg/m2 (b, e) and with a BMI greater than 30 kg/m2 (c, f). The representative images demonstrated an excellent image quality for all patients with a BMI less than 30 kg/m2
Figure 2 shows the presence of a non-occlusive PE in the right lower lobe artery on a low mA MBIR-CTPA, confirmed 3 days later by a routine FBP-CTPA.
Axial thin slice CT (0.625 mm slice thickness) demonstrating a pulmonary embolism (arrow) in the right lower lobe artery picked up on the low mA MBIR-CTPA (a), confirmed 3 days later (arrow) on a routine FBP-CTPA (b). Please note that the BMI of this patient was 36.8 kg/m2, explaining the low image quality of the low mA MBIR-CTPA. Despite this, the PE was clearly identified on the images
Discussion
Our prospective study shows that low mA MBIR-CTPA allows for a significant x-ray dose reduction of 93 %, while maintaining the same attenuation in each of the nine pulmonary arteries investigated. Moreover, as the noise was significantly lower when using MBIR, the SNR and CNR were significantly higher on the low mA MBIR-CTPA than on the routine FBP-CTPA protocol. In this paper, and despite the very low dose level achieved, we do not use the term “ultra-low dose CTPA” in accordance with the editorial by Bankier and Kressel [22], in which they state that the terms “low”, “ultra-low” and “even super-extra-nano-low-dose” should not be used anymore. Instead, we indicate the DLP, CTDIvol, effective dose and SSDE for objective comparison with other studies.
High sensitivity (83–100 %) and high specificity (89–98 %) have been reported for routine CTPA for diagnosing pulmonary embolism (PE) [23–25]. However, because of the relatively low prevalence (9–35 %) of PE among patients evaluated by CTPA, one must be extremely cautious with the dose delivered to patients [8, 26, 27], as it has been estimated that 5 fatalities per 100,000 persons per mSv could be expected [28].
Previous reports investigated the image quality of CTPA after dose reduction due to decrease kVp or mA or both [5, 29, 30] or due to lowering of the z coverage [31, 32]. All these reports showed that PE could be diagnosed with a DLP varying from 40 to 110 mGy cm. Particularly, it was shown that the use of 100 kVp was possible, without significant loss of image quality [5]. Hence, 100 kVp was used in this study for both the routine FBP-CTPA and low mA MBIR-CTPA. Our study uses a lower DLP of around 21 mGy cm. This was made possible by the use of a second generation of iterative reconstruction, the so-called model-based iterative reconstruction. This type of reconstruction has already been applied to unenhanced chest imaging, where a dose of radiation close to the one delivered by a posteroanterior and lateral chest x-rays were used [16]. The MBIR algorithm makes fewer approximations than the FBP algorithm. MBIR takes into consideration a model of noise, a model of the object and a model of the optical properties of the CT. Taking into account all these models is computationally intense and around 40 min is needed for the reconstruction to be done. It is nowadays clearly a limitation, but the time needed for the reconstruction to be done should be significantly reduced with newer and faster calculators.
It has been reported that a diagnostic CTPA examination should have a contrast-enhanced blood attenuation of 200–250 HU with a noise level below 22 HU [28, 33, 34]. Low mA MBIR-CTPA reached both criteria with a mean attenuation of 340 ± 80 HU and a background noise of 6 ± 1. The subjective image quality of all patients with a BMI less than 30 kg/m2 was considered by both readers as diagnostic, except for one patient. The subjective image quality of all patients with a BMI greater than 30 kg/m2 was graded as insufficient for diagnostic purpose (in six patients). This corresponds to 43 % of potentially non-diagnostic CTs in this category of patients. The use of low mA MBIR-CTPA protocols in obese patients (i.e. a BMI greater than 30 kg/m2) should then be strongly discouraged. Future studies should be undertaken to investigate how far the dose could be reduced in accordance with patient BMI.
Study limitation
Two different cohorts of patients were investigated and therefore a direct comparison in the same patients between low mA MBIR-CTPA and routine FBP-CTPA was not possible. It was difficult, from an ethical point of view, to inject each patient twice during the same imaging session, which is why two groups of patients have been compared.
There is only one PE in the low mA MBIR-CTPA protocols (Fig. 2), which is clearly not enough to draw conclusions of the clinical efficacy of this new low dose protocol. Nevertheless, the primary objective of this study was to establish the possibility of acquiring low mA MBIR-CTPA and to assess its image quality. Dose reduction was obtained using reduction of mA without reduction of kVp, leaving the higher attenuation of iodine at low kVp unutilized. We were afraid of having too many artefacts in the shoulder region using low kVp and low mA. This should be studied in the future.
Moreover, as the patients included in the low mA MBIR-CTPA protocol were not suspected of having PE, the probability of finding a PE was low.
Since MBIR is one of the only fully iterative algorithms commercially available [12], it is unclear if our results are applicable to other iterative reconstruction algorithms.
Conclusion
Our prospective study showed that low mA MBIR-CTPA allows a significant dose reduction while improving SNR and CNR in the pulmonary vessels, as compared with routine FBP-CTPA. This first study was a necessary step to control image quality of low mA MBIR-CTPA. The results showed that low mA MBIR-CTPA can now be studied in the clinical context of suspected PE.
Abbreviations
- CTPA:
-
Computed tomography pulmonary angiography
- DLP:
-
Dose–length product
- FBP:
-
Filtered back projection
- MBIR:
-
Model-based iterative reconstruction
- SSDE:
-
Size-specific dose estimate
References
Furlan A, Aghayev A, Chang CC et al (2012) Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology 265:283–293
Sheh SH, Bellin E, Freeman KD et al (2012) Pulmonary embolism diagnosis and mortality with pulmonary CT angiography versus ventilation-perfusion scintigraphy: evidence of overdiagnosis with CT? AJR Am J Roentgenol 198:1340–1345
Remy-Jardin M, Pistolesi M, Goodman LR et al (2007) Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society. Radiology 245:315–329
Mettler FA Jr, Huda W, Yoshizumi TT et al (2008) Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology 248:254–263
Heyer CM, Mohr PS, Lemburg SP et al (2007) Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 245:577–583
Zamboni GA, Guariglia S, Bonfante A et al (2012) Low voltage CTPA for patients with suspected pulmonary embolism. Eur J Radiol 81:e580–e584
Henes FO, Groth M, Begemann PG et al (2012) Impact of tube current-time and tube voltage reduction in 64-detector-row computed tomography pulmonary angiography for pulmonary embolism in a porcine model. J Thorac Imaging 27:113–120
Tack D, De Maertelaer V, Petit W et al (2005) Multi-detector row CT pulmonary angiography: comparison of standard-dose and simulated low-dose techniques. Radiology 236:318–325
Kalra MK, Woisetschlager M, Dahlstrom N et al (2013) Sinogram-affirmed iterative reconstruction of low-dose chest CT: effect on image quality and radiation dose. AJR Am J Roentgenol 201:W235–W244
Li Q, Yu H, Zhang L et al (2013) Combining low tube voltage and iterative reconstruction for contrast-enhanced CT imaging of the chest-initial clinical experience. Clin Radiol 68:e249–e253
Vardhanabhuti V, Loader RJ, Mitchell GR et al (2013) Image quality assessment of standard- and low-dose chest CT using filtered back projection, adaptive statistical iterative reconstruction, and novel model-based iterative reconstruction algorithms. AJR Am J Roentgenol 200:545–552
Willemink MJ, de Jong PA, Leiner T et al (2013) Iterative reconstruction techniques for computed tomography Part 1: technical principles. Eur Radiol 23:1623–1631
Deak Z, Grimm JM, Treitl M et al (2013) Filtered back projection, adaptive statistical iterative reconstruction, and a model-based iterative reconstruction in abdominal CT: an experimental clinical study. Radiology 266:197–206
Katsura M, Matsuda I, Akahane M et al (2013) Model-based iterative reconstruction technique for ultralow-dose chest CT: comparison of pulmonary nodule detectability with the adaptive statistical iterative reconstruction technique. Invest Radiol 48:206–212
Pickhardt PJ, Lubner MG, Kim DH et al (2012) Abdominal CT with model-based iterative reconstruction (MBIR): initial results of a prospective trial comparing ultralow-dose with standard-dose imaging. AJR Am J Roentgenol 199:1266–1274
Neroladaki A, Botsikas D, Boudabbous S et al (2013) Computed tomography of the chest with model-based iterative reconstruction using a radiation exposure similar to chest x-ray examination: preliminary observations. Eur Radiol 23:360–366
Christensen E (2007) Methodology of superiority vs. equivalence trials and non-inferiority trials. J Hepatol 46:947–954
Kanal KM, Stewart BK, Kolokythas O et al (2007) Impact of operator-selected image noise index and reconstruction slice thickness on patient radiation dose in 64-MDCT. AJR Am J Roentgenol 189:219–225
European Commission (1999) European guidelines on quality criteria for computed tomography. Report EUR 16262. European Commission, Brussels
Brady SL, Kaufman RA (2012) Investigation of American Association of Physicists in Medicine Report 204 size-specific dose estimates for pediatric CT implementation. Radiology 265:832–840
Brink JA, Morin RL (2012) Size-specific dose estimation for CT: how should it be used and what does it mean? Radiology 265:666–668
Bankier AA, Kressel HY (2012) Through the looking glass revisited: the need for more meaning and less drama in the reporting of dose and dose reduction in CT. Radiology 265:4–8
Coche E, Verschuren F, Keyeux A et al (2003) Diagnosis of acute pulmonary embolism in outpatients: comparison of thin-collimation multi-detector row spiral CT and planar ventilation-perfusion scintigraphy. Radiology 229:757–765
Stein PD, Fowler SE, Goodman LR et al (2006) Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 354:2317–2327
Winer-Muram HT, Rydberg J, Johnson MS et al (2004) Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography. Radiology 233:806–815
Diederich S (2003) Radiation dose in helical CT for detection of pulmonary embolism. Eur Radiol 13:1491–1493
Wells PS, Anderson DR, Rodger M et al (2001) Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med 135:98–107
Mayo J, Thakur Y (2013) Pulmonary CT angiography as first-line imaging for PE: image quality and radiation dose considerations. AJR Am J Roentgenol 200:522–528
Litmanovich D, Boiselle PM, Bankier AA et al (2009) Dose reduction in computed tomographic angiography of pregnant patients with suspected acute pulmonary embolism. J Comput Assist Tomogr 33:961–966
MacKenzie JD, Nazario-Larrieu J, Cai T et al (2007) Reduced-dose CT: effect on reader evaluation in detection of pulmonary embolism. AJR Am J Roentgenol 189:1371–1379
Kallen JA, Coughlin BF, O'Loughlin MT et al (2010) Reduced z-axis coverage multidetector CT angiography for suspected acute pulmonary embolism could decrease dose and maintain diagnostic accuracy. Emerg Radiol 17:31–35
Martillotti J, Silva N, Chhabra J et al (2013) Dose of reduced z-axis length of computed tomography angiography (CTA) of the chest for pulmonary embolism using 64-detector rows and adaptive iterative reconstruction techniques. Emerg Radiol 20:39–44
Jones SE, Wittram C (2005) The indeterminate CT pulmonary angiogram: imaging characteristics and patient clinical outcome. Radiology 237:329–337
Ramadan SU, Kosar P, Sonmez I et al (2010) Optimisation of contrast medium volume and injection-related factors in CT pulmonary angiography: 64-slice CT study. Eur Radiol 20:2100–2107
Acknowledgments
Statistical support was provided by the Clinical Research Center, University of Geneva and Geneva University Hospitals (Delphine Courvoisier). The scientific guarantor of this publication is Xavier Montet. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. No complex statistical methods were necessary for this paper. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: prospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Montet, X., Hachulla, AL., Neroladaki, A. et al. Image quality of low mA CT pulmonary angiography reconstructed with model based iterative reconstruction versus standard CT pulmonary angiography reconstructed with filtered back projection: an equivalency trial. Eur Radiol 25, 1665–1671 (2015). https://doi.org/10.1007/s00330-014-3563-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-014-3563-5